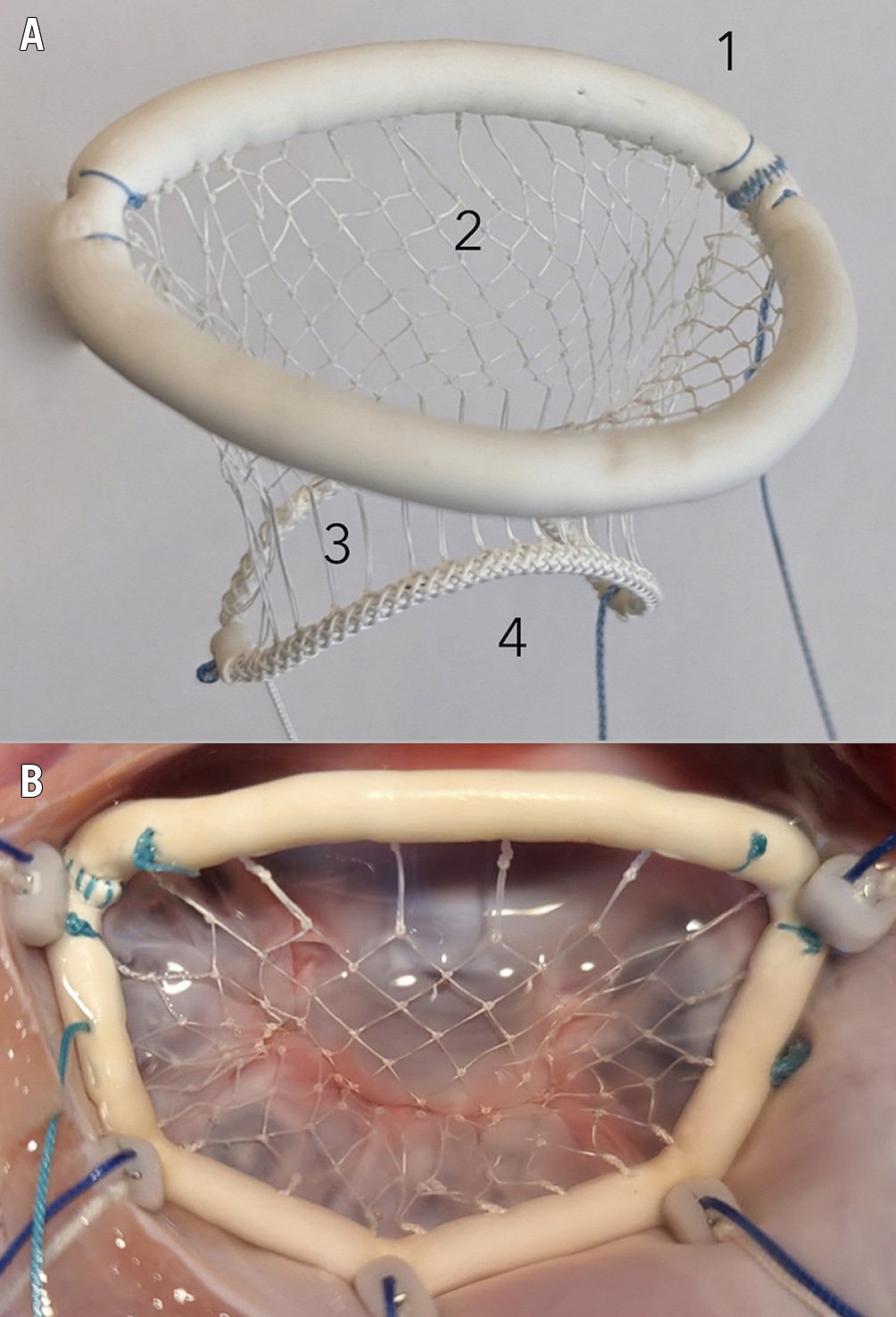
Figure 1. The Vesalius transfemoral repair device. A) Device components: 1. annuloplasty ring, 2. leaflet net, 3. multisegment neochords, and 4. adjustment arches. B) The implanted device corrects anterior leaflet prolapse and MR.
Surgical mitral valve repair for degenerative mitral regurgitation (DMR) is effective for all prolapse complexities with or without annular dilation, a goal not yet achievable with existing transfemoral solutions.
A transfemoral mitral repair device that provides both chordal replacement (for leaflet prolapse) and annuloplasty (for annular dilation) could challenge the dominance of surgical repair. If the concept is feasible for all valve segments, then for such a device the scope of treatable patients would be comprehensive and approach that of surgical repair, that is, any prolapse complexity, any annular size, and any mitral valve area.
These considerations form the design basis for the Calla transcatheter mitral valve repair (TMVr) system, a preclinical solution under development by Vesalius Cardiovascular. Their discovery that leaflet prolapse and mitral regurgitation (MR) can be corrected by a net that covers the diseased leaflet, bridging the annulus to the papillary muscle, is the exploited chordal repair concept, but the concept is extended to become a circumferential net that corrects prolapse in any valve segment. Supported by an annular ring with annuloplasty functionality, the device provides both components of surgical repair in one solution. Highly adjustable, it represents a one-size-fits-all solution for DMR patients with any disease complexity, annular size, or mitral valve area.
Figure 1A shows the device and its features. The annuloplasty ring (1) is secured to the annulus, and is expandable to fit 95% of DMR annuli, yet contractable posteriorly to achieve annular reduction, if required, for adequate leaflet coaptation. The leaflet net (2) is made of expanded polytetrafluoroethylene (ePTFE) and flexibly covers all leaflet segments. Extending from the net are multiple vertical ePTFE strands, the multisegment neochords (3), which determine the position of the net and correct prolapse in all segments. Distally, the neochordae are attached to the adjustment arches (4), which allow manipulation of the neochordae to meet the individual anatomic requirements of the patient.
Figure 1B shows the implanted device. Preclinical data demonstrate effectiveness in the correction of prolapse and MR. Tissue coverage of the device occurs within 90 days, with no evidence of injury or migration, and no thrombosis or thromboembolism. Papillary anchors carrying the load of the anterior leaflet are durable in acute “worst-case scenario” haemodynamics and chronic 300-day experiments.
Standardised delivery uses a 29 Fr transseptal approach. Tethered anchors reproducing surgical suture are placed into each papillary muscle, and five into the annulus. The repair device is delivered over the tethers to the precise anatomic position. The system gives the operator full control of all valve segments, with dial-like echo-based fine adjustability for a perfect repair, as seen in Moving image 1-Moving image 6.
The Vesalius solution is the first multisegment chordal replacement device supporting the valve from commissure to commissure, and the first solution to provide both components of a surgical repair, chordal replacement and annuloplasty, in a single device delivered transfemorally. Late recurrent prolapse and MR due to disease progression is prevented, and this unique feature may take long-term durability results to a new level.
Conflict of interest statement
P. Skarsgard is Director and Chief Scientific Officer of Vesalius Cardiovascular, which is developing the disclosed technology. C. Durkin is a clinical advisor to and shareholder of Vesalius Cardiovascular, which is developing the disclosed technology. J. Saw is a clinical advisor to and shareholder of Vesalius Cardiovascular, which is developing the disclosed technology.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation of the Calla TMVr device adjustment to correct leaflet prolapse.
Moving image 2. 3D echocardiogram of the Calla TMVr device in a beating heart.
Moving image 3. Proof of concept 1: Anterior leaflet prolapse.
Moving image 4. Proof of concept 2: Anterior leaflet prolapse and severe MR.
Moving image 5. Proof of concept 3: Calla TMVr corrects anterior leaflet prolapse.
Moving image 6. Proof of concept 4: Calla TMVr corrects severe MR.

