Abstract
Aims: To evaluate the feasibility and safety of the Nile Croco® coronary bifurcation stent system (Minvasys, Gennevilliers, France).
Methods and results: The primary endpoint was to assess the acute device success and angiographic success with the use of the Nile Croco® stent system. Secondary endpoints included in-hospital and six month major cardiac events (MACE).There were 151 consecutive patients enrolled in the Nile Croco Study at Vall Hebrón Hospital. The Nile Croco® stent was successfully implanted in 144 patients (95.4%) and final angiographic success was obtained in 100% of the patients. 138 out of the 151 (91%) patients included have accomplished the six month follow-up. There was one in-hospital MACE in the 151 recruited patients. The MACE rate at six months in the 138 patients with follow-up was 14% and the ischaemia-driven TLR rate was 7.2 %.
Conclusions: The results of our Nile Croco® Study are the first to demonstrate the safety and high performance of this dedicated stent system for the treatment of bifurcation lesions. The device can be successfully implanted in more than 95% of all cases, with a high procedural success rate and low in-hospital and six month MACE rates.
Introduction
A true bifurcated lesion in coronary atherosclerotic disease is present in approximately 15% of patients referred for percutaneous coronary intervention1. The percutaneous approach to this especially complex scenario has been treated in many different ways depending on the techniques available at the time2-4. In recent years, the introduction of drug-eluting stents (DES) has remarkably improved the outcome in bifurcation lesions compared to bare metal stents (BMS), resulting in lower adverse events and lower main branch (MB) restenosis rates5-7. However, the most suitable approach concerning the side branch (SB) remains uncertain. Although the initial “provisional” stenting technique (i.e., stenting of the SB after MB stenting only in case of suboptimal or inadequate results) is probably the prevailing approach, the two stent techniques –i.e., crush, V, T, culottes– that allow the stenting of both branches are appealing. However, even if the strategy of stenting both branches when the SB stenosis is suitable for stenting is promising it has been assessed in the literature that the clinical outcomes are better if we can avoid using the two-stent strategy routinely8.
The introduction of dedicated stents may offer an attractive strategy to approach the different types of bifurcation lesions. These stents are specifically designed to provide good deliverability, secured access to the side branch and complete coverage of the lesion site without double/triple layers of stent struts. In addition, they will soon incorporate the benefits of drug elution and ensure drug availability to all diseased surfaces. The objective of our observational prospective study was to assess the angiographic and 6-months clinical results of a cohort of patients with bifurcation lesions who underwent percutaneous intervention with dedicated stents.
Methods
Patients and lesions
From May 2005 to November 2009, all consecutive patients in whom a Nile Croco® stent was intended to be used were enrolled in the study at our institution (Hospital Universitari Vall d’Hebron in Barcelona, Spain). There were no exclusion criteria apart from the anatomy of the lesion type (0,0,1) from the Medina Classification (type 4b in Massy class)9. The intention to treat with this stent was left to the discretion of the operator, and there were neither clinical nor angiographic exclusion criteria. All the patients gave their informed consent before the use of the device for the study.
Our data from the same period shows that from May 2005 to Nov. 2009, 673 bifurcated lesions were treated (considered bifurcated if the side branch vessel involved had a visually estimated reference diameter ≥2.0 mm and at least one guidewire was placed to protect the branch). Of the 673 bifurcated lesions from 657 patients, 163 (24,8%) were urgent PCI procedures. The global stent per patient ratio was 1.71 with 732 (65%) drug eluting stents (DES) and 397 (35%) bare metal stents (BMS). Nile stents accounted for 150 out of these 397 BMS (37.8%). These numbers show that for the study period, 506 patients were not considered eligible for the Nile Stent device. The main reasons reported for not using the Nile stent were as follows: 322/506 due to a DES indication of use (63%) for the bifurcated lesion or another lesion treated in the same patient, 97/506 (19.1%) due to an obvious superior length of the main vessel lesion which would have been difficult to cover with the Nile stent, 31/506 (6.1%) eccentric localisation of the lesion that would have been unsuitable for the optimal positioning of the Nile device and the covering of the lesion and finally in 86/506 (16.9%) cases were due to the lack of experience of the operator with the device.
Study device
The Nile Croco® intracoronary stent system consists of an 18 mm balloon expandable chromium cobalt bare metal stent premounted on a dedicated delivery system with two independent balloons each with a rapid exchange lumen for the two guidewires required. The MB balloon diameter is 2.5, 3 and 3.5 mm and the SB balloon 2.0, 2.5 and 3 mm. There are five devices available combining these sizes: MB 2.5 SB 2.0 / MB 3.0 SB 2.0 / MB 3.0 SB 2.5 / MB 3.5 SB 2.5 and MB 3.5 SB 3.0. The Nile Croco® intracoronary stent is compatible with 6 Fr guiding catheters (inner diameter ≥0.70”) and is a dedicated MB stent proximally crimped over the tip of the side branch catheter (SB). The stent consists of three different segments. The distal one includes 6 to 8 cells (depending on the size), the median one includes 8 to 10 cells and the proximal one includes 7 to 9 cells. This design ensures the same metal / artery ratio along the length of the bifurcation and avoids cell overstretching, considering that the morphology of the artery at the bifurcation site is not cylindrical and thus it should accommodate the SB portal once implanted. The delivery system is based on two separate rapid-exchange balloons, one for the stent deployment in the MB and the other for opening the stent struts towards the SB. It allows final kissing balloon inflation and a strategy of MB stenting with provisional SB stenting. The design incorporates an auto-release sheath that covers both catheter shafts to avoid distal dislodgement of the tip of the balloons while pushing the entire system/stent forward to the lesion site.
Compared to other dedicated main branch stent devices for bifurcation such as the Frontier stent, the Nile system does not need a third wire nor does it leave the MB wireless. Some stents, specifically designed for side branch stenting require retrieval of the wire from one branch for placement in the other (Tryton stent; Tryton Medical, Inc., Durham, NC, USA). Even the simplest techniques for treatment of bifurcated lesions, such as provisional stenting with a tubular stent, require wire entrapment with the potential risk associated of wire breaking or hydrophilic coating delamination during the retrieval.
Stenting procedure
Consecutive patients only underwent predilatation of the target lesion after wiring both branches of the bifurcation lesion if the operator considered it necessary. A Nile Croco® intracoronary stent system of an appropriate size was selected according to the MB and SB distal reference diameters. The device was then loaded onto both wires and pushed up to the lesion (Figures 1A and 2). It was placed where the central marker of the MB balloon matched the origin of the SB. If there was no wire wrapping, the stent phased with the bifurcation by a self rotation, and it was stopped at the level of the carina. A resistance was usually felt at this time. The correct positioning was checked by angiography and occasionally by stent boost. If there were difficulties to reach the desired position, the SB wire was pulled back proximal to the carina and the SB was rewired in order to avoid or to solve the possible wire wrapping of the two wires. We classify as “criss-cross” if the rewiring solved the stent position and if not “out-of-phase” when, despite the rewiring manoeuvre, the stent position at the carina was not perfectly aligned to the opening of the SB. Wire wrapping is common when we use a device that joins two wires intended for different branches, and tactics have been widely described in the literature on how to deal with this issue. Once the stent was placed in the desired position it was deployed by inflation of the MB balloon (Figures 1B and 2). The long tip of the SB balloon emerges from the mid-part of the stent to the SB. Then, the SB balloon was moved forward and was placed at the level of the carina to perform the kissing balloon inflation at the level(s) desired (Figures 1C, 1D and 2). The special shape of the proximal part of the SB balloon is designed to avoid overexpansion of the proximal segment of the bifurcation while performing the kissing balloon thus permitting an anatomic final deployment of the stent at the carina. The delivery system was then removed (Figures 1E and 2). The SB may or may not be post-treated with additional balloon inflation or stent implantation according to the operator’s criteria.

Figure 1. Deployment sequence of the Nile Croco® stent. A) The system is pushed up to the lesion. B) The MB is inflated with deployment of the stent. C) The SB balloon is pushed and placed at the level of the carina. D) To perform the kissing balloon inflation at the desired level.
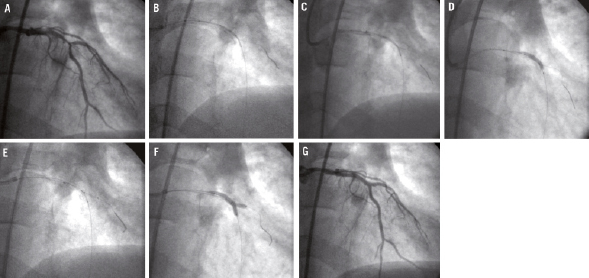
Figure 2. Implantation of a Nile Croco® in a patient with multivessel disease and bifurcation lesion at LAD / second diagonal. A) Lesion before treatment. B) Stent almost positioned onto the carina. C) Stent perfectly positioned onto the carina. D) Deployment of the stent with inflation of the MB balloon. E) The SB balloon is pushed and placed at the level of the carina. F) Kissing balloon with the Nile delivery/deployment system. G) Final result.
Patients received aspirin (>100 mg) and a loading dose of 300mg of clopidogrel. Anticoagulation therapy as well as the use of glycoprotein IIb/IIIa agents were left to the discretion of the operators. After the procedure, all patients received aspirin (≥75 mg/day) and clopidogrel (≥75 mg/day for at least one month).
Endpoints and definitions
The primary endpoints were stent placement success (the stent with the delivery system reaches the desired position at the carina of the bifurcation for the MB and the SB balloon crosses the struts with a gentle push). Angiographic success with the device for the MB and SB: we considered the Nile successful in the MB and in the SB if the Nile stent alone succeeded in achieving a good angiographic result. We considered final angiographic success the last angiographic procedure result even when other supplementary stents or devices were used. Secondary endpoints were in-hospital and 6-month events that included major adverse cardiovascular events (MACE) and other in-hospital severe adverse events such as stent occlusion or haemorrhagic complications requiring blood transfusion or surgery. MACE were defined as cardiac death, Q-wave and non Q-wave myocardial infarction and target lesion revascularisation (TLR). TLR was defined as MB and/or SB target site revascularisation by either PCI or CABG.
All patients were followed-up by phone call following previously informed consent.
Results
Between May 2005 and November 2009 we enrolled 151 patients at our institution for the study; 43 patients from this registry were reported in a previous multicentre international registry (three centres). MACE criteria remains identical in both registries.
One hundred and thirty eight (138) out of 151 (91%) patients had follow-up at 6-months. Three patients were lost to follow-up and 10 cases were included too recently to complete the scheduled time (six months).
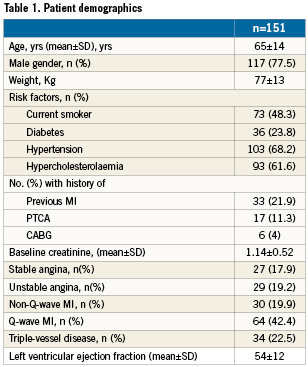
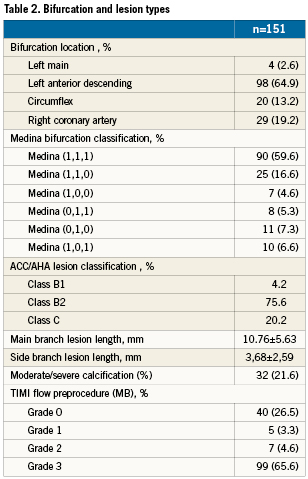
Patients demographics in the study group are summarised in Table 1 and bifurcation and lesion types are described in Table 2.
Procedural data
The radial approach was used in 114 (75%) of the cases with a 6 Fr catheter in 124 (82%). Urgent PCI was the clinical indication in 55 patients (36%). Predilatation was used in 121 cases (81%), use of debulking technique with cutting balloon in 22 (15%) and rotablator in four cases (3%). Final kissing balloon was done in more than 95% of the cases, with additional stents in MB in 19% of the cases and only 10% in the SB (Table 3).

In nine out of 151 cases (5,9 %) the stent was retrieved completely out of the guiding catheter because of difficulties in advancing the stent through the proximal part of the lesion without any wire loss. As previously reported, there was one case where the stent could not be delivered to the crux due to severe tortuousity. The lesion was treated with a conventional tubular stent. In the remaining eight cases: there was predilatation in three patients where direct Nile stenting was previously intended, and in the other five cases an aggressive second predilatation was done after which the Nile stent was well positioned at the level of the carina.
In 55 patients the procedure was done during an acute myocardial infarction so enzymes and troponins were registered each four hours during the first 24 hours. However, in the other subgroup of patients myocardial markers were registered only if complications during the procedure or residual chest pain occurred (two cases reported out of the 96 that were not AMI).
The median contrast volume used was 204 ml (98-500) and 20 minutes of fluoroscopy (7.3-52.3) with a median X-ray dose calculated as PDA (product dose-area) to the patient of 171.3 Gy/cm2 (34-849.4). During the same period, the total cohort of patients with a bifurcated lesion treated in our centre showed a mean amount of contrast of 243 ml (80-645), mean fluoroscopy time of 26.3 min (4.3-91.2), and an x-ray dose calculated at 256.6 Gy/cm2 (13.3-6665).
Despite successful pre-implantation device positioning in 95.4% of patients, criss-cross as previously described happened in 49 cases (33%) and out-of-phase in 29 (20%) patients. Optimal angiographic device success in the MB occurred in 99.3% with the Nile stent and complete angiographic success in the MB was achieved in 100% of the cases. In the SB, the system achieved an optimal result in 89.4% of the cases and despite using an additional stent for the SB in 9.7%, the final angiographic result was considered optimal (<30%) only in 96% of the cases (Table 4).

Clinical endpoint analysis
There was one confirmed in-hospital MACE in the 151 recruited patients (0, 6%). It was a death due to refractory shock in an 81 year old patient with three-vessel disease and severe mitral regurgitation who had been rejected for cardiac surgery.
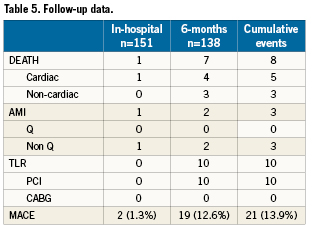
The MACE rate at 6-months in the 138 patients with complete follow-up was 14%. Clinical and/or ischaemia-driven TLR rate was 7.2 % (Table 5).
Discussion
Our study shows a high rate of device success (95%) and favourable acute and long-term clinical results, as measured by MACE at 180 days. In addition, the final procedural success achieved (100%) provides a very good index of safety considering the more complex lesions that were treated with this novel and dedicated device in non-selected patients.
A true bifurcated lesion in coronary atherosclerotic disease is present approximately in 15 to 25% of patients. The percutaneous approach to this especially complex scenario has been treated in many different ways depending on the techniques available at the time. Thus the angiographic result using the initial simple balloon technique1,2, improved with the “kissing-balloon” technique, which minimises the plaque shift to the side branch3,4. Thereafter, the athero-ablative techniques (rotational and directional atherectomy) decreased the TLR rate, but increased the procedural complications after the percutaneous approach5-7. The use of stents for the scaffolding of the plaque treated became the treatment of choice. Although the use of one or two bare metal stents for the treatment of bifurcation lesions was initially successful, it was associated with high restenosis rates regardless of the technique used8,9.
The first bifurcated stents appeared in the 1990s, but their rigidity and poor profile were associated with a low success rate10. Consequently, the idea of treating the MB while protecting the SB with a single dedicated stent and delivery system emerged. The first controlled study with angiographic controlled follow-up was carried out with the Multi-Link Frontier coronary bifurcation stent system (Abbott Laboratories. Abbott Park, Il, USA)11. This data, using a first generation stent, showed a device success rate of 91% and a very good safety profile (2,9% MACE at one month and 17.1% at 6-months, only 11,4% ischaemia driven TLR at 6-months). Furthermore, other devices developed by various companies have also been studied, showing similar results in the initial feasibility studies12-15.
The patients included in this study were older than initially expected (mean 65±12 years; 11% of patients above 80 years) and more than one-third (36%) treated by urgent PCI in an evolving myocardial infarction. One probable explanation is a possible selection bias during the recruitment. Thus the patients included would be those who were not optimal candidates for a DES. Although we did not record all prognostic factors to accurately determine the population and arteries risk profile, the prevalence of diabetic patients (22%) as well as the mean diameter of the vessels treated indicate a low risk and thus a favourable population for angiographic success and low clinical outcomes. On the contrary, the rather low prevalence of bifurcation lesions involving the LAD (67%) is clearly lower than that reported in previous studies (Lèfevre et al11 – 80%) or initial experiences. This should have led to poorer results in our series (which was not the case), since the LAD bifurcation lesion has shown in other studies to be an independent predictor factor for angiographic and clinical success.
The SB was stented in 9.7% of cases despite the fact that 71% of the lesions involved the SB (lesions Medina 90 [1,1,1] +8 [1,0,1]) + 10 [0,1,1]). The rate of final kissing balloon (97%) suggests an easy SB access that would encourage the operator to stent the SB if the angiographic result had been poor. However, the residual stenosis after implantation of the Nile stent at the SB was visually estimated below 30% in 89.4% of the cases. This remarkable beneficial effect of the device in the SB may be related to the design of the dedicated stent. Its shape and structure may avoid some spurious angiographic visual effects that are occasionally visualised with the tubular single stents. For instance, a stenosis involving the SB ostia may be misleadingly interpreted as struts along the SB ostia when using tubular single stents. Another additional reason may be the small protrusion of the struts that face the SB before the kissing balloon, scaffolding the very proximal part of the SB after the kissing balloon.
Clinical outcome
The Nile Croco® stent provided favourable acute and long-term clinical results, as measured by MACE at 180 days compared with other studies11-13,16,17. The rate of the secondary endpoint event, MACE at six months, was 13.9%. Ischaemia-driven TLR was necessary only in 7.2% of the cases, which is a better outcome rate then that reported in previous studies undertaken with the T-provisional stenting approach16,17,20-22. It is very important to note that due to the lack of exclusion criteria in the study the profile of the patients recruited is clearly biased towards acute phase patients with a bigger mortality index in the short- and mid-term follow-up. Clinical outcomes of this registry will be compared to bifurcation studies in the DES era, some of them with very promising results such as NORDICI (Table 6). Nevertheless we should take into account that one of the major difficulties is that we compare non-dedicated DES to a dedicated BMS system in different populations, with different inclusion criteria and different levels of disease located at the bifurcated lesion.

Future
Stent thrombosis has emerged recently as a major drawback of DES and it appears that bifurcation compared to non-bifurcation lesion treatment with DES is associated with a higher stent thrombosis rate23-25; and treatment of bifurcation with tubular DES is not an approved FDA indication and should therefore be considered with caution. Some dedicated DES devices for bifurcation have been studied with promising preliminary results26, however, the general expectations are that bifurcation treatment will become easier when the best platform for drug delivery is finally associated with a dedicated stent. The Nile Croco® stent shows promising results in the acute- and mid-term and that should encourage its use as a platform for dedicated DES.
Study limitations
The Nile Croco® registry is a study designed to examine procedural safety and clinical feasibility of one particular dedicated stent. It does not compare other bifurcation lesion treatments, and does not have the statistical power to address issues such as predictive factors of MACE during follow-up.
Conclusions
Bifurcation lesions have been treated for a long time with a large variety of stenting approaches, from the simplest to the most complex. Since tubular or conventional stents are not designed to treat bifurcation lesions, these devices frequently jeopardise the SB during and after the procedure thus increasing the risk of MACE when compared to non-bifurcation lesions. Therefore, the dedicated bifurcated DES stents may be a real improvement for the management of these complex lesions.
Conflict of interest statement
The authors have no conflict of interest to declare.

