Abstract
Abstract: Stroke remains a devastating complication of transcatheter aortic valve replacement (TAVR), with the incidence of clinically apparent stroke seemingly fixed at around 3% despite TAVR’s significant evolution during the past decade. Embolic showers of debris (calcium, atheroma, valve material, foreign material) are captured in the majority of patients who have TAVR using a filter-based cerebral embolic protection device (CEPD). Additionally, in systematic brain imaging studies, the majority of patients receiving TAVR exhibit new cerebral lesions. Mechanistic studies have shown reductions in the volume of new cerebral lesions using CEPDs, yet the first randomised trial powered for periprocedural stroke within 72 hours of a transfemoral TAVR failed to meet its primary endpoint of showing superiority of the SENTINEL CEPD.
The present review summarises the clinicopathological rationale for the development of CEPDs, the evidence behind these devices to date and the emerging recognition of cerebral embolisation in many non-TAVR transcatheter procedures. Given the uniqueness of each of the various CEPDs under development, specific trials tailored to their designs will need to be undertaken to broaden the CEPD field, in addition to evaluating the role of CEPD in non-TAVR transcatheter heart interventions. Importantly, the cost-effectiveness of these devices will require assessment to broaden the adoption of CEPDs globally.
Introduction
Current American1 and European2 valvular heart disease guidelines favour transcatheter aortic valve replacement (TAVR) via transfemoral access for patients with severe aortic stenosis (AS) across the entire surgical risk spectrum (>65 years in US guidelines, >75 years in European Union [EU] guidelines). Stroke remains a devastating complication for TAVR recipients, imparting a 6-fold higher 30-day mortality compared with those without stroke post-TAVR345. The burden of stroke has a substantial effect on patients, their families, health services and society in terms of morbidity and mortality along with a significant socioeconomic impact67. Its prevention therefore represents a priority objective across all levels of healthcare in many geographies. From a patient’s perspective, stroke represents the most feared complication of TAVR89. Contemporary stroke rates related to TAVR remain at 2-4%31011, with no significant reduction in recent times34. Furthermore, the detection of clinical strokes and silent cerebral lesions post-TAVR is highly dependent on the intensity of the neurological examination and imaging modality used.
Cerebral embolic protection devices (CEPD) were developed to mitigate TAVR-related stroke along with the burden of cerebral embolic debris and have been shown to be safe in various clinical settings. However, their true efficacy in stroke prevention during TAVR remains to be demonstrated. Table 1 summarises the evidence to date of the safety and efficacy of various CEPD systems used in the TAVR population.
Table 1. Success and complication rates of CEPDs in major TAVR studies.
| Device | SENTINEL | TriGUARD | ProtEmbo | Emblok | Emboliner | Embrella | FIH CAPTIS | FLOWer | POINT-GUARD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study name | MISTRAL-C29 | CLEAN-TAVI23 | SENTINEL26 | PROTECTED TAVR37 | DEFLECT III40 | REFLECT II25 | PROTEMBO SF TRIAL (FIH)43 | PROTEMBO C TRIAL42 | Latib A et al41 | SafePass clinical program444546 | PROTAVI-C Pilot Study96 | CAPTIS47 | Embrace /NAUTILUS FIH study4849 | CENTER FIH trial50 |
| Study design | RCT | RCT | RCT | RCT | RCT | RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT |
| Number of patients | 65 (device arm: 32; control arm: 33) | 100 (device arm: 50; control arm: 50) | 363 (device safety arm: 123; device imaging arm: 121; control arm: 119) | 3,000 (device arm: 1,500; control arm: 1,500) | 85 (device arm: 46; control arm: 39) | 220 (device arm: 162 [41 roll-ins plus 121 randomised]; control arm: 58) | 4 | 41 (37 intention-to-treat cohort, of which 31 per-protocol cohort) | 20 | 63 (series of 3 single-arm feasibility studies: SafePass FIH: 13; SafePass 2: 31; SafePass 3: 19) | 52 (device arm: 41; control arm: 11) | 20 (data available for 11 patients) | 75 | 4 |
| Device success | 93.0% | 92.0% | 94.4% | 94.4% | 88.9% | 71.0% | 100% | 94.6% | 100% | 100% | 100% | 100% | 100% | 100% |
| Primary endpoint | Incidence of new brain lesions 5 to 7 days after TAVR, assessed by DW-MRI | Numerical reduction in positive post-procedure DW-MRI brain lesions relative to baseline at 2 days following TAVR in potentially protected territories | 1) Safety: incidence of MACCE at 30 days: all death, all strokes (disabling and non-disabling), and acute kidney injury (AKI; stage 3) according to VARC-2. 2) Efficacy: reduction in median total new lesion volume in protected territories between the device and control arms, as assessed by DW-MRI at 2 to 7 days after TAVR |
Clinical stroke within 72 hours after TAVR or before discharge (whichever came first) | In-hospital procedural safety (MACCE), a composite of all-cause mortality, all stroke (disabling and non-disabling), life-threatening (or disabling) bleeding, AKI (stage 2 or 3), and major vascular complications | 1) Safety at 30 days: a composite of all-cause death, stroke, life-threatening or disabling bleeding, stage 2-3 AKI, coronary artery obstruction requiring intervention, major vascular complications, and valve-related dysfunction requiring repeat procedure. 2) Efficacy: a hierarchical composite of (i) all-cause mortality or any stroke at 30 days, (ii) NIHSS worsening from baseline to 2-5 days post-procedure or MoCA worsening (decrease of 3 points or more from baseline) at 30 days, and (iii) total volume of cerebral ischaemic lesions detected by DW-MRI performed 2-5 days post-procedure |
Feasibility and safety | 1) Safety: MACCE at 30 days, defined as a composite of all-cause mortality, all stroke, life-threatening or disabling bleeding, major vascular complications in the access vessels or aorta, or AKI (stage 2 or 3), all according to VARC-2. 2) Performance: rate of technical success, defined as the ability to safely deliver, deploy, and remove the device; the ability to secure and stabilise the position of the device throughout the procedure; and to deflect embolic material, defined by coverage of the 3 cerebral vessels without impeding blood flow |
1) Technical success, defined as the successful insertion, placement, and removal of the Emblok device, and device performance was evaluated during and after completion of the TAVR index procedure.2) Immediate cerebral embolic burden after TAVR, defined as the number and volume of new brain lesions as detected with DW-MRI at days 2-5 post-TAVR compared with baseline | 1) Safety: incidence of 30-day MACCE (death, stroke, and stage 3 AKI) compared with a 12% historical performance goal. 2) Performance: the ability to successfully deploy and retrieve the device |
Feasibility, safety, and exploratory efficacy | Safety: incidence of mortality or cerebrovascular event at 72 hours and device-related complications | 1) Safety: incidence of 30-day MACCE (death, stroke, and stage 3 AKI) compared with a 14.3% historical performance goal. 2) Performance: technical success and system usability; debris captured and histopathological evaluation. 3) Clinical benefit: brain imaging (DW-MRI) at baseline and within 2-5 days after TAVR; neurocognitive protection assessed by NIHSS, MOCA and mRS at baseline, 2-7 days and 30 days after TAVR |
Safety and performance |
| Complications | ||||||||||||||
| Stroke/TIA | Device arm: 0; control arm: 2 patients within 30 days (disabling) | Device arm: 10%; control arm: 10% within 7 days (all non-disabling) | Device arm: 5.6%; control arm: 9.1% within 30 days (p=0.25) | Device arm: 2.3%; control arm: 2.9% within 72 hours (p=0.30) | Device arm: 2.2%; control arm: 5.1% within 72 hours (p=0.30) | Device arm: 6.4%; control arm: 5.3% in hospital (p=1.000) | 0% at 30 days | 1 (2.7%) patient (CEPD retrieved prematurely because of interaction with the TAVR catheter) | 0% at 30 days | 2 (6.5%) patients (at day 1 and at day 17 post-TAVR) in SafePass 2, and 1 (5.2%) patient in SafePass 3 (still to be adjudicated) | 2 strokes and 1 TIA in the device arm | 0% at 30 days | 3 (5.2%) strokes at 30 days (data from 58 patients) | Not reported |
| Vascular complications | Minor: device arm 39%; control arm 41% at 30 days (p=0.904). Major: CEPD arm 0%; control arm 19% (p=NA) | 1 (10%) patient in each treatment group with a life-threatening bleed | Device arm: 8.6%; control arm: 5.9% at 30 days (p=0.530). CEPD-related: 0.4%; TAVR-related: 8.2% | 1 (0.1%) patient in the device arm | Device arm: 15.2%; control arm: 15.4% (p=0.85) | Device arm: 7.0%; control arm: 0% in hospital (p=0.039). CEPD-related: 1.9%; TAVR-related: 4.5%; aortic vascular injury: 1.3% | 0% at 30 days | 8.1% (3/37): 2 haematomas and 1 dissection treated with balloon inflation | 1 (5.0%) patient at site of valve insertion | 1 patient with a minor oozing at the device access site in SafePass 2 | Device arm: 5 (12.2%); control arm: 1 (9.1%) (p≥0.999), at 30 days | 1 (5.0%) | Not reported | Not reported |
| AKI: acute kidney injury; CEPD: cerebral embolic protection device; DW-MRI: diffusion-weighted magnetic resonance imaging; FIH: first-in-human; MACCE: major adverse cardiac and cerebrovascular events; MoCA: Montreal Cognitive Assessment; mRS: Modified Rankin Scale; NA: not applicable; NIHSS: National Institutes of Health Stroke Scale; RCT: randomised controlled trial; TAVR: transcatheter aortic valve replacement; TIA: transient ischaemic attack; VARC-2: Valve Academic Research Consortium-2 | ||||||||||||||
Aetiology and timing of strokes during TAVR
TAVR-related stroke pathogenesis is multifactorial (Figure 1) and can be broadly categorised into acute − linked more with the TAVR procedure per se − and longer-term stroke events − related more to the patient’s atherosclerotic disease and overall comorbidity burden. A prior history of stroke, arterial/valvular calcium burden, bicuspid aortic valves, aortic valve pre-/postdilatation and valve-in-valve procedures have been identified as risk factors for periprocedural stroke311, while reduced renal function, diabetes mellitus and increasing age were found to be related to the incidence of late stroke12. Whether both periprocedural and longer-term stroke rates are directly related to the implantation procedure or the underlying type of aortic bioprosthesis (surgical vs transcatheter, intra-annular vs supra-annular transcatheter heart valves, deflectable/steerable vs non-deflectable/-steerable delivery systems) remains to be proven. This said, randomised data in lower-risk patients demonstrate lower stroke rates in TAVR versus surgical aortic valve replacement (SAVR) recipients13. To further complicate matters, clinical manifestations of stroke are broad and unpredictable, ranging from major stroke with typical disabling sequelae to more subtle episodes of postprocedural transitory delirium or an acute confusional state, to clinically silent cerebral embolisation (silent brain infarction) that can only be detected on brain imaging.
Most periprocedural TAVR-related ischaemic strokes are caused by athero- or thromboembolic events provoked by the disruption of atheromatous or calcific debris arising during several procedural steps14, as summarised in Figure 1.
Approximately 50% of events occur during the acute periprocedural phase (~72 hrs) and in 80% of patients the stroke is detected within the first week post-TAVR31011. Symptoms typically appear once periprocedural anticoagulation wears off and embolised debris forms a nidus for in situ thrombosis.
Numerous studies have shown the presence of “silent” ischaemic brain lesions, detected by diffusion-weighted magnetic resonance imaging (DW-MRI), in almost all patients who undergo TAVR/SAVR1516 (Table 2). Data from a systematic review and meta-analysis including 42 studies with a total of 2,632 patients showed that the incidence of new silent brain infarcts during TAVR (4.58±2.09) is greater than the incidence during SAVR (2.16±1.62), on- and off-pump coronary artery bypass graft surgeries (2.11±0.25), percutaneous coronary interventions (1.88±1.02), and mixed cardiothoracic surgeries (3.38±0.72)17. This reinforces the importance of mitigating strategies for cerebral embolisation during TAVR.
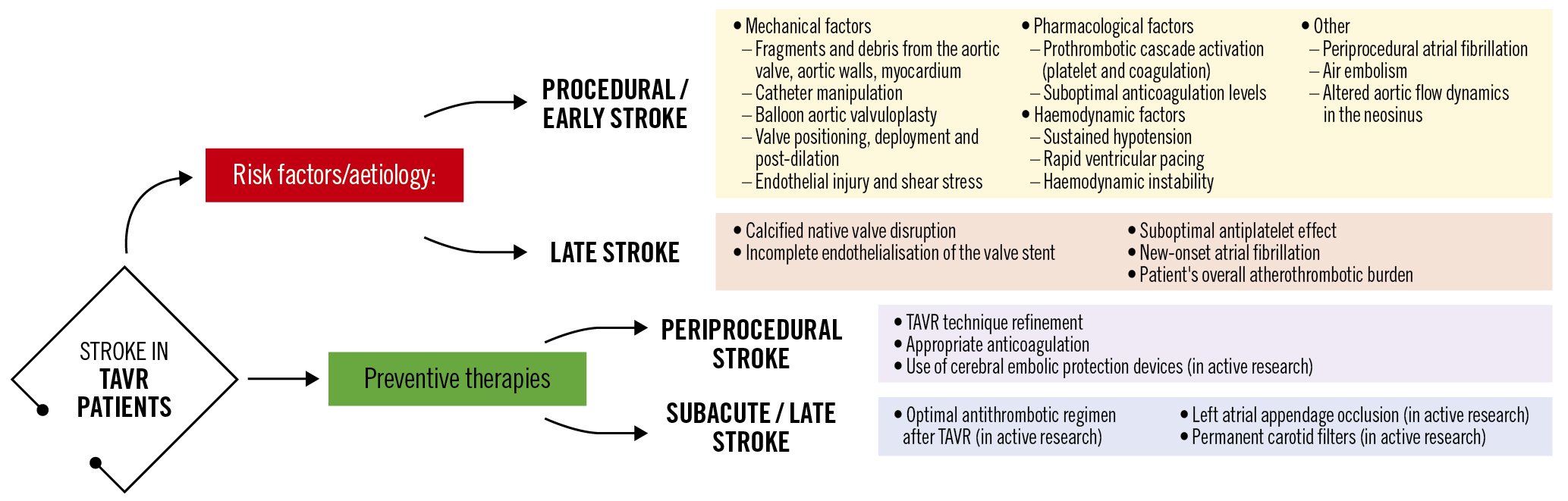
Figure 1. Potential mechanisms related to stroke during and after TAVR and preventive strategies. TAVR: transcatheter aortic valve replacement
Table 2. Brain MRI results and cognitive outcomes of CEPDs in major TAVR studies.
| SENTINEL | TriGUARD | ProtEmbo | Emblok | Emboliner | Embrella | FIH CAPTIS | FLOWer | POINT-GUARD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MISTRAL-C29 | CLEAN-TAVI23 | SENTINEL26 | PROTECTED TAVR37 | DEFLECT III40 | REFLECT II25 | PROTEMBO SF TRIAL (FIH)43 | PROTEMBO C TRIAL42 | Latib A et al41 | SafePass clinical program444546 | PROTAVI-C Pilot Study96 | CAPTIS47 | Embrace /Nautilus FIH study 4849 | CENTER FIH trial50 | |
| Brain DW-MRI results | New brain lesions at 5-7 days: 78% (73% vs 87%; p=0.31). Median total new lesion volume: 95 mm3 (10-257) vs 197 mm3 (95-525) (p=0.17). Median total lesion volume in protected areas: 0 mm3 (0-102) vs 76 mm3 (40-221) (p=0.057). Absence of new lesions: 13% vs 27% (p=0.31) | New brain lesions at 2 days: 98%. Median new lesion number: 4 (3.3-7.25) vs 10 (6.75-17.00) (p<0.001). Median total new lesion volume: 242 mm3 (159-353) vs 527 mm3 (364-830) (p=0.001) | Median total new lesion volume at 2-7 days: device arm: 102 mm3; control arm: 177 mm3 (p=0.33) | Not performed | New brain lesions at 30 days: 80.8%; device arm: 73.1%; control arm: 88.5% (per-treatment analysis) | Median total new lesion volume at 2-5 days: device arm: 215.39 mm3; control arm: 188.09 mm3 (p=0.405) | 50% reduction in number of new lesions compared to historical cohort, and 87% reduction of new lesions for the protected vs unprotected TAVR at 3 and 30 days | Median number of new lesions at 2-7 days: 8 (IQR 3-16). Median total new lesion volume: 210 mm3 (137-456 mm3). Average new lesion volume: 34 mm3 (24-45 mm3). Freedom from brain lesions >150 mm3: 87%.Freedom from brain lesions >350 mm3: 97% | New brain lesions at 2-5 days: 95%. Median number of new lesions: 10 (4.75-15.25). Median total new lesion volume: 199.9 mm3 (83.9-447.5). Mean new lesion volume per lesion: 42.5 mm3 (21.5-75.6) | Not performed | New brain lesions at 7 days: device arm: 100%; control arm: 100%. Median number of new lesions: device arm: 7.5 (3-13); control arm: 4 (2-8) (p=0.413). Median total new lesion volume: device arm: 305 mm3; control arm: 180 mm3 (p=0.909). Median lesion volume: device arm: 30 mm3 (20-50 mm3); control arm: 50 mm3 (30-70 mm3) (p=0.003). Lesion location | Not performed | Median total new lesion volume at 2-5 days: device arm: around 500 mm3 (32 patients); control arm: around 1,450 mm3 (4 patients) | Pre- and post-procedure DW-MRIs were conducted, no results reported |
| New neurocognitive deficit | Device arm: 4%; control arm: 27% (p=0.017) | No differences between both groups regarding NIHSS, mRS and moCA at 2, 7, and 30 days | No differencein overall composite scores at baseline, 30 days, or 90 days between device and control arms | No differences between both groups in NIHSS, mRS, MoCA and CAM-ICU scores | Worsening in NIHSS score from baseline with DW-MRI evidence of ischaemia: device arm 3.1%; control arm 15.4% (p=0.160) | No differences in NIHSS score worsening at discharge (14.1 vs 7.6, p=0.18) nor at 30 days post-procedure (7.8 vs 3.6; p=0.31), in the device vs control arm, respectively | No differences in MoCA at 30-day follow-up | No significant worsening of NIHSS in any ofthe patients at 30-day follow-up | 0% at 30 days (NIHSS) | Not reported | No differences in the median scores (NIHSS scale, the modified Rankin Scale and theBarthel Index) compared with baseline examination (p>0.15) | No increase of NIHSS score at follow-up. 1/11 patients had an increase in mRS score at 72h and 2/10 patients at 30 days | Not reported | Not reported |
| Other findings | Baseline brain MRI assessment confirmed ischaemic lesions in 11% of patients. Total lesion volume was greater in patients with self-expanding TAVR vs balloon-expandable TAVR (693 mm3 vs 266 mm3; p=0.067).In particular, the lesion volume in the posterior lobes was significantlygreater with self-expanding THVs (405 mm3 vs 92 mm3; p=0.037) | The mediannumber of periprocedural HITS was 3,196 (IQR 2,522-4,010) in the filter group and 3,674 (IQR, 2,551-5,217) in the control group | MACCE at 30 days: device arm: 7.3%; control arm: 9.9% (p=0.25). The change in neurocognitive scores from baselineto 30-day follow-up correlated with the median newlesion volume in protected territoriesand all territories | Disabling stroke: device arm: 0.5%; control arm: 1.3% (p=0.02)-Non-disabling stroke: device arm: 1.7% control arm: 1.5% (p=0.67)-Acute kidney injury: device arm: 0.5%; control arm: 0.5%-NNT for prevention of disabling stroke =125 | In-hospital MACCE (all-cause mortality, all stroke, life-threatening/disabling bleeding, acute kidney injury [stage 2 or 3]): device arm: 21.7%; control arm: 30.8% (p=0.34). New post-TAVR ischaemic lesions at 30-day follow-up DW-MRI were detected in 11.5% of device arm and 9.1% of control subjects (both mean single and maximum lesion volumes were 5.2±17.9 vs 3.3±11.9 mm3; p=0.78). All subjects undergoing TAVR with or without TriGUARD device showed DW-MRI ischaemic lesions when the CoreValve prosthesis was used | Primary safety endpoint at 30 days (all-cause mortality, all stroke, life-threatening/disabling bleeding, coronary artery obstruction requiring intervention, acute kidney injury [stage 2 or 3], major vascular complications, valve-related dysfunction requiring intervention): device arm: 15.9%; control arm: 34.4% (p=0.0001).Primary efficacy endpoint at 30 days (all-cause mortality, all stroke, NIHSS worsening, absence of DW-MRI lesions post-procedure, total volume of cerebral lesions by DW-MRI): device arm: 45.7%; control arm: 54.3% (p=0.857) | All three side branch vessels of the aortic arch were covered in all patients. Minimal to no interaction with TAVR catheters was reported. | MACCE at 30 days (all-cause mortality, all stroke, life-threatening/disabling bleeding, acute kidney injury (stage 2 or 3). The largest single lesion volume detected in any of the patients was 402 mm3. Dwell time of the device: 30.2±13.4 min (IQR: 16 to 69)Average time for device deployment: 4.5±4.9 min | No MACCE was observed at 30 days. Debris captured in 90% of cases.Patients with full protection showed significant lower new lesion volume compared to patients without full protection (median 176.1 mm3 [IQR 60.7 to 90.6 mm3] vs 519.7 mm3 [IQR 400.1 to 459.9 mm3]; p= 0.0402 (post hoc analysis). No difference in terms of reduction of new lesions was seen (8.0 [IQR 4.5 to 15.5] vs 13.0 [IQR 3.0 to 15.0], respectively; p=0.179).19 (95%) patients had new ischaemic defects at postprocedural DW-MRI. Median number of new lesions per patient: 10.00 (IQR 4.75 to 15.25), total new lesion volume: 199.9 mm3 (IQR 83.9 to 447.5 mm3), mean lesion volume per lesion: 42.5 mm3 (IQR 21.5 to 75.6 mm3). Dwell time of the device: 24.0 mins (IQR 19.2 to 27.7)Median time for device deployment: 2.0 mins (IQR 0.0 to 6.75) | MACCE at 30 days (death, stroke, and stage 3 acute kidney injury): 2 (6.5%) patients. Debris captured in 100% of cases. Average ~280 particles ≥150 μm and 2,151 particles ≥60 μm captured per patient. 66% of patients had at least 1 particle ≥1 mm size (data from SafePass 2 trial). Average 287 particles ≥150 μm and 3,175 particles ≥60 μm captured per patient (partial and preliminary results from the SafePass 3 trial, pending final adjudication) | There was 1 radial thrombosis with no clinical consequences and 1 pseudoaneurysm of the brachial artery that required surgical repair. At 30 days, the reported incidence of life-threatening bleeding, renal insufficiency/failure, and mortality occurred in 3 patients (7.3%) for each event (p>0.999).Total number of HITS during the procedure: device arm: 632; control arm: 279 (p<0.001). Median time for device deployment: 2 min (IQR 1 to 3) | No MACCE (all-cause mortality, all TIA and stroke) was observed at 30 days. Acute kidney injury at 72 hours (or discharge): 1 (5.0%).Debris was collected in all cases, with an average total number of collected particles of 1,448, with 112 particles ≥150 μm in size, 95 particles of 150-500 μm, 11 particles of 500-1,000 μm, and 6 particles of >1,000 μm. Median deployment and retrieval time of 8 and 4 minutes,respectively | Debris collected in all cases, with an average total number of collected particles of 420 per patient, with around two-thirds being particles ≤150 μm in size (data from 15 patients) | All 4 filters showed evidence of debris capture. 4 non-device-related adverse events were reported |
| CAM-ICU: Confusion Assessment Method for Intensive Care Unit Patients; CEPD: cerebral embolic protection device; DW-MRI: diffusion-weighted magnetic resonance imaging; HITS: high-intensity transient signals; IQR: interquartile range; MACCE: major adverse cardiac and cerebrovascular events; MRI: magnetic resonance imaging; mRS: modified Rankin Scale; MoCA: Montreal Cognitive Assessment; NIHSS: National Institutes of Health Stroke Scale; NNT: number needed to treat; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve; TIA: transient ischaemic attack; VARC-2: Valve Academic Research Consortium-2 | ||||||||||||||
Pathological insights of TAVR-induced debris and its impact upon neurocognitive functioning
Prior studies conducted in a high surgical risk population and recent evaluations in patients at low and intermediate surgical risk have shown the presence of captured material or debris in the vast majority of patients undergoing TAVR and transcatheter mitral valve interventions when using the SENTINEL CEPD (Boston Scientific) (Table 3). These analyses reported significant heterogeneity of debris type captured within the filters, consisting mainly of acute thrombus, arterial wall remnants, valve tissue, calcific debris, myocardial tissue, and even foreign material. Most of the captured particles were <500 μm in size, but large particles >1,000 μm (comprising nearly 5% of all captured particles) were detected in more than 2/3 of low-risk patients. This is relevant because, while small particles are linked to silent cerebral lesions detected on DW-MRI, larger particles (>1,000 μm) may account for clinically apparent strokes. The risk for larger particle embolisation has been found to be more common in patients with bicuspid aortic valves (odds ratio [OR] 2.91, 95% confidence interval [CI]: 1.20-7.03; p=0.02), with transcatheter heart valve repositioning being associated with a greater quantity of captured debris (OR 2.96, 95% CI: 1.42-6.16; p=0.004). Overall, the capture rate of debris, observed tissue types, and size/distribution of debris are comparable across the entire surgical risk spectrum (Table 3).
The potential impact of silent cerebral lesions on cognitive function and its prognostic relevance at medium- to long-term follow-up is still not well defined1819. Despite the lack of overt symptoms of stroke, silent brain infarcts are associated with subtle impairments in physical function and cognitive ability that usually pass unnoticed. The presence of silent strokes in an elderly population has been thought to increase the risk of major stroke and dementia along with a faster onset of cognitive impairment20. Although some reports suggest cognitive decline post-TAVR, others have reported that cognitive improvement can be achieved among TAVR recipients with pre-existing impaired cognitive function prior to the procedure, possibly related to haemodynamic improvement following resolution of aortic stenosis21. However, the initial data of neurocognitive tests were primarily obtained from elderly patients who had a high or prohibitive risk of surgery and a pre-existing impaired baseline neurocognitive function, which made the detection of subtle neurocognitive changes due to TAVR extremely challenging. Additionally, the population currently evaluated with neurocognitive testing remains small, the tests used to assess cognitive function are heterogeneous across studies, and clinical follow-up remains too short to be able determine long-term impact. Conclusive data are therefore currently lacking to tie the link between cerebral embolisation and longer-term cognitive decline. However, emerging data indicate that silent brain lesions may not be benign and that their mitigation in the younger TAVR population may ultimately prove clinically relevant.
Table 3. Summary of studies using CEPDs reporting histopathological analysis.
| Study | Device | N | Valve type | Percentage of patients with particles captured | Histopathological analysis/other clinical findings |
|---|---|---|---|---|---|
| AORTIC PROCEDURES | |||||
| Van Mieghem et al97 Circulation. (2013) |
SENTINEL | 40 | CoreValve: 36 (90%), SAPIEN: 4 (10%) |
75% | • The captured material varied in size between 0.15 mm and 4.00 mm.• Amorphous calcified material of 0.55-1.80 mm diameter (5/30, 17%) consisted of degenerative and calcified aortic valve leaflets.• Collagenous and proteoglycan matrix with elastic tissue, the longest segment of 0.25-4.00 mm, (8/30, 27%) consisting in endothelial cells of the aortic surface above the calcified area.• Pure collagenous material without any blood clot (4/30, 13%).• Thrombotic material consisting of platelets, fibrin, and erythrocytes, with and without neutrophils of 0.15 to 2.00 mm (21/30, 70%). Of those, 13 (62%) were acute thrombi and 8 (38%) were chronic thrombi (organising thrombus).• Foreign body material consisting of polymer used in catheters during TAVR (4/30, 13,3%).• Two (5%) cerebrovascular events were reported (1 major stroke in the left occipital cerebral lobe and 1 TIA). |
| Van Mieghem et al98JACC Sentinel Cardiovasc Interv. (2015) |
SENTINEL | 81 | CoreValve: 56 (69%), SAPIEN XT: 24 (30%), Other: 1 (1%) |
86% | • The median size of debris was 1 mm (IQR 0.6 to 1.5 mm) and varied between 0.1 mm and 9.0 mm.• Fibrin and thrombotic material of 0.2 to 6.2 mm (60/81, 74%).• Tissue-derived debris (51/81, 63%).• Degenerative aortic valve leaflets of 0.2 to 5.5 mm consisting in amorphous calcified material and collagenous and proteoglycan matrix with elastic tissue surrounded by endothelial cells (27/81, 33%).• Collagenous tissue of 0.2 to 1.5 mm of undetermined origin (either vessel wall or aortic valve leaflet) (14/81, 17%).• Endothelium strands of size 0.2 to 9 mm (39/81, 48%).• Myocardial tissue of 0.1 to 1.7 mm (13/81, 16%).• Small foreign body polymer material (8/81, 10%).• Captured tissue fragments were less frequent with CoreValve than SAPIEN XT (56% vs 79%; p=0.05).• There were no differences between patients with versus without captured debris regarding pre- and post-dilatation or access route (TA/TF/TS).• The type of transcatheter valve used and valve oversizing were found as predictors of tissue embolisation (multivariate analyses).• Two strokes after TAVR and one TIA with concomitant new onset atrial fibrillation were reported. |
| Schmidt et al99J Am Heart Assoc. (2016) | SENTINEL | 161 | SAPIEN 3: 86 (53%), SAPIEN/SAPIEN XT: 28 (17%), CoreValve/Evolut R: 20 (12%), Lotus: 8 (5%), Direct Flow Medical system: 8 (5%), Portico: 7 (4%), JenaValve: 3 (2%), Centera: 1 (1%) |
97% | • The most common captured debris in either the proximal or distal filter were thrombi (147/161, 91%), arterial wall (109/161, 68%), valve tissue (85/161, 53%), calcification (74/161, 46%), and foreign material (49/161, 30%).• Distribution of debris by filter location was not different.• Patients with aortic valve predilatation more commonly showed valve tissue captured (in either or both filters) compared with patients without predilatation (67% vs 43%; p=0.0294).• Valve tissue was found to be significantly increased in either or both filters in procedures with pre- and/or post-dilation (64% vs 41%; p=0.0313).• A trend towards more valve tissue captured was found in female versus male patients (63% vs 43%; p=0.0688), with more valve tissue in the distal filter in female patients (51% vs 28%; p=0.0304).• Female sex (p=0.0287) and diabetes mellitus (p=0.0116) were predictive of higher rates of debris in the filters.• Two (1%) cerebrovascular events were reported: 1 stroke at day 1 after TAVR (the filter of the left carotid artery was reported with malposition) and 1 TIA on day 3 after TAVR in a patient who developed new onset atrial fibrillation. |
| Mieghem et al29 EuroIntervention (2016) | SENTINEL | 65 (SENTINEL use: 32) | SAPIEN 3: 35 (54%), CoreValve: 16 (25%), SAPIEN XT: 10 (15%), Others: 4 (5%) |
100% | • The captured debris consisted of fibrin thrombus (26/30, 87%), endothelium (25/30, 83%), collagenous (11/30, 37%), valve tissue (10/30, 33%), myocardium (10/30, 33%), foreign body (9/30, 30%), amorphous calcium (8/30, 27%).• New brain lesions were found in 78% of patients at 5 days on follow-up MRI.• The CEPD group had numerically fewer new lesions and a smaller total lesion volume (95 mm3 [IQR 10-257] vs 197 mm3 [IQR 95-525]).• Patients with self-expanding valves had greater total lesion volume (693 mm3 [IQR 459-744]) compared to those with balloon-expandable valves (266 mm3 [IQR 155-358]; p=0.067). In addition, the posterior lobes of patients with self-expanding valves showed higher lesion volume (405 mm3 [IQR 332-530] vs 92 mm3 [IQR 40-240]; p=0.037).• Overall, there were more patients in the CEPD group with no new lesions (27% vs 13%; p=0.31), and less patients with ten or more new brain lesions (20% vs 0%; p=0.03) and with neurocognitive deterioration (4% vs 27%; p=0.017).• Two (7%) strokes at 30 days after TAVR were reported, both in the control (no device) arm. |
| Van Gils et al83Catheter Cardiovascular Interv. (2017) | SENTINEL and Wirion carotid filter (Cardiovascular Systems, Inc) | 11 | Lotus: 8 (72%), SAPIEN 3: 2 (18%), CoreValve: 1 (9%) |
100% in all filters | • Three-vessel protection was accomplished by using the SENTINEL and Wirion filters.• All filters captured debris.• Fibrin/thrombus in 100% of filters, endothelium (SENTINEL 9/11 patients vs Wirion 7/9 patients), foreign body material consisted of blue gel or coloured fibres, likely derived from catheters or the TAVR delivery system (SENTINEL 6/11 patients vs Wirion 6/9 patients).• No differences were observed between the SENTINEL and Wirion filter for any type of debris/tissue captured.• Captured debris size, counts, and types were similar between SENTINEL and Wirion filters.• 2/11 patients didn’t receive the Wirion filter: in 1 patient selective cannulation of the left vertebral artery was not possible because of anatomical difficulties and in 1 patient a dissection of the left vertebral artery developed (sealed with a stent).• One patient had an ischaemic stroke 8 days after TAVR. |
| Kapadia et al [100JACC (2017) | SENTINEL | 363 (225 with SENTINEL) |
Lotus: 8 (72%), SAPIEN XT: 2 (17.8%),SAPIEN 3: 2 (52.4%), CoreValve: 1 (3.9%), Evolut R: (25.9%) |
100% in all filters | • The captured debris consisted of acute thrombus (99%), arterial wall (94%), valve tissue (50%), calcification (50%), foreign body (36%), myocardium (16%), and organising thrombus (6%).• More than 80% of debris was 150 to 500 µm in maximum diameter; <5% was >1,000 µm.• After variables adjustment, there was a significant difference in new lesion volume favouring neuroprotection in both protected territories (p=0.025) and unprotected territories (p=0.050). |
| Schmidt et al101JACC Cardiovasc Interv. (2018) | SENTINEL | 246 | SAPIEN 3: 145 (59%), Evolut R: 40 (16%), Lotus: 36 (15%), SAPIEN XT: 25 (10%) |
99% | • The captured debris consisted of acute thrombus (~95%), arterial wall (~85%), valve tissue (~55%), calcification (~55%), foreign body (~45%), organising thrombus (~15%), myocardium (~15%), and necrotic core (~3%).• 90% of all patients had large particles (≥500 μm), and 53% had particles ≥1 mm.• Patients treated with an Evolut R or a Lotus valve had larger particles (≥1,000 μm) and greater total captured particle area compared to those treated with a SAPIEN 3 or SAPIEN XT valve.• Transcatheter valve type was the only significant contributor to the morphometric results (multivariate analysis). |
| Seeger et al102JACC Cardiovasc Interv. (2018) | SENTINEL | 100 | SAPIEN 3: 42 (42%), Evolut R: 35 (35%), Lotus: 23 (23%) |
99% | • The captured debris consisted of acute platelet-rich thrombus (99%), arterial wall (84%), valve tissue (84%), calcification (58%), foreign body (33%), myocardium (14%), necrotic core (12%), and organising thrombus (7%).• The tissue/debris pattern was similar in both filters.• Patients treated with a SAPIEN 3 valve had larger particles (≥1,000 μm) compared to those treated with an Evolut R or a Lotus valve.• The total tissue area was significantly greater in patients treated with a SAPIEN 3 (21.3±15.1 mm²) and Evolut R (20.1±19.0 mm2) valve compared to those treated with a Lotus valve (7.1±6.3 mm2) (p=0.0014).• Two strokes within 48 hours after TAVR were reported, 1 non-disabling stroke on day 1 in a patient treated with the Lotus valve and 1 disabling stroke on day 2 in a patient treated with the Evolut R. |
| Latib et al41JACC Cardiovasc Interv. (2020) | Emblok | 20 | Evolut R: 11 (55%), ACURATE neo: 4 (20%), Portico: 2 (10%), Evolut PRO: 1 (5%), SAPIEN 3: 1 (5%) |
90% | • Red blood cells in 18 filters (100%), fibrin strands in 15 (75.0%), collagen strands in 9 (45.0%) with a mean strand length of 1,216±153 mm; calcium debris in 5 (25.0%), and arterial wall in 1 (5%).• No neurological events were reported.• DW-MRI indicated 95% (19) of patients with new brain ischaemic defects at 2 to 5 days post-TAVR. |
| Kroon et al103EuroIntervention (2021) | SENTINEL | 328 | Evolut R/PRO: 123 (37.5%), SAPIEN 3: 113 (34.5%), Lotus: 92 (28%) |
98% | • The captured debris consisted of thrombus (90%), aortic valve tissue (70%), foreign material (49%), endothelium (30%), collagen (25%), calcification (22%), and myocardium (10%).• The treatment of native (functional) bicuspid valves (OR 2.91; p=0.02) and the use of the Lotus valve (OR 2.44; p=0.02) were significantly associated with the highest risk for dislodging particles ≥1,000 μm.• Valve repositioning was independently associated with larger amounts of captured debris (OR 2.96; p=0.004).• Four patients suffered a post-TAVR stroke, all with the Lotus valve, and particles >1,000 μm were present in the filters of all patients. |
| Donà et al104J Pers. Med. (2022) | SENTINEL | 213 | Self-expanding valves (63.9%): Portico (13.6%), ACURATE neo (34.0%), Evolut R (14.6%), Centera valve (1.0%), Allegra valve (0.7%). Balloon-expandable valves or mechanically expanded valves (36.1%, data not specified). |
91% | • Debris was captured in 91% of filters, and in 64%, histopathological analysis was conducted (only in captured particles >2 mm in size).• The captured tissue consisted of valve tissue (62.5%), arterial wall (37.5%), atherosclerotic plaque (32.5%), myocardium (10.0%), acute thrombus (7.5%), organised thrombus (7.5%), and foreign material (2.5%).• 24 (11.3%) patients received only the brachiocephalic trunk filter.• At 90-day follow-up, 20 (4.9%) cerebrovascular events occurred, of which 10 (2.4%) were disabling strokes.• In the CEPD group, 5 cerebrovascular events were recorded: 2 (2/189, 1.1%) occurred in patients with correctly positioned CPS, both affecting the territory of the left vertebral artery (not covered by the SENTINEL), and 3 events occurred (3/24; 12.5%) in patients with incorrectly positioned CPS; two of them in the territory of the left carotid or vertebral arteries (which were not covered by the distal filter), and one in the right mid-cerebral artery territory, indicating incomplete coverage of the right brachiocephalic trunk/the right carotid artery.• The incidence of cerebrovascular events was reduced in the CEPD group by 70% (CEPD 2.3% vs no CEPD 7.6%; p=0.02). |
| Kawakami et al105Circ Cardiovasc Interv. (2022) | SENTINEL | 49 | Evolut PRO: 27 (55%), SAPIEN 3: 22 (45%) |
100% | • Arterial wall (98% of cases), acute thrombus (96%), valve tissue (71%), calcification (55%), and foreign material (43%), myocardial tissue (20%), organising thrombus (4%), and necrotic core (4%).• Most captured debris had a size of <500 μm (78% were 150-500 μm). Nearly 5% of the captured particles were ≥1,000 μm, and these were detected in 67% of cases.• Transcatheter heart valve type did not impact the debris tissue types, particle numbers, or sizes.• Calcified particles were more common in patients treated with predilatation (71% vs 43%; p=0.046). However, predilatation or post-dilatation did not alter particle numbers or sizes.• Low risk: 86%, and intermediate risk: 14%.• The rates of death or stroke within 30 days post-TAVR were 2% (1/49, due to myocardial infarction) and 4% (2/49), respectively. |
| MITRAL PROCEDURES | |||||
| Frerker et al51JACC Cardiovasc Interv. (2016) | SENTINEL | 14 | MitraClip for severe functional mitral regurgitation | 100% | • The most common tissue types were acute thrombus and small foreign material (hydrogel) in 12 patients (87.5% each), fibroelastic tissue (valve tissue and/or superficial atrial wall tissue) in 9 (64.3%), organising thrombus in 4(28.6%), any thrombus in 2 (14.3%), and myocardium in 2 (14.3%).• Combination of thrombus, valve/atrial wall tissue and foreign material in 8 (57%), and any thrombus plus foreign material only in 3 (21%).• Calcification was not seen in any filter.• The median cumulative particle area per patient of 2.46 mm2 (IQR 0.44 to 3.67), median maximum particle diameter of 295 μm (IQR 104 to 509).• Larger particles were identified more frequently in proximal versus distal filters• Patients treated with 2 clips had a larger maximum particle diameter compared with 1 clip (median 402 vs 134 μm; p<0.0001)• Cumulative areas of debris captured in proximal and distal filters were 1.35 mm2 (IQR 0.30 to 2.09) and 1.07 mm2 (IQR 63 to 442) per patient of 2.46 μm (IQR 0.44-3.67), respectively, with maximum diameters of 346 μm (IQR 211-555) and 217 μm (IQR 63-442), respectively (p<0.0001).• No cerebrovascular events were reported. |
| COMBINED AORTIC AND MITRAL PROCEDURES | |||||
| Schmit et al55Heart (2016) | SENTINEL | 15 | Evolut R: 8 (62%), CoreValve: 3 (23%)SAPIEN 3: 1 (8%), SAPIEN XT: 1 (8%) |
100% in proximal filters and 87% in distal filters | • 13 degenerated aortic and 2 degenerated mitral surgical or transcatheter bioprostheses were treated with transcatheter valve-in-valve (ViV) using cerebral protection• All patients underwent a ViV TAVR procedure.• For ViV in the aortic position (CoreValve, n=3; Evolut R, n=8; SAPIEN XT, n=1; SAPIEN 3, n=1).• For ViV in the mitral position (SAPIEN XT, n=2).• Acute thrombus was the most common type of debris, captured in all 15 proximal filters and 12 (80%) distal filters; arterial wall tissue in 80% (12/15), calcification in 73% (11/15), valve tissue in 60% (9/15), foreign material in 27% (4/15), organised thrombus in 33% (5/15), and myocardium in 13% (2/15).• Platelet aggregation to the biological debris was seen in all patients in whom acute thrombus and biological debris (arterial wall, calcification, and valve tissue) were found. Also, all samples containing acute thrombus and foreign material showed platelet aggregation.• Combination of all types of debris was the most common combination (n=5, 33%) followed by thrombus in combination with arterial wall and calcification (n=4, 27%).• Overall, 2,862 particles were found in all filters, with 455 particles >150 mm.• The median of particles per patient in the filter pairs was 123, with a median of 20 particles per patient being >150 mm.• The average median particle diameter per patient was 47 mm (minimum of 32 mm, maximum of 88 mm).• A trend towards higher total particle counts in proximal compared with distal filters (median 78 vs 39, respectively; p=0.065) was reported.• No differences between proximal and distal filters were observed for average particle diameters or the number of particles ≥150 mm.• No neurological event was seen during hospitalisation.• One (7%) stroke occurred 5 months after the procedure. |
| CEPD: cerebral embolic protection device; CPS: cerebral protection system; DW-MRI: diffusion-weighted magnetic resonance imaging; IQR: interquartile range; NR: not reported; OR: odds ratio; TA: transaortic; TAVR: transcatheter aortic valve replacement; TF: transfermoral; TIA: transient ischaemic attack; TS: transsubclavian; ViV: valve-in-valve | |||||
Cerebral embolic protection devices
Given the ubiquitous showering of debris during TAVR and transcatheter mitral interventions (Table 3), adjunctive use of a CEPD during transcatheter heart valve procedures to mitigate cerebral embolisation (along with its clinical consequences) seems intuitively beneficial. Stroke reduction and lessening the extent of neurological damage notionally seem to be sound clinical rationale for promoting CEPD use during TAVR. Despite the above, its use during TAVR remains infrequent. The SENTINEL device was used in 7.1% of TAVR procedures across 551 sites in the USA between 2018 and 201922. Although multiple patient and hospital characteristics have been associated with CEPD use, TAVR case volume seems to be the predominant factor associated with its use in the USA, rather than its eligibility for the Centers for Medicare & Medicaid Services (CMS) TAVR reimbursement or the CMS new technology add-on payment. This suggests that in the absence of definitive data for CEPD efficacy, reimbursement alone did not drive its use. In addition, the added risk burden due to use of a CEPD per se, such as thrombotic or vascular complications, should be considered, especially with CEPDs requiring larger-bore femoral access. Prior studies have shown the feasibility and safety of the currently available CEPDs2324252627. Their use is associated with reductions in the number of new lesions on brain DW-MRI along with the total volume of brain injured, which may possibly reduce the risk of developing cognitive impairment or accentuating pre-existing neurological pathologies23242526. Yet their efficacy for clinical stroke prevention remains to be clearly demonstrated28. Table 4 highlights the main aspects of an “ideal” CEPD. According to their mechanism of action, CEPDs can be primarily categorised into 2 groups: devices that capture (totally or partially) debris within the aorta before it reaches the brain, renal or peripheral arteries; or deflectors of debris from the aortic arch and its branches. The former may be positioned along the aortic arch and/or descending aorta or within the brachiocephalic trunk and/or common carotid arteries, while the latter are typically positioned along the roof of the aortic arch (Central illustration). Devices can also be classified into partial capture devices (SENTINEL), full capture devices (Emblok [Innovative Cardiovascular Solutions], Emboliner [Emboline], FLOWer [AorticLab]), primarily deflective devices (with small capture capacity) (TriGUARD 3 [Keystone Heart/Venus Medtech], ProtEmbo [Protembis], POINT-GUARD [Transverse Medical]), and deflection and capture devices (CAPTIS [Filterlex]). Figure 2 summarises the main characteristics of a range of CEPDs currently approved for clinical use and those in the preliminary clinical phase or in preclinical development. Currently, there are 4 devices with published data in peer-reviewed journals, two of which are in clinical use, and several others remain under active investigation in early phase studies for their potential applications in TAVR and structural heart interventions (Central illustration).
Table 4. Main features of an ideal CEPD.
| Provide complete protection to the cerebral circulation |
| Easy to use, deploy, and retrieve |
| Harmless and safe |
| Effective to prevent small and large emboli |
| Feasible for all anatomies (one size fits all) |
| No/minimal interaction with the TAVR delivery systems |
| Optimal visibility under fluoroscopy |
| Maintained stability throughout the procedure |
| Absence of cerebral flow obstruction |
| Low thrombogenicity |
| Low risk of causing dissections, carotid or aortic plaque displacement or rupture during device deployment, positioning, and retrieval, and during passage of the TAVR delivery system in the aortic valve and ascending aorta |
| As low profile as possible to avoid access site complications |
| Low cost |
| Captures and removes all cerebral and non-cerebral debris |
| Versatile to be used for other left heart procedures |
| CEPD: cerebral embolic protection device; TAVR: transcatheter aortic valve replacement |
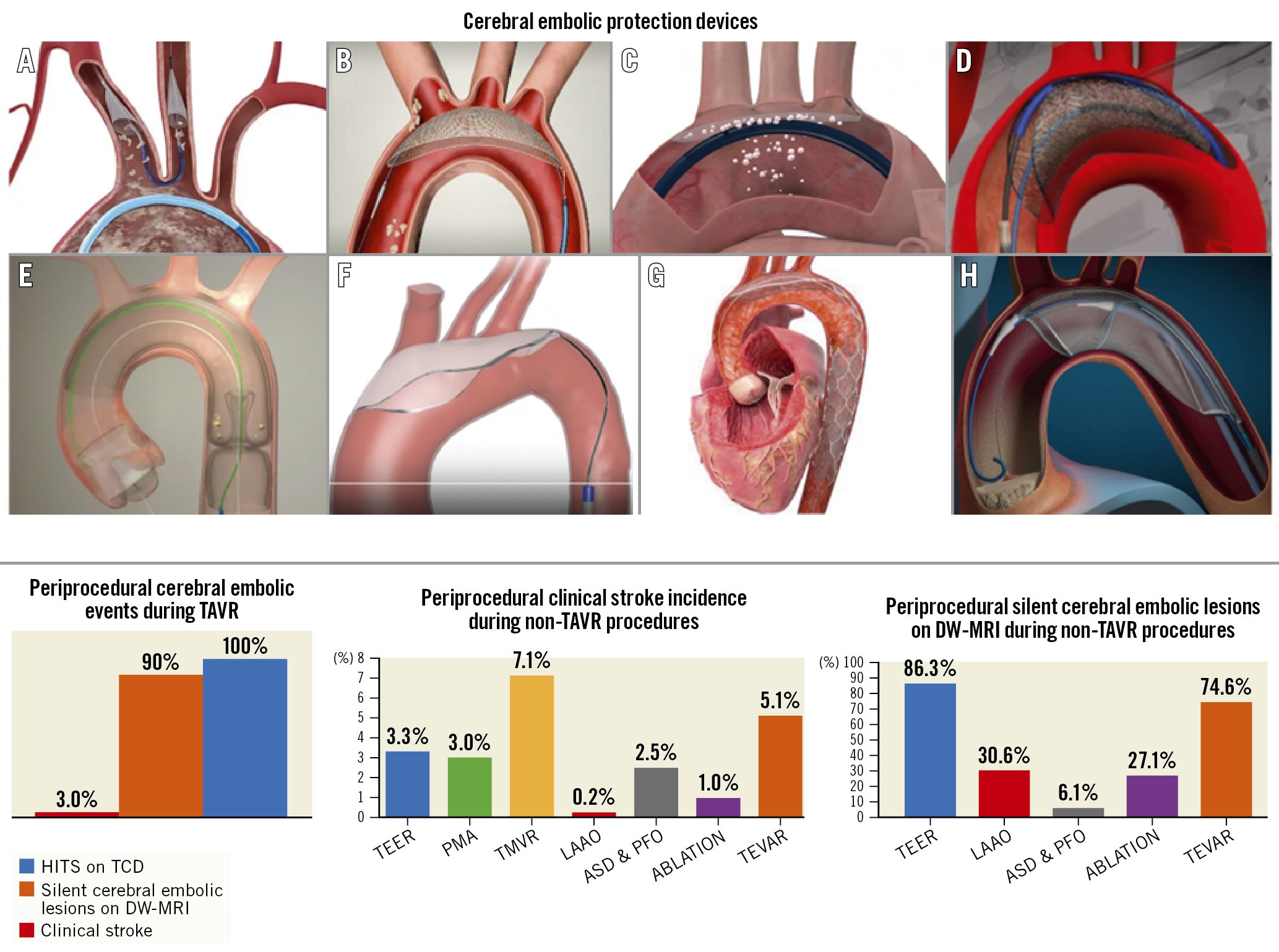
Central illustration. Cerebral embolic protection devices and data on TAVR and non-TAVR procedures. A) SENTINEL; B) TriGUARD 3; C) ProtEmbo; D) Emblok; E) Emboliner; F) POINT-GUARD; G) CAPTIS; H) FLOWer ASD: atrial septal defect; DW-MRI: diffusion-weighted magnetic resonance imaging; HITS: high-intensity transient signal; LAAO: left atrial appendage occlusion; PFO: patent foramen ovale; PMA: percutaneous mitral annuloplasty; TAVR: transcatheter aortic valve replacement; TCD: transcranial Doppler; TEER: transcatheter edge-to-edge repair; TEVAR: thoracic endovascular aortic repair; TMVR: transcatheter mitral valve replacement
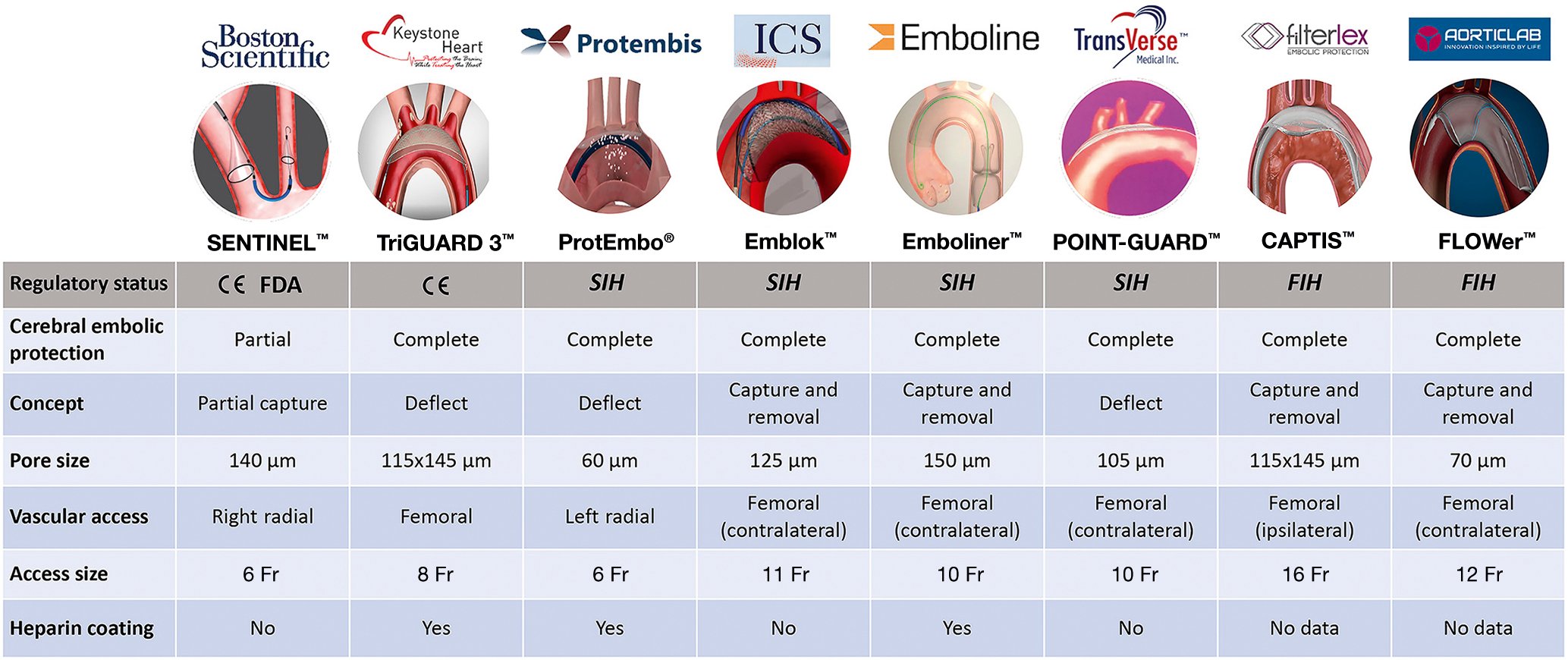
Figure 2. Cerebral embolic protection devices: main features and regulatory status. CE: European conformity; Fr: French; FDA: U.S. Food and Drug Administration; FIH: first-in-human; SIH: second-in-human
What is the evidence for using specific CEPDs during TAVR?
The SENTINEL Cerebral Protection System
The SENTINEL Cerebral Protection System consists of 2 polyurethane filters with 140 mm diameter pores fixed in a flexible nitinol radiopaque frame, advanced from a 6 Fr sheath through the right radial or right brachial artery and deployed into the ostia of brachiocephalic trunk and left common carotid artery2329. It is designed to capture emboli passing into the cerebral circulation in 2 of the 3 branches of the aortic arch. The proximal filter is aimed to be positioned at the ostium of the brachiocephalic trunk, whereas the distal filter is placed in the left common carotid, but leaving the left subclavian open, and the left vertebral circulation is unprotected2329. The device has a CE (European conformity) mark and U.S. Food and Drug Administration (FDA) approval for use within vessels measuring 9-15 mm in diameter and is, to date, the most widely used CEPD in TAVR.
The MISTRAL-C29 (n=65 patients) and the CLEAN-TAVI (n=100 patients) randomised trials23 showed fewer new lesions and smaller total lesion volumes in the SENTINEL-protected group. Neurocognitive deterioration was more frequent in the patients treated without protection.
The US SENTINEL IDE Study26 was a larger multicentre study (n=363 patients) with a 1:1:1 randomisation into a safety device arm (n=123), an imaging device arm (n=121), and an imaging control arm (n=119). The authors reported debris in 99% of the filters. Despite a numerical reduction in all-cause stroke at 30 days, statistical significance was not met (5.6% for the SENTINEL group vs 9.1% in the control group; p=0.25). Also, the median total new lesion volume in protected territories evaluated by DW-MRI 2-7 days post-TAVR did not differ significantly between the control and the CEPD groups. The CEPD group demonstrated a reduction in stroke within 72 hrs after TAVR (classified as procedural stroke by the Neurologic Academic Research Consortium [NeuroARC] definitions)30 when compared to the unprotected group (3.0% vs 8.2%; p=0.053). Although the topline results of this trial failed to demonstrate a statistically significant clinical reduction in stroke events and new lesion volume, several caveats of the trial warrant consideration. Baseline abnormal brain signal volume (indicative of prior stroke) is known to impact new lesion volume assessment31; additionally, there is a dynamic fluctuation of T2-weighted-Fluid-Attenuated Inversion Recovery (T2/FLAIR) lesion volume from day 2 post-TAVR to day 7 post-TAVR. With this in mind, reanalysing new lesion volume in protected and unprotected brain regions when controlling for baseline cerebral infarction volume did show a protective effect of the SENTINEL CEPD in both protected (p=0.025) and unprotected territories (p=0.05)26. Despite the above, the results of this post hoc analysis can be considered only as hypothesis-generating. The loss of brain MRI follow-up in 25% of cases was also a significant confounder for result interpretation. Moreover, the SENTINEL IDE Study was never powered to show a significant difference in clinical indices.
Three large-scale non-randomised retrospective studies from Medicare, Transcatheter Valve Therapy (TVT) and National Inpatient Sample (NIS) registry data analysed the outcomes of TAVR patients and CEPD use, with the SENTINEL CEPD, in real-world practice283233. There was a lack of concordance amongst these studies in demonstrating a consistent stroke reduction benefit of the SENTINEL CEPD during TAVR. Other retrospective analyses from Alkhouli et al34, Seeger et al35, and an analysis from Ulm36 were similarly discordant in their findings of benefit (or not) with the use of a CEPD during TAVR.
The recently published PROTECTED TAVR (Stroke PROTECTion With SEntinel During Transcatheter Aortic Valve Replacement) trial37 was the first randomised, open-label, multicentre, all-comer trial powered for clinical endpoints. It enrolled 3,000 patients undergoing transfemoral TAVR with all commercially available transcatheter heart valves, to receive TAVR plus the SENTINEL device (CEPD group: 1,501 patients) or TAVR with no CEPD (control group: 1,499 patients). The primary endpoint was all stroke (haemorrhagic, ischaemic, or undetermined status; disabling or non-disabling) up to 72 hours post-TAVR procedure or hospital discharge using NeuroARC definitions30. Successful device deployment was achieved in 94.4% of patients, and 1 patient (0.1%) developed a vascular complication at the CEPD access site. SENTINEL use did not significantly reduce the incidence of stroke within 72 hours post-TAVR or before hospital discharge compared to the control group (2.3% vs 2.9%; p=0.30). Disabling stroke (a secondary endpoint) was less frequent in the CEPD group (0.5%) compared to the control group (1.3%; difference −0.8 percentage points; 95% CI: −1.5 to −0.1). Also, the incidence of all-cause death (0.5% vs 0.3%), stroke, transient ischaemic attack (TIA), or delirium (3.1% vs 3.7%), and acute kidney injury (AKI; 0.5% vs 0.5%) were similar between groups. The number needed to treat to prevent 1 disabling stroke was 125 patients. Although the device proved to be safe and a possible effect on disabling strokes (a secondary endpoint) was seen, the lack of efficacy and clear clinical benefit might challenge its use in daily practice, and questions continue to arise regarding its cost-effectiveness, especially in single-payer health systems. Figure 3 summarises the stroke reduction obtained by the SENTINEL CEPD in clinical trials.
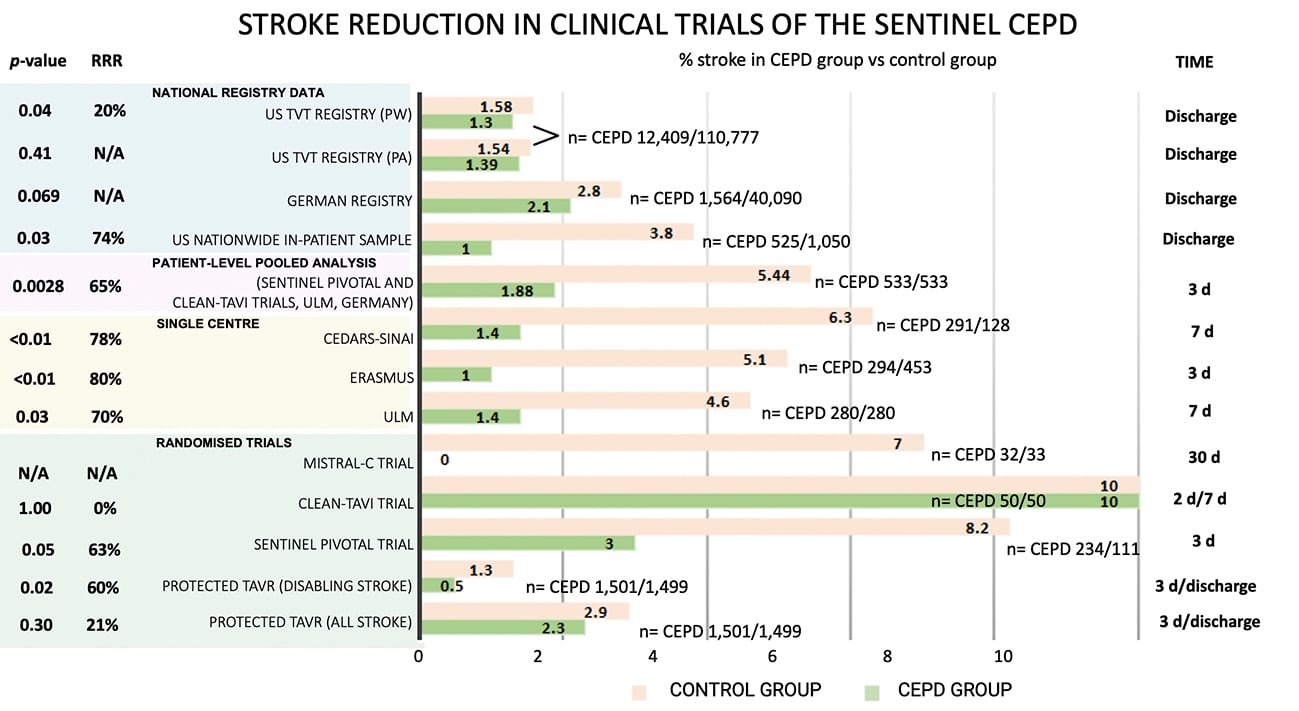
Figure 3. Stroke reduction obtained by SENTINEL CEPD in clinical trials. CEPD: cerebral embolic protection devices; d: days; N/A: not applicable; PA: primary analysis; PW: propensity-weighted; RRR: relative risk reduction
THE TRIGUARD 3 CEREBRAL PROTECTION DEVICE
The TriGUARD 3 Cerebral Protection Device is a deflection device positioned to protect all 3 branches of the aortic arch, including the left subclavian artery. It is placed via a transfemoral 9 Fr femoral arterial sheath which allows for concomitant use of a 6 Fr pigtail catheter. The device is composed of a semipermeable nitinol mesh with pores of 115x145 mm which deflect particles larger than 140 μm. The mesh is heparin-coated to reduce the risk of thrombus formation. The circumferential pressure of the device in the aortic arch and the nitinol device shaft holds the device in position during TAVR2425.
The feasibility and safety of this device was investigated in the DEFLECT I38 and DEFLECT II39 trials, which were prospective, single-arm studies (n=37 and 14 patients, respectively). DEFLECT I38 showed a similar presence of new cerebral ischaemic lesions on DW-MRI to historical controls (82 vs 76%; p=not significant). Similarly, in the DEFLECT II trial39, comparing the DW-MRIs of these patients to a historical control group showed no reduction in the number of lesions (median 5.5 vs 5.0; p=0.86). DEFLECT III40 (n=85 patients) was a single-blind multicentre randomised trial in which TAVR patients were randomised to either a CEPD (n=46) with the TriGUARD system or to no CEPD (n=39). Device success was achieved in 88.9% (40/45) of patients. The primary in-hospital procedural safety endpoints (death, stroke, life-threatening or disabling bleeding, stage 2 or 3 AKI, or major vascular complications) were not statistically different (21.7% of the TriGUARD HDH group compared to 30.8% of the control group; p=0.34).
The REFLECT I trial (n=258 patients of the initially planned 375 patients) was a multicentre (20 US and 6 European centres), randomised controlled trial that evaluated the safety, efficacy, and performance of the TriGUARD system in patients undergoing TAVR24. Prior to completing enrolment, the study was suspended at the recommendation of the data safety monitoring committee. The primary efficacy endpoint was a hierarchical composite of (i) all-cause mortality or any stroke at 30 days, (ii) National Institutes of Health Stroke Scale worsening at 2-5 days or Montreal Cognitive Assessment worsening at 30 days, and (iii) total volume of cerebral ischaemic lesions detected by DW-MRI at 2-5 days. Complete coverage of all 3 cerebral vessels throughout the TAVR procedure was achieved in 57.3% (78/136) of cases. The primary safety outcome was met compared with the performance goal (21.8% vs 34.4%; p<0.0001). The primary hierarchical efficacy endpoint was not significantly different between groups.
The REFLECT II US trial25 enrolled 220 of the planned 345 patients (63.8%) across 18 US sites. After the FDA suggested that enrolment be suspended for unblinded safety data assessment, the sponsor discontinued the study early. Complete cerebral coverage was achieved in 59.7% (94/157) of cases, device interaction was reported in 9.6% (15/157). The trial met its primary safety endpoint compared with the performance goal (15.9 vs 34.4%; p<0.0001). The primary hierarchal efficacy endpoint at 30 days (death or stroke at 30 days, National Institutes of Health Stroke Scale worsening in hospital, and cerebral ischaemic lesions on DW-MRI at 2 to 5 days) was not met.
CEPDs currently under development
Emblok Embolic Protection System
The Emblok Embolic Protection System is a device designed to protect all supra-aortic vessels by a full circumferential coverage of the aortic arch. The delivery system is 11 Fr, compatible to be deployed through a single access site supported by a 0.035” guidewire and incorporates an integrated 4 Fr radiopaque pigtail catheter which provides constant visualisation, from which an aortogram can be performed both for the CEPD and valve deployment41. The filter is made of a polyurethane mesh with a pore size of 125 μm, supported by a nitinol frame positioned just proximal to the brachiocephalic trunk. Once the transcatheter aortic valve is advanced to the level of the ascending aorta and before the transcatheter valve is positioned across the native aortic valve, the conical filter is unsheathed, then, the filter frame is pulled back towards the aortic wall to maximise apposition and sealing. Once the transcatheter valve is deployed, the Emblok system must be recaptured in order to retrieve the transcatheter delivery system from the body41. Early human experience is summarised in Table 1.
ProtEmbo Cerebral Protection System
The ProtEmbo Cerebral Protection System is a temporary, intra-aortic embolic deflection filter used during transcatheter heart interventions and is the only current device that can be positioned through a 6 Fr left radial access42. ProtEmbo is designed to provide complete cerebral protection; it is inserted at the beginning of the procedure prior to TAVR and removed following TAVR completion. The device consists of (i) a heparin-coated mesh with 60 μm pores (currently the smallest pore size of CEPDs under development), (ii) a self-expanding nitinol frame that provides complete coverage of all supra-aortic vessels with radiopaque markers for fluoroscopic visualisation and precise device placement and (iii) a delivery unit. The device is fully retrievable and recapturable; it is delivered unexpanded and deployed by unsheathing the self-expanding filter to cover the orifice of all 3 cerebral vessels (brachiocephalic trunk, left common carotid, and left subclavian arteries). A handle provides a simple user interface for preparation, delivery, deployment and removal of the device. The device is loaded into a commercially available delivery catheter and placed into the aortic arch using a commercially available guiding sheath via the left radial or brachial artery42. The first-generation ProtEmbo device was shown to be safe and feasible in the first-in-human PROTEMBO SF Trial (n=4 patients; ClinicalTrials.gov: NCT03325283) at 2 clinical sites in Europe43. The PROTEMBO C Trial (n=41 patients at 8 European centres) interim results have been published42 and are summarised in Table 1. The company will conduct the ProtEmbo IDE trial in up to 500 patients in the USA and Europe starting in the third-quarter (Q3) of 2023.
Emboliner total embolic protection device
The Emboliner total embolic protection device is a cylindrical nitinol mesh filter with a pore size of 125 μm that circumferentially conforms to the aortic arch to cover all 3 cerebral branch vessels. The device captures and removes debris generated during TAVR. The delivery system is 10 Fr and integrates a 6 Fr pigtail and an expandable access port to allow passage of the transcatheter valve44. SafePass clinical program results4546 are summarised in Table 1. Although the study results are yet to be published, the company launched a US-based pivotal trial this year for FDA and CE approval comparing Emboliner versus SENTINEL in 500 TAVR patients (plus 40 Emboliner roll-in cases) with a 1:1 randomisation (ClinicalTrials.gov: NCT05684146). The primary endpoint is the 30-day combined major adverse cardiac and cerebrovascular event (MACCE) rate (all death, stroke and stage 3 AKI). Results are expected in December 2024.
CAPTIS embolic protection system
The CAPTIS embolic protection system has a combined mechanism of action: deflection of embolic particles at the level of the aortic arch and capture and removal of debris at the level of the descending aorta. It is composed of a filter made of PEEK (polyether-ether-ketone), with a pore size of 115x145 μm, attached to a nitinol frame with a deflector configuration in the aortic arch covering the 3 cerebral branch vessels and collector pockets adapted circumferentially to the descending aorta anatomy, joined by a nitinol rail and an anchoring segment as a stabilisation mechanism. The 16 Fr delivery system uses the same femoral access as TAVR without the need for an extra vascular access47. The first-in-human (FIH) results, presented at EuroPCR in 2022, are summarised in Table 1.
FLOWer embolic protection device
The FLOWer device, formerly the Embrace filter, is an embolic protection system designed to protect cerebral and peripheral vessels. The device is a cylindrical mesh filter with a pore size of 70 μm attached to a frame that adapts circumferentially to the aortic arch to cover all the cerebral branch vessels. The device comes in 3 different sizes and aims to capture and remove debris produced during TAVR. The delivery system is 12 Fr and integrates a 5 Fr pigtail and a port compatible with all TAVR delivery systems. An in vitro test reported by the company (not published) showed 99% of particles >150 μm captured at the level of the aortic arch (cerebral protection) and 84% distal to the aortic arch towards downstream circulation (systemic protection), with a calculated in vitro pressure drop of 6.6 mmHg at 4.5 L/min cardiac output through the Embrace filter48. Preliminary data from the NAUTILUS FIH study (ClinicalTrials.gov: NCT04704258), presented at EuroPCR in 202349, are summarised in Table 1.
POINT-GUARD Dynamic Cerebral Embolic Protection
The POINT-GUARD Dynamic Cerebral Embolic Protection is a second-generation device aimed to provide full aortic arch protection with maximum coverage of all great arch vessels and inlets to the brain. The device consists of an ultrathin flexible filter mesh attached to a dynamic frame assembly with dual-edge perimeter seal, proximal and distal anchoring segments to provide stability, an anchor on the proximal drive shaft to prevent position shift, and a proximal pouch for embolic capture. The device has an adaptive asymmetric configuration that allows it to adapt to the angulation of the aortic arch and provide complete anterior and posterior coverage. The first-generation device was tested in the POINT-GUARD First-In-Human CENTER Study (n=4 patients) during elective transfemoral TAVR (no published data yet available)50 (Table 1).
Non-TAVR use of CEPDs
The risk of debris embolisation (atheroma or thrombus) during non-TAVR structural heart procedures (i.e., transcatheter mitral valve therapies, left atrial appendage occlusion [LAAO]), is not negligible515253 (Table 5). Similar to TAVR, cerebral embolisation may be asymptomatic and detected solely on brain imaging or may present as major clinical events impacting morbidity and mortality. However, existing studies evaluating cerebral embolisation in non-TAVR procedures have lacked formal neurological adjudication. Consequently, the real incidence of neurological events may be underreported (Central illustration).
Table 5. Periprocedural cerebral embolic events during non-TAVR procedures.
| Transcatheter edge-to-edge repair (TEER)55565758106 | Transcatheter mitral valve replacement (TMVR)107108109110111 | Percutaneous mitral annuloplasty (PMA)112113114 | Left atrial appendage occlusion (LAAO)606162115116117118119120121 | Atrial septal defect (ASD) & patent foramen ovale (PFO)122123124 | Catheter ablation63656667125126127128129130131132 | Thoracic endovascular aortic repair (TEVAR)70-73,75,76,79,81,134,135] | |
|---|---|---|---|---|---|---|---|
| Study design | Retrospective, RCT, systematic review and meta-analysis | Prospective, single-arm | Prospective, single-arm | Prospective observational, RCT, systematic review | Prospective, single-arm | Retrospective, prospective observational | Retrospective, prospective observational |
| Stroke/TIA | 3.3% | 7.1% | 3.0% | 0.2% | 2.5% | 1.0% | 5.1% |
| Number of patients | 1,176 | 14 | 101 | 39,775 | 81 | 62,154 | 980 |
| Number of events | 39 | 1 | 3 | 81 | 2 | 608 | 50 |
| Cerebral embolic lesions on DW-MRI | 86.3% | NR | NR | 30.6% | 6.1% | 27.1% | 74.6% |
| Number of patients | 51 | NR | NR | 72 | 65 | 317 | 59 |
| Number of patients with new lesions | 44 | NR | NR | 22 | 4 | 86 | 44 |
| HITS on transcranial Doppler | 100% | NR | NR | NR | 100% | NR | 100% |
| Number of patients | 54 | NR | NR | NR | 16 | NR | 80 |
| Number of HITS | 152 [IQR 94-280] | NR | NR | NR | 31.5 [IQR 3-113] | NR | 268 [IQR 71-521] |
| DW-MRI: diffusion-weighted magnetic resonance imaging; HITS: high-intensity transient signals; IQR: interquartile range; NR: not reported; TIA: transient ischaemic attack; TAVR: transcatheter aortic valve replacement | |||||||
Transcatheter edge-to-edge repair and mitral valve replacement
In mitral valve interventions, stroke rates are considerably lower than those reported in TAVR, yet still significant. In the COAPT trial54, the stroke rate was 0.7% at 30 days, while in the MITRA-FR trial55, authors reported a periprocedural incidence of 1.4%. A recent systematic review and meta-analysis56 showed an incidence of 2.9% of periprocedural strokes in 941 patients who underwent transcatheter edge-to-edge repair (TEER). In a small study (14 patients) using the SENTINEL system during TEER51, microscopic debris were detected in all 28 filters, consisting mainly of acute thrombus (85.7%) or fragments of foreign material such as hydrophilic device coating (85.7%) (Table 3). As in TAVR, the spectrum of brain damage ranges from overt stroke to silent ischaemic brain injury. In a small study, new brain lesions were detected using DW-MRI in 23 (85.7%) of 27 patients after TEER57. These data are consistent with what was recently reported by Braemswig et al58, who observed an incidence up to 87% (21 out of 24 patients) of cerebral ischaemic injuries in TEER procedures, assessed by pre-and postprocedural DW-MRI, and a 16.6% incidence of overt stroke (9 out of 54 patients). Using periprocedural transcranial Doppler (TCD) monitoring, the authors identified that the procedural step with the highest numbers of microembolic signals towards the brain was device interaction with the mitral valve.
Although few published data evaluate the incidence of stroke during percutaneous mitral annuloplasty and transcatheter mitral valve replacement (TMVR), an acute stroke incidence of around 3% has been reported (Table 3). There are no reports so far of CEPD use during TMVR.
Left atrial appendage occlusion
The incidence of stroke during left atrial appendage occlusion (LAAO) procedures is between 0.2% and 1.2% across a range of LAAO devices (Table 3). Limited data exist on outcomes after LAAO in the presence of persistent thrombus within the left atrial appendage (LAA) in patients considered to be poor candidates for long-term oral anticoagulant (OAC) use or in patients where OACs have failed to dissolve LAA thrombus. In a systematic review of patient-level data of cases using a CEPD in 17 of 58 (29%) patients, no periprocedural stroke was reported59. Microembolic signals have been found in 100% of patients during LAAO with the WATCHMAN device (Boston Scientific), alongside new silent cerebral embolic events in over one-third of patients within 24 hours after LAAO, assessed by TCD monitoring and DW-MRI, respectively60. Data from different studies using a variety of LAAO devices reported an incidence of new brain lesions between 4.8% and 52.0% within 2 days after LAAO6162.
Catheter ablation
The reported rates for periprocedural stroke or transient ischaemic attack range from 0.38% to 7.00% during ablation procedures6364. The first 24 hours post-ablation represent a high-risk period, which continues for an additional 2 weeks64. Also, new brain ischaemic lesions have been detected on postprocedural DW-MRI in up to 58% of patients undergoing routine left ventricular ablation procedures65 and as high as 38% after left atrial catheter ablation6667.
The evidence for CEPD use during cardiac ablation is limited to a small series of cases in patients undergoing ventricular tachycardia ablation with confirmed high risk of periprocedural stroke (left atrial appendage thrombus, left ventricular apical thrombus, complex aortic plaque)6869. No cerebrovascular events were reported, but debris was captured in most cases.
Thoracic endovascular aortic repair procedures
Stroke occurs in 3 to 8% of patients undergoing thoracic endovascular aortic repair (TEVAR)7071; microembolic signals assessed by TCD are detected in 100% of patients during the procedure72, and in over 80% of cases, postprocedural DW-MRI demonstrated the presence of silent stroke7374. This has been associated with significant neurocognitive decline and linked with considerably increased morbidity and mortality75. It is estimated these numbers are higher in ascending aortic TEVAR. Consequently, efforts have been made to reduce the risk of periprocedural stroke during TEVAR7677. There are several case reports and series of patients who have undergone TEVAR with a CEPD787980. The authors noted that the use of CEPD appeared to reduce the size and distribution of new cerebral infarcts compared with previously reported neuroimaging studies of unprotected TEVAR procedures, suggesting improved protection for the anterior (protected) circulation7381. Interestingly, a greater number of high-intensity signals were detected during CEPD manipulation and deployment (median 124 [interquartile range [IQR 59-146] compared to during stent manipulation and TEVAR deployment (median 82 [IQR 73-142])79.
Permanent cerebral embolic protection
The Vine filter (Javelin Medical) is a permanent, bilateral, common carotid artery filter made of a single nitinol wire with a circular cross-section (diameter 240 μm) developed to prevent emboli >1.4 mm in size from reaching the cerebral anterior circulation (Figure 4). The implantation is ultrasound-guided using a 22 gauge insertion needle via a completely percutaneous approach. Preliminary data have been promising82. A large clinical trial to further evaluate the efficacy and safety of this approach is planned (ClinicalTrials.gov: NCT05723926).
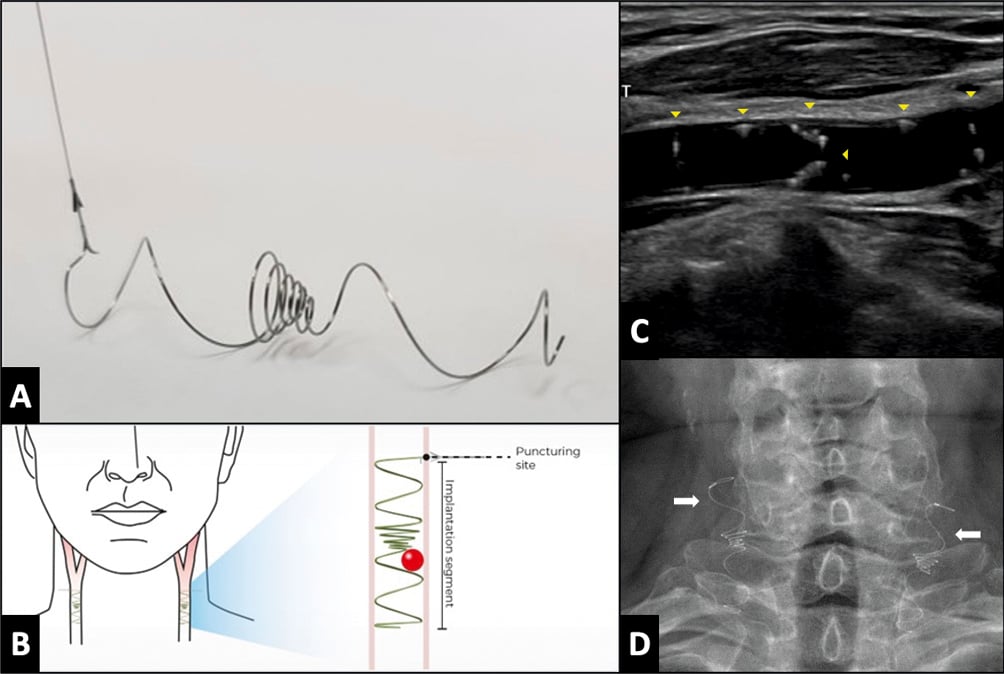
Figure 4. The Vine (Javelin Medical) filter. A) A permanent, bilateral, common carotid artery (CCA) filter. B) Illustration of the filter position in both carotid arteries. C) Transcarotid echography in longitudinal view showing the filter implanted in the right common carotid artery (yellow arrowheads). D) Fluoroscopic view showing both carotid filters in the anteroposterior view (white arrows).
Current controversies
Complete versus incomplete cerebral embolic protection
Current data notionally support the use of CEPDs that offer complete brain protection during transcatheter heart interventions, when feasible, since the left vertebral artery accounts for up to 20% of total brain perfusion838485. The amount and size of debris that passes through the left vertebral artery during TAVR is comparable to the amounts that pass through the brachiocephalic trunk and left common carotid artery15168386. The clinical and surrogate endpoint (MRI, cognitive impairment) outcomes are anticipated to be superior with complete versus partial CEPDs. However, this concept will require confirmation in prospective randomised studies.
Deflection versus collection of debris during transcatheter cardiac interventions
Likewise, it stands to reason that entrapping and extracting debris produced during transcatheter heart interventions may offer some advantage over deflection of particles away from the brain towards the distal circulation. However, this concept has yet to be demonstrated in any study to date, and it is not supported by the literature in terms of a lower incidence of clinical events, such as acute renal failure or distal limb complications arising from embolic debris. In addition, the risk/benefit ratio (in terms of excess vascular complication rates arising from a large-bore contralateral femoral access site versus stroke/peripheral embolic protection/mitigation) must be preserved in the setting of using larger-calibre devices via the femoral artery to extract as much debris as possible during transcatheter interventions for total body protection. A current niche area that is anticipated to grow exponentially in the future involves techniques to resect native or transcatheter heart valve leaflets87. These procedures carry a high risk of large-sized debris embolisation wherein devices designed for full body embolic protection and total debris entrapment would notionally stand to play an important role. Furthermore, given the similar incidence of periprocedural stroke during transcatheter mitral edge-to-edge repair therapies (large-bore venous procedures) as compared with TAVR, avoiding large-bore femoral arterial access would be ideal if CEPDs were to ultimately find a place for stroke/brain protection during these procedures.
CEPD use during non-femoral TAVR procedures
The increasing application of TAVR in populations with complex iliofemoral vascular access (severe calcification and/or tortuosity), particularly in patients harbouring an anatomy incompatible with the sizes of current delivery systems, has resulted in alternate access TAVR procedures being performed in a non-negligible proportion of patients88. To date, there are no data on the preferential choice of any specific type of CEPD based on the vascular access route. This choice should consider the technical characteristics of TAVR, taking special care when using CEPDs via the radial route in patients with transcarotid, axillary or subclavian TAVR and with CEPDs via the femoral route in patients with transcaval or transaortic TAVR. The effectiveness and safety of CEPDs can be altered by their interaction with the various catheters used in TAVR and with modifications to perform TAVR through alternative vascular access.
CEPD during SAVR
The reported stroke rates after SAVR range widely (from 1 to 17%)899091. In a propensity-matched study including 1,204 pairs of patients undergoing TAVR or SAVR from the PARTNER trials, Kapadia et al92 found a greater 30-day major stroke incidence after SAVR compared to TAVR (3.9% vs 2.2%, respectively; p=0.018) and an associated decline in quality of life at 1 year in all patients who suffered a major stroke from both groups. Also, the incidence of subclinical brain lesions is considerable (up to 60%)90. A randomised trial compared 2 types of CEPD (n=118 for suction-based extraction and n=133 for intra-aortic filtration device) versus a standard aortic cannula (control; n=132) at the time of SAVR93. The study failed to demonstrate prevention of either clinical stroke or imaging-based cerebral infarction, despite capturing embolic debris in the majority of the embolic protection devices. Although attractive, the use of transcatheter CEPDs has not been reported in this setting.
Upcoming trials
A large, randomised trial (ongoing) is likely to provide definitive evidence on the efficacy of the SENTINEL CEPD in the clinical prevention of stroke during TAVR, as well as several other studies which will provide data for various CEPDs on the prevention of procedural stroke and ischaemic brain injury by DW-MRI (Figure 5).
The BHF PROTECT-TAVI (British Heart Foundation Randomised Trial of Routine Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation) (n=7,730), is an open-label, outcome-adjudicated, multicentre, all-comer randomised clinical trial in the UK that will randomise patients undergoing TAVR by any access route to CEPD (with the SENTINEL CEPD) or no CEPD, with no specific exclusion criteria. The primary outcome measure is stroke at 72 hours post-TAVR. Amongst a range of secondary outcome measures, a cost-effectiveness analysis at 12 months will be performed. As of 30 June 2023, 59% of the total population (4,534 patients) have been enrolled in the trial94. An interim analysis is planned after the inclusion of 3,865 participants (50% of sample size). Final results are expected in July 2026. One of the main differences with the PROTECTED TAVR trial is there is no mandatory neurological evaluation by a neurologist or by a stroke specialist, this may decrease non-disabling stroke detection. Also, enrolment bias could be diminished since the device is not approved for daily clinical use in the UK.
A pooled patient-level meta-analysis from both studies (PROSPERO registry) encompassing over 10,000 TAVR patients is planned and could provide a definitive answer on the SENTINEL device’s efficacy in stroke prevention during TAVR95. Although it will be very important information, the PROTECTED TAVR and BHF PROTECT-TAVI trials results are only relevant for SENTINEL CEPD. Hence, the results are not definitive for CEPD as a class of devices. Newer generations of CEPD will require their own specific clinical validation.
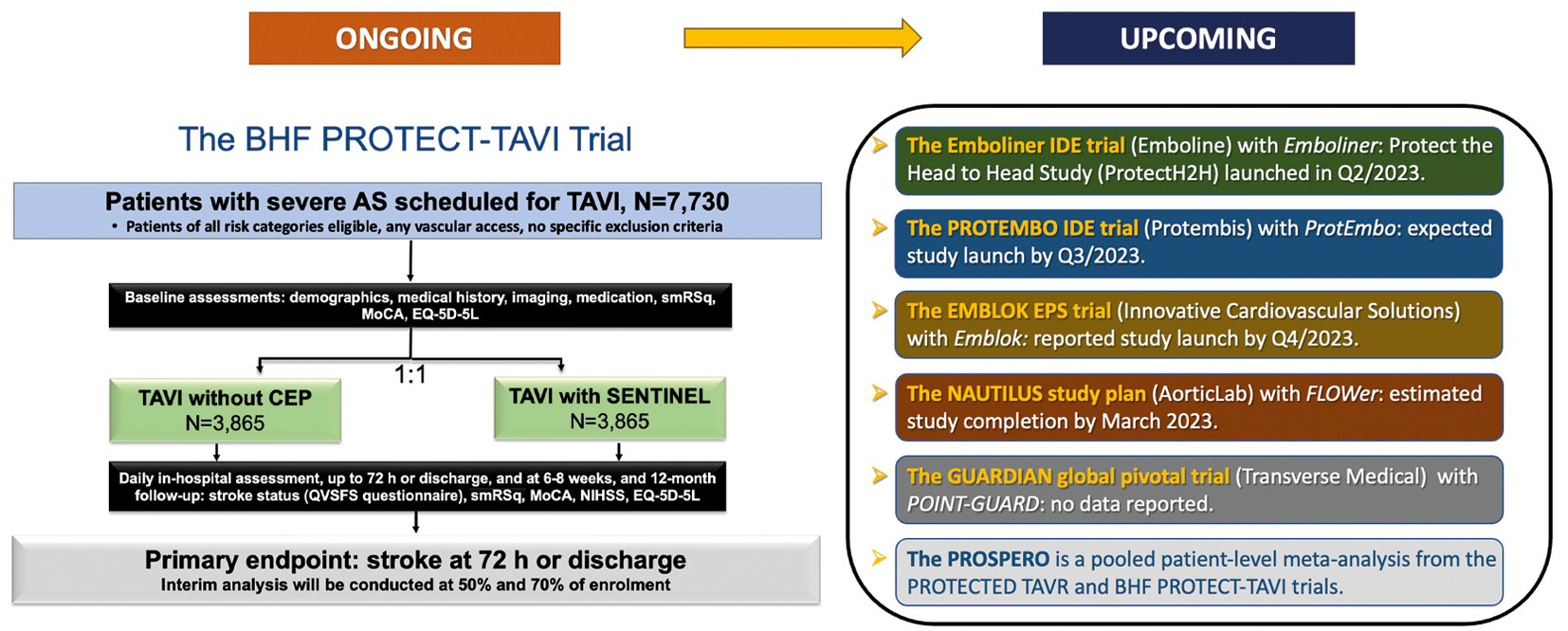
Figure 5. Main ongoing and upcoming clinical trials with CEPD. AS: aortic stenosis; BHF: British Heart Foundation; CEP(D): cerebral embolic protection (device); IDE: investigational device exemption; MoCA: Montreal Cognitive Assessment; NIHSS: National Institute of Health Stroke Score; Q: quarter; QVSFS: Questionnaire for Verifying Stroke-Free Status; smRSq: simplified modified Rankin Scale questionnaire; TAVI: transcatheter aortic valve implantation
Conclusions
Stroke following TAVR and various other transcatheter heart interventions remains largely unpredictable. In current TAVR practice, the rate of overt stroke during or soon after TAVR remains constant at between 2% and 4%45, despite significant improvements in transcatheter heart valve design and TAVR techniques. Emerging evidence points to cerebral embolisation during other commonly performed left-sided transcatheter heart procedures, particularly mitral edge-to-edge repair interventions. Although cerebral embolisation appears ubiquitous during TAVR, its longer-term consequences upon cognition remain uncertain. While the first pivotal trial powered for clinical superiority of the SENTINEL CEPD failed to meet its primary endpoint, a much larger randomised trial is expected to provide further evidence of whether the current SENTINEL CEPD design significantly reduces the incidence of stroke during TAVR. Meanwhile, numerous other CEPDs remain under development offering a range of protective mechanisms and deployment strategies. There remains limited, yet emerging, data on the use of CEPDs in non-TAVR transcatheter heart and vascular procedures. Ultimately, the protective benefit of CEPDs in certain clinical scenarios or high-risk catheter-based vascular and structural interventions posing significant risk of stroke will require clinical evidence before CEPDs ultimately find their way onto the shelves of many cardiac catheterisation laboratories worldwide. Further studies are needed to determine CEPD’s role and economic value in the rapidly expanding field of non-TAVR transcatheter interventions that are known to liberate considerable debris towards the brain.
Conflict of interest statement
V.A.J. Diaz is co-founder of Protembis GmbH and co-inventor of ProtEmbo. D. Mylotte is a consultant for Medtronic, Boston Scientific, and MicroPort. A. Lansky reports institutional research support from Emblok, Emboline, and AorticLabs. R. Puri is a consultant to Protembis GmbH. The other authors have no conflicts of interest to declare.

