Abstract
Antiplatelet therapy is key to reducing local thrombotic complications and systemic ischaemic events among patients undergoing percutaneous coronary interventions (PCI), but it is inevitably associated with increased bleeding. The continuous refinement in stent technologies, together with the high incidence of ischaemic recurrences after PCI and the understanding of prognostic implications associated with bleeding, have led to a substantial evolution in antiplatelet treatment regimens over the past decades. Numerous investigations have been conducted to better stratify patients undergoing PCI according to their ischaemic and bleeding risks and to implement antithrombotic regimens accordingly. Evidence from these investigations have resulted in a number of antithrombotic treatment options as recommended by recent guidelines. In this State-of-the-Art review we provide the rationale, summarise the evidence, and discuss current and future directions of antiplatelet treatment regimens after PCI.
Introduction
In patients with coronary artery disease (CAD), percutaneous coronary interventions (PCI) are the cornerstone of treatment for those presenting with an acute coronary syndrome (ACS); PCI has also been largely adopted in patients with chronic coronary syndromes (CCS)1. Adjunctive pharmacotherapy, in particular antithrombotic therapy, has a pivotal role in optimising outcomes in patients undergoing PCI23. In fact, patients undergoing PCI may develop both acute and long-term ischaemic events4. Therefore, antithrombotic drugs, in particular antiplatelet agents, are key to the treatment and prevention of both local and systemic thrombotic complications23. Over the past four decades there has been an evolution in antiplatelet treatment regimens being used in patients undergoing PCI2. This is attributed to a number of factors including refinement in stent technologies, leading to safer (i.e., less thrombogenic) stent platforms, the development of new antiplatelet drugs, as well as an understanding of the prognostic implications associated with bleeding, the most feared trade-off associated with the use of antiplatelet therapies25. Moreover, numerous investigations have also helped to develop a profile of patients at increased risk of ischaemic and bleeding complications2. These profiles, paralleled with an understanding of how individuals may have variable reactions to specific antiplatelet agents, have also laid the foundation for personalised treatment regimens with the goal of optimising efficacy and safety outcomes6. In this State-of-the-Art review, we provide the rationale, discuss the evidence and summarise the current and future directions of antiplatelet treatment regimens after PCI, with a specific focus on oral antiplatelet agents.
Rationale for the use of antiplatelet therapy in patients undergoing percutaneous coronary intervention
Arterial thrombus formation is a complex and dynamic pathological process that is initiated within an injured blood vessel wall or by contact activation on a foreign surface7. Platelets play a key role in thrombus formation and their initial tethering is mediated by the interaction between the complex glycoprotein (GP) Ib-IX-V and Von Willebrand factor and by collagen receptors present on the platelet surface such as GPVI (Figure 1)789. The crosstalk between platelets, the haemostatic system and inflammatory pathways are key to atherosclerotic development and thrombotic complications occurring after plaque destabilisation10111213141516. In ACS patients, when plaque rupture or erosion occurs, the activation of coagulation cascade and platelets can lead to either subocclusive or occlusive thrombosis, causing a symptomatic event. Alternatively, a plaque healing phenomenon occurs when thrombus formation is contained; repeated cycles of this process promote disease progression among patients with CCS (Figure 1)141718. Therefore, the rationale for using antiplatelet agents in patients with CAD is not only for the treatment of acute thrombotic events, but also to modulate the progression of atherosclerotic disease141718.
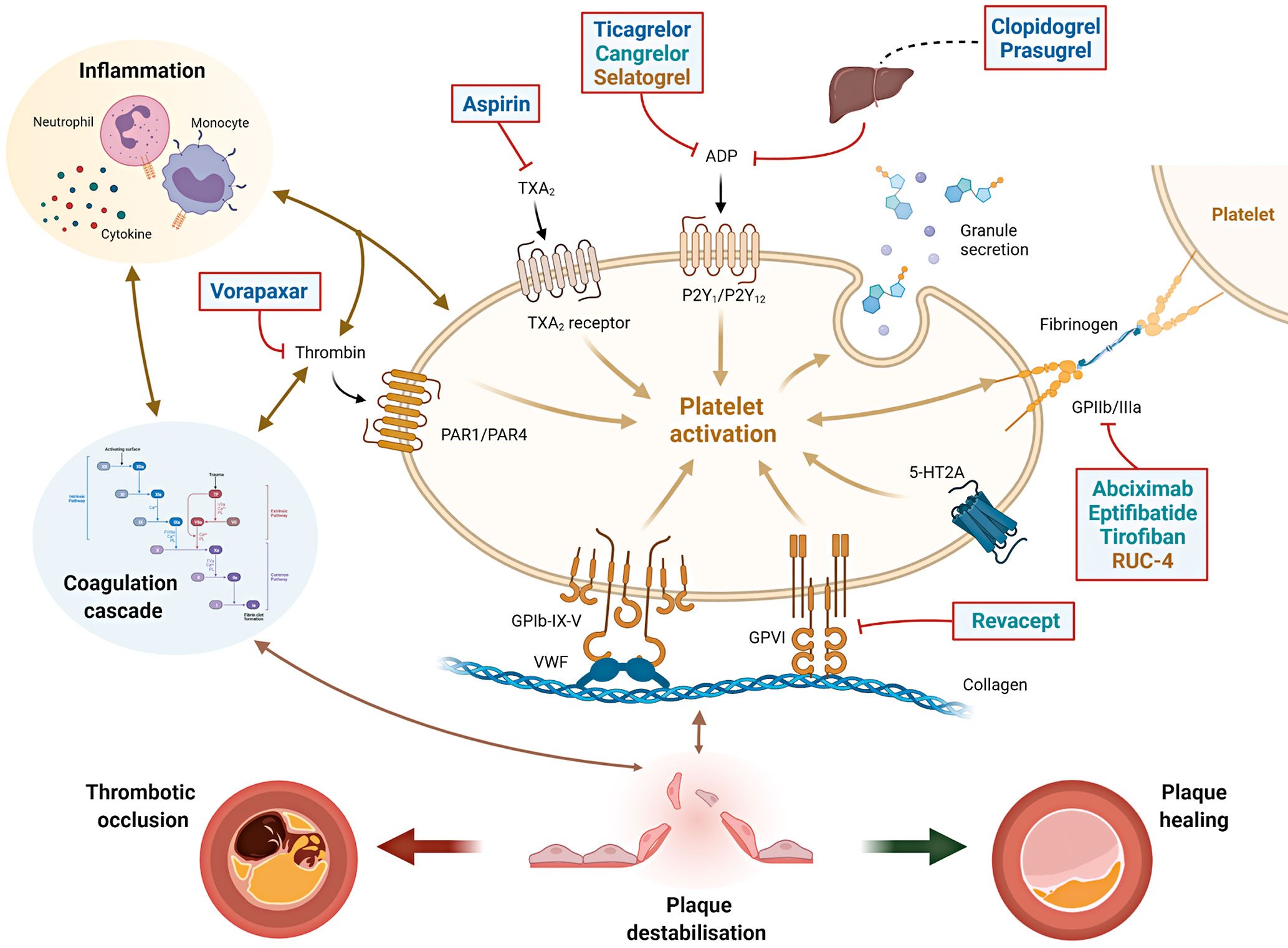
Figure 1. Interplay between platelets, coagulation and inflammation in atherothrombosis and sites of action of antiplatelet agents. The interplay between platelets, coagulation and inflammation is a key modulator of atherosclerosis and its thrombotic complications. When plaque destabilisation occurs, due to plaque rupture or erosion, platelets and coagulation factors, as well as subsequent inflammatory processes, it may lead to either a thrombotic occlusion of the coronary artery (leading to acute coronary syndromes) or plaque healing (favouring plaque progression with stable coronary artery disease). Initial platelet tethering is mediated by the interaction between the complex glycoprotein (GP) Ib-IX-V and Von Willebrand factor (vWF) and by other collagen receptors present on the platelet surface such as GPVI. Thrombin is a key linking factor between platelet and coagulation cascade. Sites of action of common antiplatelet agents are reported: aspirin inhibits thromboxane A2 (TXA2); clopidogrel, prasugrel, ticagrelor and cangrelor are inhibitors of the ADP P2Y12 receptor. Clopidogrel and prasugrel require hepatic activation. Vorapaxar is a thrombin receptor inhibitor (protease-activated receptor, PAR-1); abciximab, eptifibatide, tirofiban and RUC-4 are GPIIb/IIIa receptor inhibitors. Revacept is a competitive antagonist of the collagen-GPVI signalling. Routes of administration: Blue=oral; Green=intravenous; Yellow=subcutaneous/intramuscular. ADP: adenosine diphosphate; GP: glycoprotein; PAR-1: platelet protease-activated receptor-1; TXA2: thromboxane A2; vWF: von Willebrand factor; 5HT2A: serotonine receptor.
Antiplatelet therapy reduces thrombotic-related periprocedural myocardial damage associated with blood vessel wall trauma such as dissections or plaque ruptures, embolisation or side branch occlusions. In addition, it decreases the risk of stent thrombosis (ST), which is more frequent in the acute or subacute phase of PCI (Figure 2)192021. Importantly, peri-PCI thrombotic complications impact long-term prognosis, to the extent that their occurrence has challenged the long-term clinical benefit of PCI as compared to medical therapy among patients with CCS2223. Intravenous antiplatelet agents, including glycoprotein IIb/IIIa inhibitors (GPIs) and cangrelor, reduce the risk of peri-PCI thrombotic complications24. A detailed description of intravenous antiplatelet agents goes beyond the scope of this manuscript and is summarised elsewhere24.
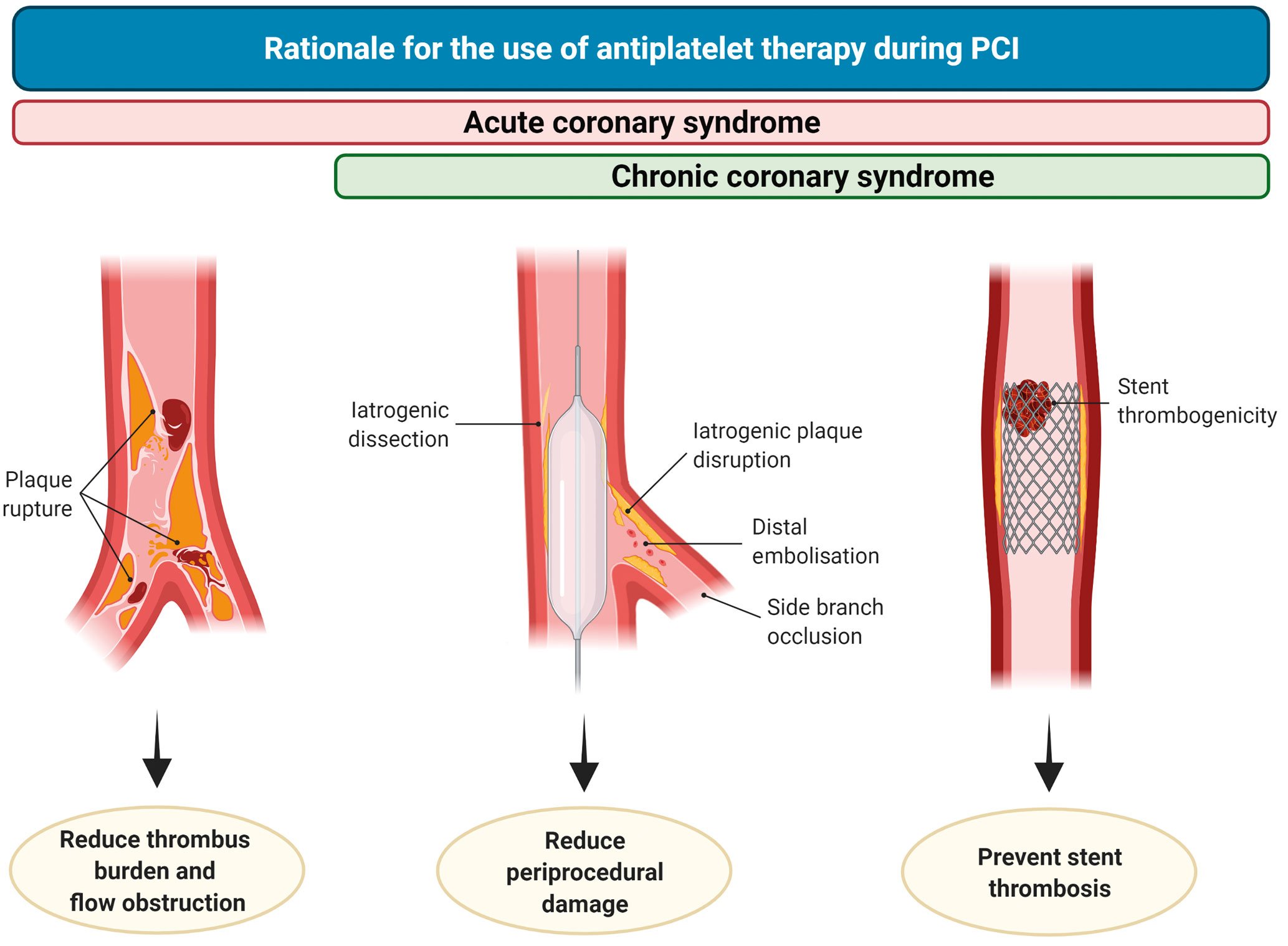
Figure 2. Rationale for the use of antiplatelet therapy during PCI. In patients undergoing PCI in the setting of chronic coronary syndrome (CCS), antiplatelet therapy reduces the occurrence of intraprocedural or very early stent thrombosis (right), periprocedural damage caused by iatrogenic dissections, plaque disruption, distal embolisation or side branch occlusion (middle). During acute coronary syndrome, antiplatelet therapy also plays a role in reducing thrombus burden and flow obstruction (left). PCI: percutaneous coronary intervention.
Oral antiplatelet agents are essential to both short- and long-term management after PCI23. Aspirin is an irreversible inhibitor of platelet cyclooxygenase (COX)-1 and traditionally has been a backbone treatment for patients with atherosclerotic disease manifestations25. In patients undergoing PCI, the association of aspirin plus a P2Y12 inhibitor, a strategy known as dual antiplatelet therapy (DAPT), has represented the cornerstone of treatment for patients undergoing PCI23. The clinical development of DAPT is based on investigations showing that the adjunctive use of a P2Y12 inhibitor with aspirin is associated with synergistic platelet inhibitory effects, resulting in improved antithrombotic efficacy in the setting of ACS and patients undergoing PCI262728293031. Ticlopidine, a first-generation thienopyridine, was characterised by several drawbacks (i.e., bone marrow suppression) and replaced by clopidogrel, a second-generation thienopyridine, due to its more favourable safety profile30. Clopidogrel remains the most widely studied P2Y12 inhibitor. The key role of platelet P2Y12 signalling in platelet activation and amplification processes explains why a blockade of this pathway in patients undergoing PCI results not only in a reduction of acute, local, thrombotic events (i.e., ST) but also prevents long-term ischaemic recurrences due to atherosclerotic plaque progression and destabilisation both in the coronary and extra-coronary vasculature9.
Despite its undisputed benefits, several investigations have revealed heterogeneity in individual response profiles to clopidogrel, with a considerable number of patients yielding inadequate platelet inhibitory effects, resulting in increased risk of thrombotic events post-PCI63233. These observations have prompted the development of newer-generation oral P2Y12 inhibitors including prasugrel, a third-generation thienopyridine, and ticagrelor, a first-in-class cyclopentyltriazolopyrimidine, characterised by potent and predictable antiplatelet effects resulting in greater antithrombotic efficacy compared to clopidogrel in the setting of ACS, albeit at the expense of increased bleeding34. The time course of benefit and risk associated with DAPT in patients undergoing PCI are summarised in Figure 3.
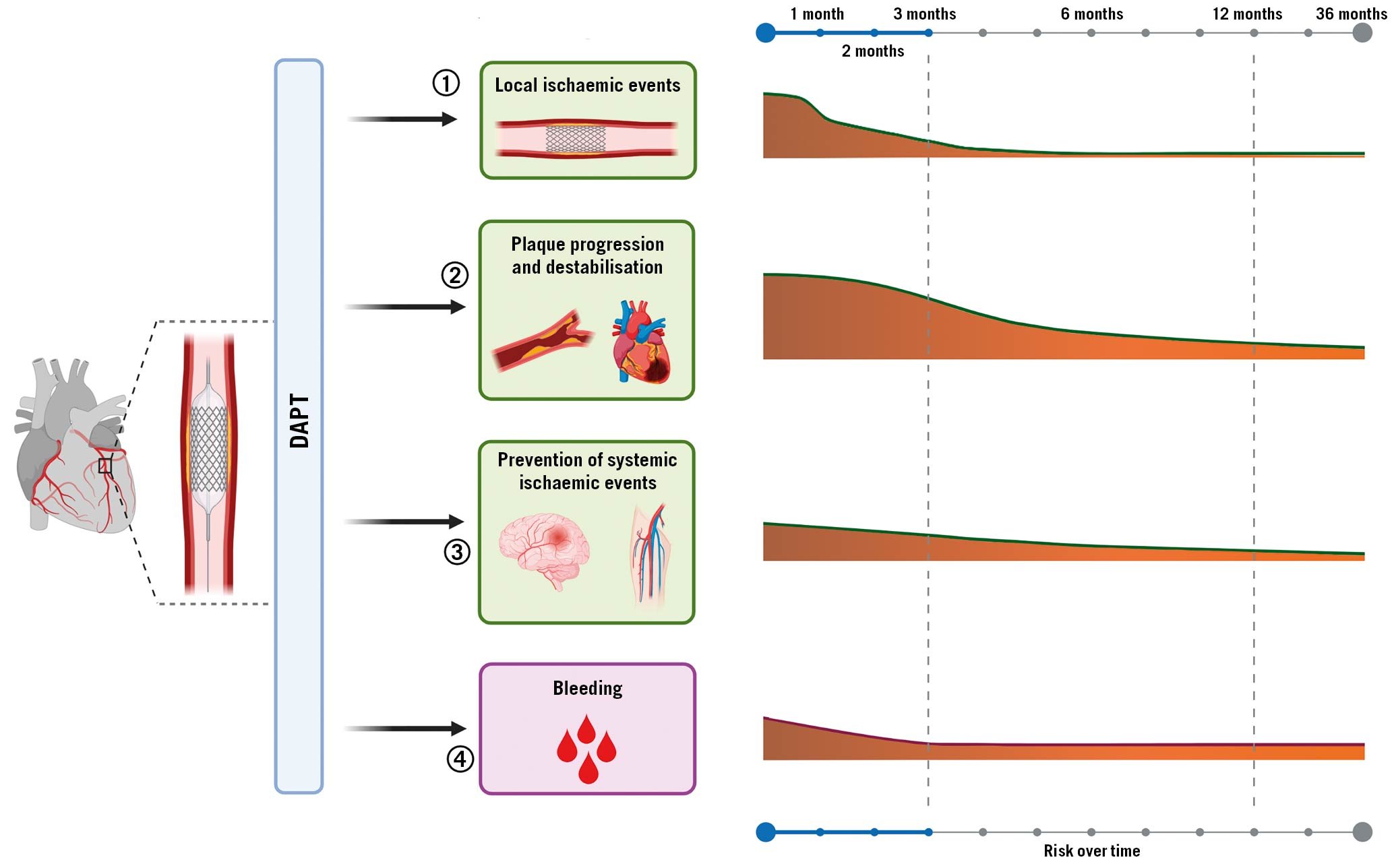
Figure 3. Time course of benefit and risk of antiplatelet therapy after PCI. Antiplatelet agents administered after PCI may 1) reduce the incidence of stent-related ischaemic events such as stent thrombosis or target vessel revascularisation; 2) reduce the incidence of cardiovascular ischaemic recurrence and their consequences, such as myocardial infarction and cardiovascular death; 3) prevent cerebrovascular events in other areas affected by atherosclerotic disease, such as peripheral and carotid arteries; 4) be inevitably associated with increased risk of bleeding. Importantly, the potential benefit of antiplatelet agents varies over time, with the greatest benefit in terms of less ischaemic events maximised during the first months after PCI and decreasing over time, while bleeding events remain stable over time. DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention.
Indeed, bleeding is the main drawback of antithrombotic therapies, particularly given its association with adverse prognosis35. Multiple mechanisms can explain the adverse outcomes, including ischaemic events and mortality, associated with bleeding. These include, but are not limited to: interruption of antiplatelet treatment as a reaction to the bleeding event, activation of coagulation and inflammation in case of bleeding, and depletion of 2,3-diphosphoglyceric acid and nitric oxide triggered by blood transfusion which modulates oxygen exchange at the tissue level and favours vasoconstriction and platelet aggregation3637. The risk of bleeding is proportional to the intensity of antithrombotic treatment38. This explains why bleeding complications are highest in the early phase post-PCI, given that patients warrant vascular access and are exposed to adjuvant intraprocedural antithrombotic therapy. Whilst both ischaemic and bleeding risks are highest in the periprocedural phase, the risk of bleeding tends to be stable over time while ischaemic risk decreases after 1 to 3 months post-PCI, albeit with variability according to the clinical presentation of the patients and complexity of the PCI39. These considerations have stimulated interest for tailoring antiplatelet regimens according to the ischaemic and bleeding risk of the individual patient.
Current recommendations on the use of oral antiplatelet therapy after PCI
DAPT is the standard of care for patients undergoing PCI140414243. Aspirin (loading dose of 160-325 mg orally or 250-500 mg intravenously, followed by an oral maintenance dose of 75-100 mg once daily [od]) should be administered in all patients. The DAPT regimen, including the choice of P2Y12 inhibitor, varies according to the clinical setting (i.e., ACS or CCS) as well as the thrombotic and bleeding risk of the individual patient. Guideline recommendations from the European Society of Cardiology (ESC) are provided in Figure 4.
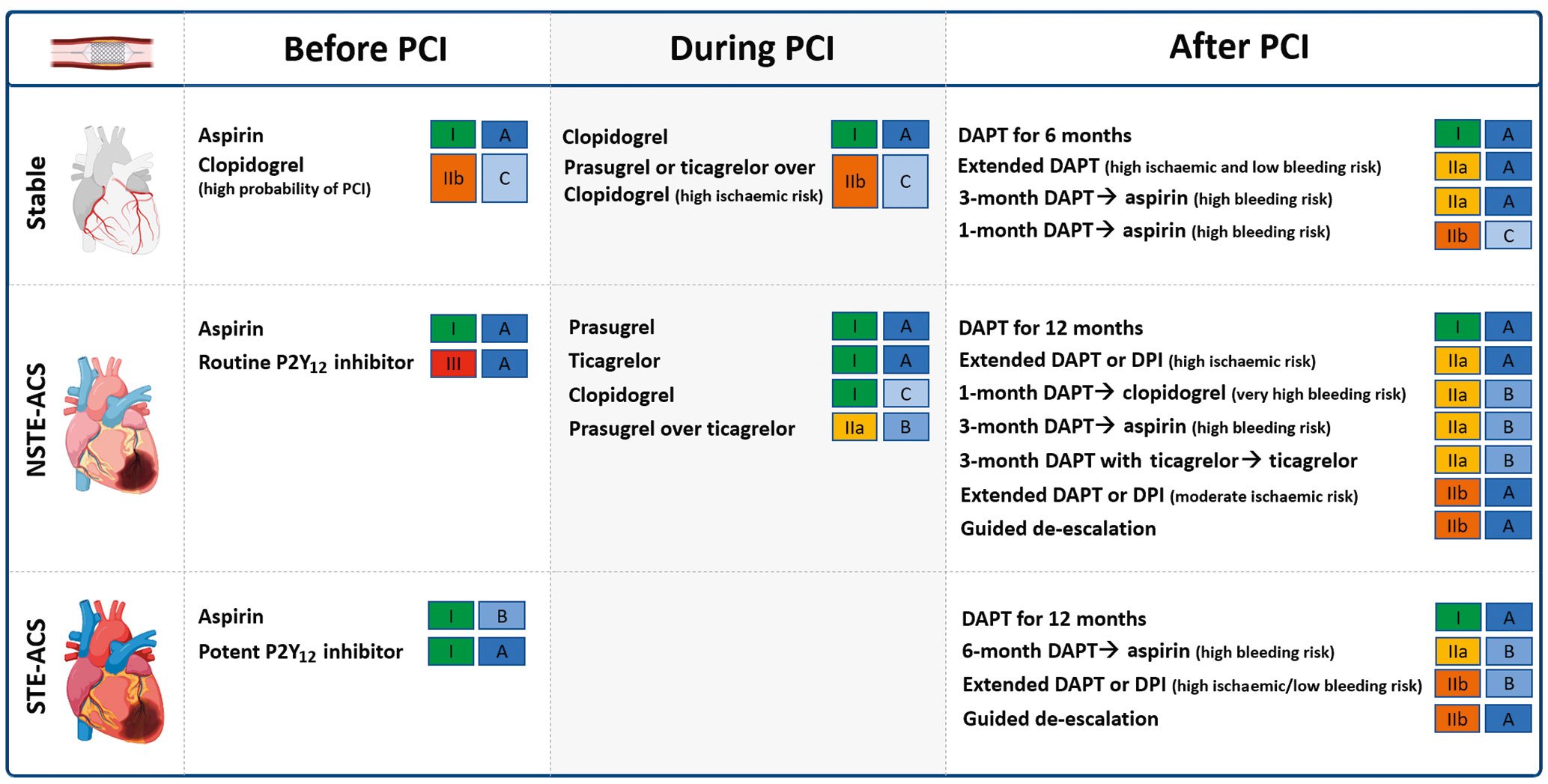
Figure 4. Current Guidelines of the European Society of Cardiology recommendations for oral antiplatelet agents among patients undergoing PCI. DAPT: dual antiplatelet therapy; DPI: dual pathway inhibition; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STE-ACS: ST segment-elevation acute coronary syndrome
Chronic coronary syndrome
In patients with CCS, initiation of oral P2Y12 inhibitors is usually delayed until the coronary anatomy is defined4043. Clopidogrel, administered as a 600 mg loading dose followed by a 75 mg maintenance dose, is the P2Y12 inhibitor of choice in CCS patients undergoing PCI4043. After PCI, 6-month DAPT, followed by long-life aspirin monotherapy is the default recommendation with the option of shortening DAPT duration to either 1 or 3 months according to the bleeding risk4043.
Acute coronary syndromes
In patients with ACS, while pre-treatment with an oral P2Y12 inhibitor has the theoretical advantage of providing greater antiplatelet protection by the time of PCI, not all patients undergoing coronary angiography undergo PCI. This inevitably may expose patients unnecessarily to adjunctive antiplatelet treatment and increase their risk of bleeding44. This also represents a major drawback for patients requiring surgical revascularisation who then need to wait a washout period before undergoing their procedure, prolonging hospital stay and costs44. Ultimately, most recent randomised controlled trials (RCTs) have failed to show any benefit of pre-treatment versus in-lab use of P2Y12 inhibitors454647. More specifically, pre-treatment versus in-lab treatment with prasugrel in patients with a non-ST-elevation ACS (NSTE-ACS) did not reduce ischaemic events but increased major bleeding complications47. In turn, pre-treatment with prasugrel is contraindicated in NSTE-ACS4143. Moreover, pre-treatment with ticagrelor compared with in-lab treatment with prasugrel favoured the latter45. Accordingly, the latest NSTE-ACS guidelines do not recommend routine pre-treatment with a P2Y12 inhibitor, stating that this strategy can only be considered (using ticagrelor 180 mg followed by 90 mg twice daily [bid] or clopidogrel 600 mg followed by 75 mg/od) for patients at low bleeding risk who are not scheduled to undergo an early invasive strategy41. On the contrary, among STE-ACS patients, the use of a potent P2Y12 inhibitor at the time of diagnosis is encouraged, as primary-PCI is the treatment of choice in these patients4243. It is however important to note that, particularly in the setting of ST-segment elevation myocardial infarction (STEMI), the onset of action of oral P2Y12 inhibitors is significantly delayed, requiring up to 4-6 hours to achieve full antiplatelet effects, underscoring the need for strategies to bridge this gap in platelet inhibition, such as the use of intravenous antiplatelet therapies48.
In ACS patients undergoing PCI, the standard recommendation is 12 months of DAPT including a potent P2Y12 inhibitor414243. Prasugrel and ticagrelor are preferred over clopidogrel in patients with NSTE-ACS in the absence of contraindications, with prasugrel (60 mg followed by 10 mg/od) being recently recommended over ticagrelor4149. NSTE-ACS guidelines also introduce the use of ticagrelor monotherapy 3 months after PCI among patients at low ischaemic risk41. Among patients with moderate or high bleeding risk, clopidogrel should be preferred over more potent P2Y12 inhibitors and DAPT can be shortened to either 1 month, when followed by clopidogrel monotherapy, or to 3 months in NSTE-ACS and to 6 months in STE-ACS when followed by aspirin monotherapy4142. Moreover, guided or unguided de-escalation of P2Y12 inhibitors can also be considered41. Conversely, among patients with high or moderate ischaemic risk and low bleeding risk, prolonged antithrombotic regimens can be considered 12 months after PCI for ACS41. Treatment options are the prolongation of DAPT with either clopidogrel (75 mg/od), prasugrel (10 mg/od), or ticagrelor (60 mg/bid) in addition to aspirin, or alternatively, to consider a strategy of dual pathway inhibition (DPI) with the use of a vascular dose of rivaroxaban (2.5 mg/bid) in addition to aspirin4142.
Special scenarios
Among patients who undergo PCI but who also have an indication to be treated with oral anticoagulants (OAC), such as those with atrial fibrillation (AF), recommendations have varied over the years50. The latest recommendations now indicate that the default strategy to be used is triple therapy (TT), consisting of aspirin, a P2Y12 inhibitor (preferably clopidogrel) and a novel oral anticoagulant (NOAC) up to 1 week post-PCI, followed by dual antithrombotic therapy (DAT) with a P2Y12 inhibitor and an NOAC (i.e., discontinue aspirin)4151. The duration of TT could be prolonged up to 1 month, but not beyond, among patients with high ischaemic risk but at low risk for bleeding4151. DAT should be maintained for up to 12 months after which antiplatelet therapy should be discontinued, except in those patients deemed to be at increased risk of long-term ischaemic recurrences4151. Discontinuation of antiplatelet therapy and maintaining OAC treatment alone may be considered sooner (i.e., at 6 months) if patients are at increased risk of bleeding or at low ischaemic risk4151. Although clopidogrel is the P2Y12 inhibitor of choice, potent P2Y12 inhibitors may be considered in patients at high thrombotic risk and low bleeding risk. However, the use of aspirin should not go beyond one week4151.
Qualitative and quantitative approaches for risk stratification
A careful stratification at a single patient level represents the foundation of a personalised selection of antiplatelet strategies among patients undergoing PCI52. This can be achieved by an integrated assessment of 3 key factors: bleeding risk, ischaemic risk and responsiveness to an antiplatelet agent (Figure 5).
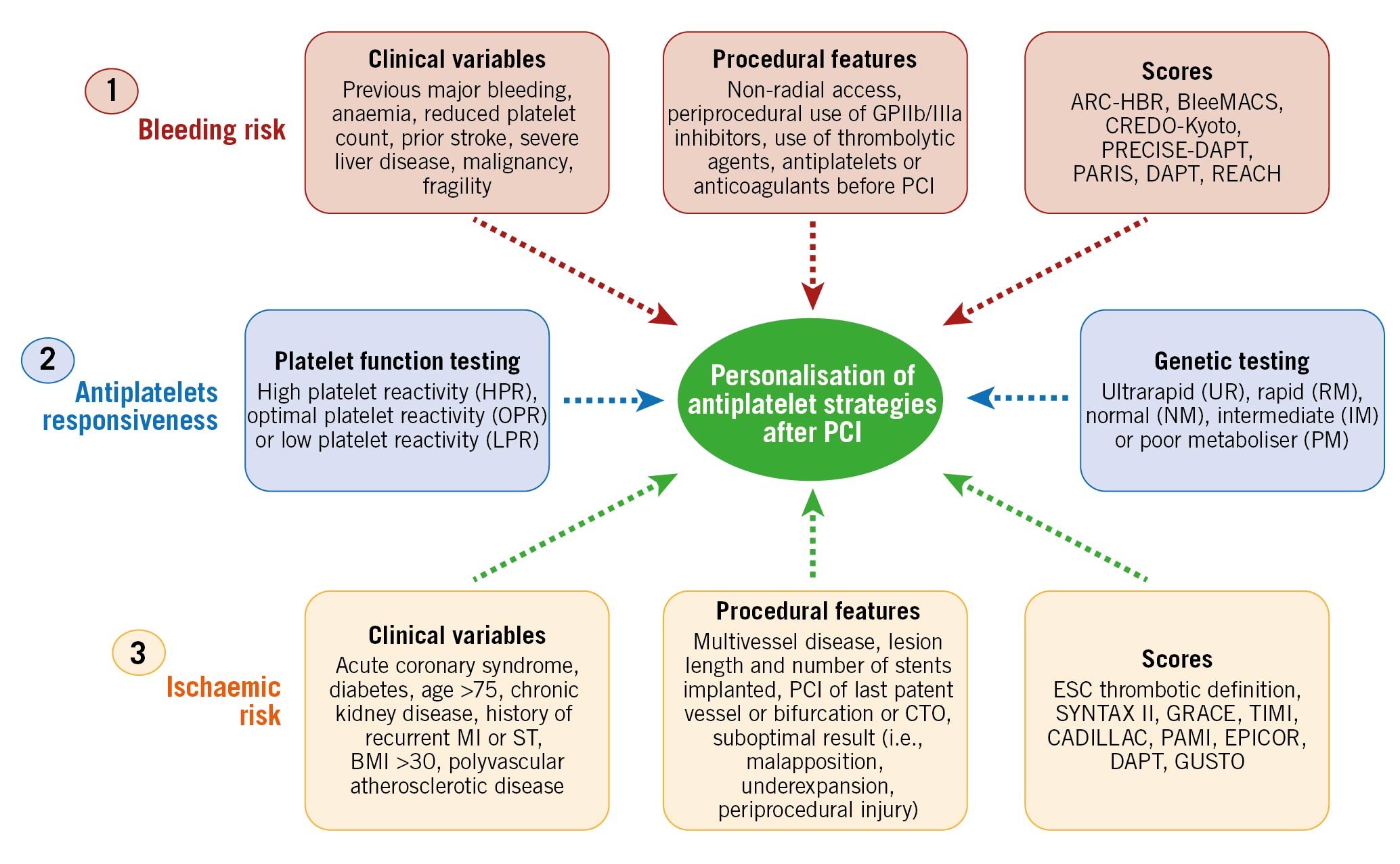
Figure 5. Risk assessment to guide antiplatelet therapy among percutaneous coronary disease patients. Bleeding and ischaemic risk assessments are based on clinical variables, procedural features and the use of scores/definitions. Antiplatelet responsiveness can be assessed by either platelet function or genetic testing. BMI: body mass index; CTO: chronic total occlusion; GP: glycoprotein; MI: myocardial infarction; PCI: percutaneous coronary intervention; ST: stent thrombosis.
Bleeding and ischaemic risk assessment
The assessment of bleeding and ischaemic risks is achieved by the evaluation of clinical and procedural features which can be defined qualitatively as well as quantified by means of a scoring system (Figure 5). Clinical variables, including patient history, fragility and general status as well as comorbidities and laboratory exams represent the cornerstone for risk assessment.
Nevertheless, in the setting of patients undergoing PCI, procedural and technical features play an important role in determining ischaemic or bleeding risks and should be taken into consideration535455. Several clinical, procedural and laboratory factors that have been found to be associated with increased bleeding or ischaemic risk in retrospective studies have been included in the scores and definitions for risk assessment, in the hope of providing a prognostic stratification and predicting bleeding and/or ischaemic events to help guide the choice of antiplatelet therapy after PCI, as well as to improve the standardisation of the design of clinical trials and ease their interpretation525556. The most commonly adopted ischaemic scores (i.e., GRACE or TIMI) are mainly used for prognostic stratification. Concerning the use of scores or definitions for ischaemic risk stratification to guide the selection of antiplatelet therapy, the recent ESC guidelines provide a thrombotic risk stratification for intensified antithrombotic treatment after the standard DAPT duration by defining patients at high or moderate thrombotic risk (Table 1)4041.
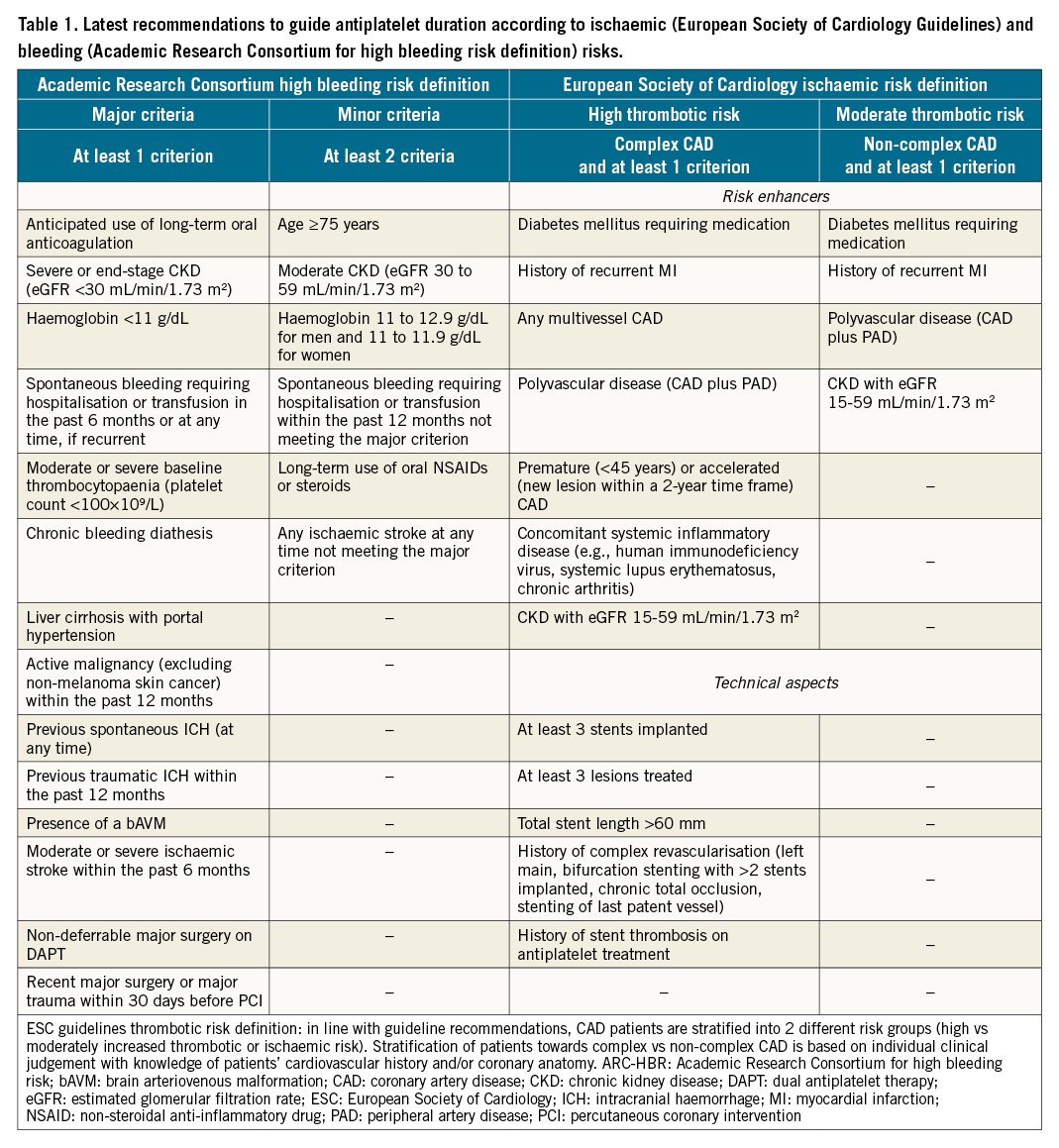
Specific risk scores have been developed aimed at managing antithrombotic duration after PCI according to both bleeding and ischaemic risks. Among these, the DAPT and the PRECISE-DAPT scores are calculated at patient discharge and at one year from the index event, respectively5758. The DAPT score includes clinical and procedural features and supports DAPT extension up to 30 months when the score is ≥257. Similarly, the PRECISE-DAPT score, which includes clinical and laboratory features, suggests the shortening of DAPT when the score is ≥25, as in these patients a longer DAPT duration was associated with increased bleeding without reducing ischaemic events58. Of note, both scores were developed and validated in patients without an indication to oral anticoagulation, although limited evidence is available in this setting5960. It is important to note that many patients are at risk of both increased bleeding and ischaemic events; when these are concordant, observational data suggest that bleeding, more than ischaemic risk, should inform the decision-making on the duration of DAPT61. Guidelines also recommend that the bleeding risk of an individual patient be the key determinant in defining DAPT duration1341. To date however, there are no studies that have provided definitive evidence of the advantage of bleeding risk stratification as compared to ischaemic risk stratification as a guide for the intensity and duration of DAPT.
Prompt identification of high bleeding risk patients by a standardised score or definition could play an important role in defining patients for whom a reduction in intensity of antiplatelet therapy could be advantageous. Over the years, a number of scores have been proposed to address this. Recently, the Academic Research Consortium for High Bleeding Risk (ARC-HBR) proposed a consensus for the definition of high bleeding risk, including 14 major and 6 minor criteria (Table 1)62. High bleeding risk is defined by at least 1 major or 2 minor criteria. Although retrospective studies have validated this definition, prospective studies using ARC-HBR criteria to stratify patients to specific antiplatelet regimens are warranted63. Although risk scores and definitions are useful for standardisation purposes, their use should always be integrated with other factors such as clinical and procedural characteristics as well as antiplatelet drug response52.
Antiplatelet drug response
Compared to prasugrel and ticagrelor, clopidogrel is characterised by less potent platelet inhibition, slower onset of action and wide interindividual variability in response profiles. This leads to high on-treatment platelet reactivity (HPR) in approximately 30% of patients, although this prevalence may vary based on distribution of risk factors and patient ethnic background632. Importantly, HPR status is associated with an increased risk of thrombotic complications63264. Of note, while HPR is a modifiable marker of thrombotic risk, low platelet reactivity (LPR), which is more common with prasugrel and ticagrelor, is associated with increased risk of bleeding without any reduction of ischaemic events among patients responding to clopidogrel32. Individual responsiveness to P2Y12 inhibitors can be assessed through platelet function tests665. Although platelet function testing has the advantage of defining the platelet phenotype which is associated with ischaemic outcomes, its implementation in clinical practice has inherent challenges6566. Indeed, although commercially available “point-of-care” or “near-patient-based assays”, which are more user-friendly than some more complex laboratory-based methods, have the advantage of providing results in a timely fashion, they are still limited by the variability in the results obtained and by the fact that patients need to be on clopidogrel to define responsiveness6. Genetic testing represents an alternative tool to assist with the guidance of antiplatelet therapy667. Indeed, genetic polymorphisms of the hepatic cytochrome P450 (CYP) 2C19 enzyme required to transform clopidogrel into its active metabolite, has an important role, with carriers of loss-of-function (LoF) alleles being characterised by reduced active metabolite generation, increased HPR rates and enhanced thrombotic risk, including ST66869. CYP2C19*2 and *3 are the most common LoF alleles667. It is important to note that CYP2C19 genotypes are not the sole contributors to clopidogrel response and thus may not always identify HPR status. For this reason, integrating clinical variables with genotypes to predict HPR status has been suggested to identify clopidogrel-HPR status more accurately70.
Strategies focused on reducing ischaemic events
Several strategies aimed at reducing the residual burden of ischaemic events among patients undergoing PCI, especially among high-risk cohorts of patients, have been tested over the years and are discussed below. In the Central illustration we illustrate a detailed timeline of the RCTs that focused on antiplatelet agents over the past 40 years. Table 2 summarises the strategies focused on the reduction of ischaemic events. The major drawback of these strategies is their trade-off in bleeding risk. Strategies that are no longer recommended by guidelines, such as use of ticlopidine, cilostazol or double-dose clopidogrel will not be discussed. RCTs and meta-analyses, as opposed to observational or registry studies, will be discussed as they represent the highest level of evidence. Moreover, it is important to acknowledge that amongst the strategies aimed at reducing ischaemic events in patients with ACS, that adding a NOAC to standard antiplatelet treatment regimens, including DAPT, has also been tested7172. Although a detailed description of this topic goes beyond the scope of this manuscript which is focused on antiplatelet therapies, it is important to acknowledge that a number of NOACs have been tested in this context, but only the low-dose rivaroxaban was shown to meet the objectives of this strategy in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects With Acute Coronary Syndrome ACS 2–Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2-TIMI 51) trial73. In particular, for patients with a recent ACS (n=15,526), rivaroxaban (twice-daily doses of either 2.5 mg or 5 mg) on top of standard of care antiplatelet therapy, most commonly aspirin and clopidogrel, reduced the risk of the composite endpoint of death from cardiovascular (CV) causes, myocardial infarction (MI), or stroke, at the expense of an increased risk of major bleeding and intracranial haemorrhage, but not fatal bleeding. The twice-daily 2.5 mg dose of rivaroxaban was associated with less bleeding. Despite the ischaemic benefit, this strategy has had limited uptake in clinical practice due to concerns surrounding the increased bleeding and the fact that the trial was not reflective of current recommendations that prefer P2Y12 inhibition with prasugrel and ticagrelor in patients with ACS. In contrast, a strategy of DPI with the use of rivaroxaban 2.5 mg/bid, in addition to aspirin, for long-term secondary prevention in patients with stable atherosclerotic disease, has received a stronger endorsement from practice guidelines and has been more broadly adopted41.
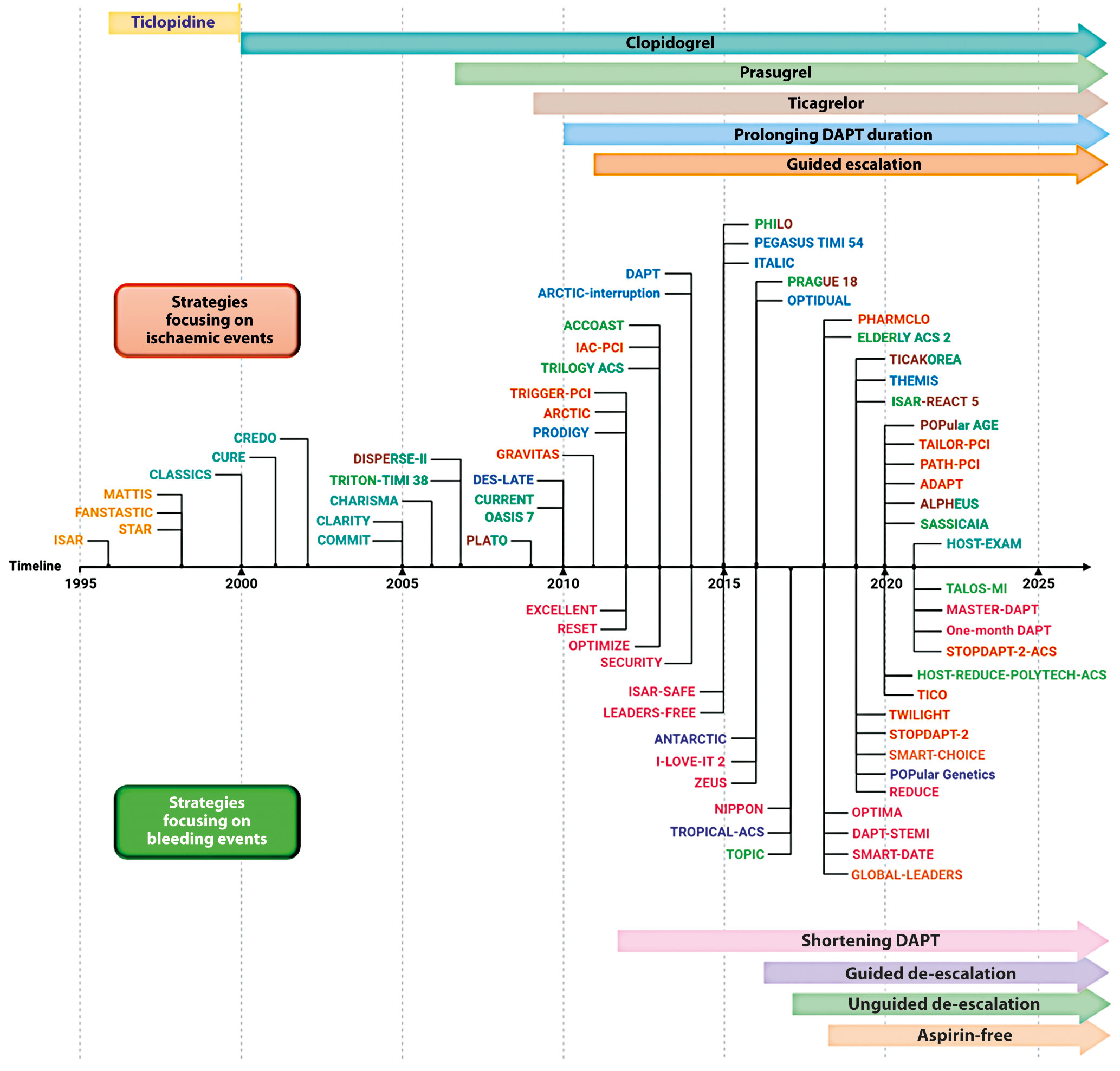
Central illustration. Timeline of randomised controlled trials on antiplatelet therapy focusing on strategies aiming at reducing ischaemic (upper) or bleeding (lower) events. DAPT: dual antiplatelet therapy
Potent P2Y12 inhibiting therapy
The first strategy proposed to reduce the occurrence of ischaemic events among patients undergoing PCI, was to use more potent P2Y12 inhibitors as compared to clopidogrel34.
Prasugrel
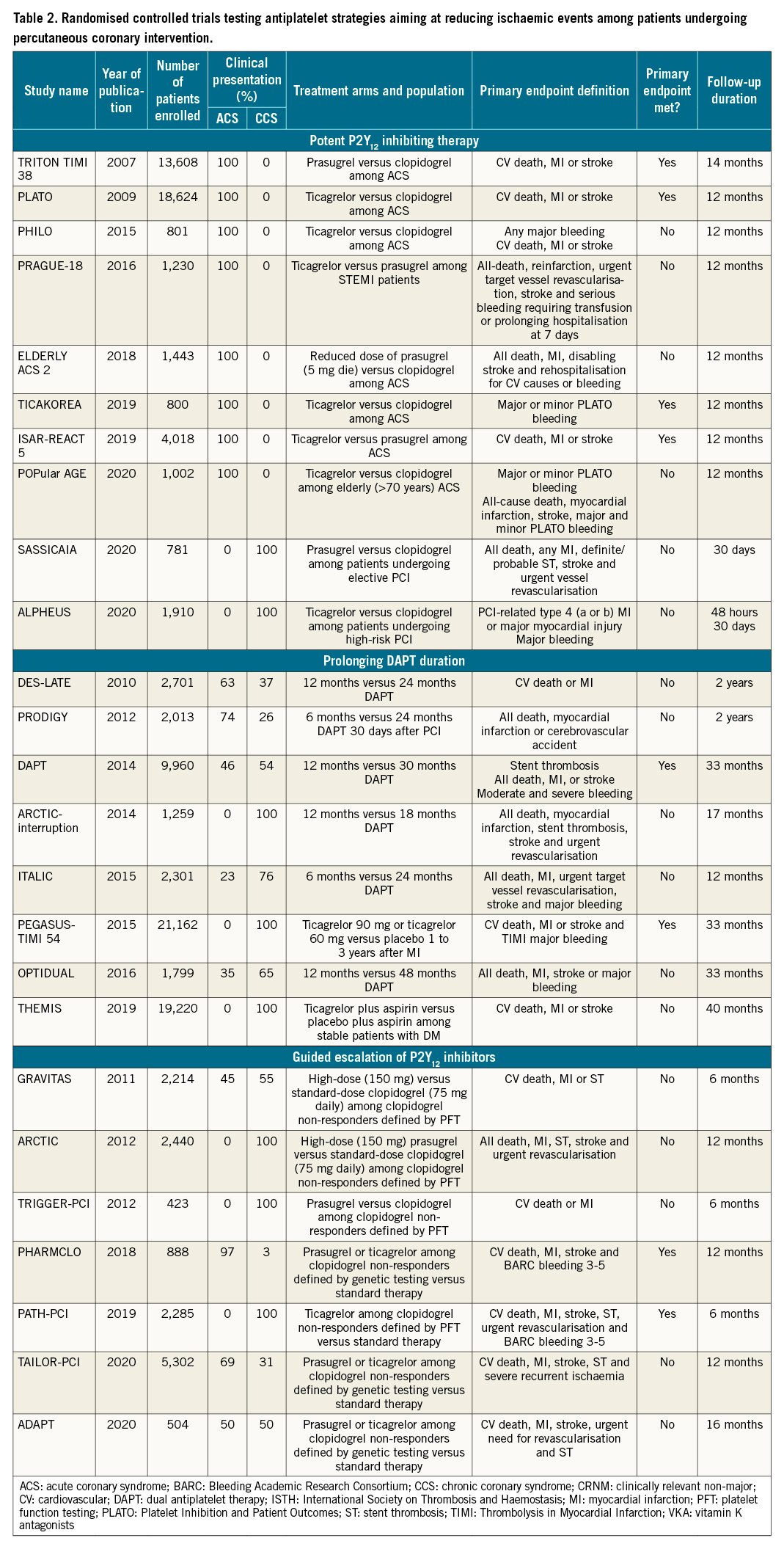
In 2007, the pioneering Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) trial showed prasugrel reduced the incidence of the primary endpoint of major adverse cardiovascular events (MACE) by 19% at 15 months, compared to clopidogrel, among 13,608 patients with ACS undergoing PCI74. These differences were driven by a reduction in MI; prasugrel also reduced rates of stent thrombosis74. However, prasugrel was also associated with a significant 32% increase in non-coronary artery bypass graft (CABG)-related Thrombolysis In Myocardial Infarction (TIMI) major bleeding, as compared to clopidogrel. Such an increased risk of bleeding offsets the benefits of prasugrel in certain patient cohorts, resulting in neutral effects in the elderly (aged ≥75 years) and low body weight (<60 kg) patients, and net harm to those with a history of stroke or transient ischaemic attack74. Although data from pharmacokinetic/pharmacodynamic (PK/PD) modelling suggested the use of a lower maintenance dose regimen (i.e., 5 mg/od) in the elderly and low-weight patients7576, to date there are no studies supporting superior efficacy of this regimen over clopidogrel77. The ischaemic benefits of prasugrel in ACS patients undergoing PCI has been confirmed in meta-analyses showing reduced MI and ST but yielding a 25-30% increase in major bleeding compared to clopidogrel78. Studies conducted in patients with medically-managed ACS or high-risk CCS undergoing PCI have failed to show any benefit of prasugrel7980.
Ticagrelor
In 2009, the Platelet Inhibition and Patient Outcomes (PLATO) trial, showed a 16% reduction of the composite primary endpoint of MACE at 12 months, with ticagrelor compared to clopidogrel among 18,624 patients with ACS managed either invasively or non-invasively81. These differences were driven by a reduction in MI and CV death81. The efficacy of ticagrelor was consistent among patients undergoing revascularisation; ticagrelor also reduced rates of stent thrombosis among patients undergoing PCI8283. Although the overall incidence of PLATO major bleeding was similar in the 2 groups, ticagrelor treatment was associated with a significant 25% increase in non-CABG-related TIMI major bleeding and an increase of fatal intracranial bleeding81. Moreover, a common non-bleeding adverse effect of ticagrelor was dyspnoea, which occurred in 15-22% of patients and was the most common cause of drug withdrawal81.
Although in PLATO there weren’t any subgroups in which the bleeding risk offset the efficacy of ticagrelor, absolute bleeding rates increased among the elderly treated with ticagrelor81. In the Ticagrelor or Prasugrel Versus Clopidogrel in Elderly Patients With an Acute Coronary Syndrome and a High Bleeding Risk: Optimization of Antiplatelet Treatment in High-risk Elderly (POPular AGE) study conducted in NSTE-ACS patients aged >70 (n=1,002), clopidogrel was associated with fewer bleeding events compared with ticagrelor and without an increase in the combined endpoint of all-cause death, MI, stroke, and bleeding84. Parallel findings were observed in several registry studies of ACS patients8586. The safety and efficacy of ticagrelor compared with clopidogrel among Asian patients with ACS was tested in 2 relatively small RCTs showing no ischaemic benefit and more bleeding, questioning the effectiveness of ticagrelor in this population or the potential need for dose adjustments, although neither study was sufficiently powered to demonstrate efficacy8788.
Subsequent meta-analyses in ACS patients showed ticagrelor to be the only P2Y12 inhibitor associated with a reduction in all-cause and CV death, but yielding a 20-30% increase of major bleeding compared to clopidogrel78. This finding can potentially be attributed to the pleiotropic mechanisms of ticagrelor, including increased plasma levels of adenosine, and supports the concept that there may be a mortality benefit independent from a reduction of ischaemic events89. However, such observations have not been observed in other studies45859091. Ultimately, in patients with CCS undergoing high-risk elective PCI, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis92.
Prasugrel versus ticagrelor
Whether 1 of the potent P2Y12 inhibitors is superior to the others has been a topic of debate for years93. Although earlier investigations were inconclusive94, The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 5) trial, conducted in 4,018 ACS patients, showed that prasugrel administered at the time of PCI after defining coronary anatomy, compared with pre-treatment with ticagrelor before defining coronary anatomy, was associated with a significant 26% reduction of the primary endpoint of MACE at 1 year without any increase in bleeding45. These findings were consistent, irrespective of clinical presentation (NSTE-ACS and STEMI)9596. Mechanisms that can explain these findings include the more potent platelet inhibitory effects of prasugrel over ticagrelor as confirmed in the pharmacodynamic substudy of the trial, as well as better compliance to treatment given that ticagrelor is more commonly associated with treatment discontinuation due to dyspnoea97. A network meta-analysis including both direct and indirect comparisons of oral P2Y12 inhibitors in ACS showed no significant differences between prasugrel and ticagrelor, but when compared to clopidogrel, only prasugrel reduced MI, while only ticagrelor reduced all-cause and CV death78.
Guided escalation of P2Y12 inhibitors
The use of tools to guide the selection of antiplatelet therapy can lead to an escalation of P2Y12 inhibiting potency (i.e., prasugrel or ticagrelor) among patients identified to have clopidogrel-HPR (i.e., using platelet function tests) or CYP2C19 LoF alleles (i.e., using genetic testing)6567. From a clinical standpoint, an escalation strategy is aimed at reducing ischaemic events without a trade-off in bleeding6. Two lines of research have investigated the impact of a guided escalation among patients undergoing PCI: in the first, escalation was compared to standard therapy selectively among patients with clopidogrel-HPR or carriers of the CYP2C19 LoF allele, and in the second, this was tested as a strategy (i.e., to assess the clinical benefit of testing versus no-testing in all-comers PCI). In the first line of research, several trials were performed but failed to demonstrate any benefit9899100. However, these trials were characterised by design limitations including poor definition of clopidogrel-HPR, implementation of strategies inadequate to overcome clopidogrel-HPR, and inclusion of low-risk patients. These observations have allowed for subsequent studies with ameliorated designs that support the clinical benefit of the use of guided escalation as a strategy (Table 2)101102103.
The second line of research tested the use of platelet function or genetic testing as a strategy among the entire population of patients undergoing PCI. These latter studies are of clinical interest since they tested the effectiveness of implementing a guided selection of antiplatelet therapy in clinical practice101102103104. Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI) randomised 5,302 patients undergoing PCI to either a guided or a standard selection of antiplatelet therapy103. The primary analysis was in patients with CYP2C19 LoF variants, and the secondary analysis included all randomised patients. This trial thus provided evidence on both the first and the second line of research. Nevertheless, despite a 34% reduction of MACE at 12 months found in both settings, but favouring the guided selection over the standard selection of antiplatelet therapy, this was not statistically significant given the choice of a very ambitious 85% power to show a 50% reduction of the primary endpoint with guided therapy103. Collectively, the negative results of RCTs in this setting were largely driven by their limited sample sizes. A recent meta-analysis overcoming such limitations found a strategy of a guided escalation of antiplatelet therapy to be associated with a 26% reduction of composite ischaemic events and no difference in bleeding as compared to standard selection of antiplatelet therapy among patients undergoing PCI33.
Prolonging DAPT duration
The observation that patients with CAD, particularly those with prior MI, remain at risk for long-term, even beyond one year, ischaemic recurrences has laid the foundation for investigations evaluating the safety and efficacy of prolonging DAPT (Table 2). Although earlier investigations were designed based on concerns surrounding the long-term safety (i.e., ST) of earlier-generation drug-eluting stents (DES), these concerns have been largely overcome with newer-generation DES platforms25. Hence, the focus of studies evaluating prolonged antithrombotic regimens shifted from being centred on “stent” outcomes to “patient” outcomes. Indeed, a number of earlier studies, relatively small in sample size, failed to demonstrate any benefit of prolonging DAPT105106107108109. This prompted the design of the larger-scale Dual Antiplatelet Therapy (DAPT) study which enrolled 9,961 patients and found that, compared to 12-month DAPT, an additional 18 months of DAPT (with either clopidogrel 75 mg/od or prasugrel 10 mg/od) significantly reduced the primary endpoint of MACE by 29%. ST rates were also significantly reduced. However, this benefit occurred at the expense of a significant increase in moderate and severe bleeding110. Of note, the reduction of major adverse cardiac and cerebrovascular events (MACCE) with prolonged DAPT was enhanced among patients with a prior history of MI compared with those without (p-interaction=0.03)111. Subsequent meta-analyses showed an overall reduction of MI and ST with prolonged, as compared to standard, DAPT but at the cost of increased bleeding112113114115.
The available evidence from post hoc analyses of larger studies that suggested that patients with prior MI potentially benefit from prolongation of intensified antiplatelet regimens, prompted a selective investigation in patients with prior MI111116. In particular, the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial tested a P2Y12 inhibiting therapy with either ticagrelor 90 mg/bid or 60 mg/bid on top of aspirin versus a placebo. The trial included 21,162 patients with MI 1 to 3 years earlier who were at least 50 years of age, and had 1 of the following additional high-risk features: age ≥65, diabetes mellitus requiring medication, a second prior MI, multivessel CAD, or chronic renal dysfunction90. At 33 months, both ticagrelor doses significantly reduced the incidence of the primary endpoint of MACE by 15%. However, this occurred at the expense of an increase in major bleeding with both ticagrelor regimens. Results were consistent irrespective of prior PCI, which represented 83% of the study population117. However, the safety profile, both in terms of bleeding and non-bleeding side effects (i.e., dyspnoea) was more favourable with the 60 mg/bid dose, and is the reason for which this dosing regimen is recommended for long-term, beyond 1 year, secondary prevention in practice guidelines4190. The benefits of intensified antiplatelet therapy among high-risk patients without any prior history of an acute ischaemic event (prior MI or cerebrovascular event) was tested in The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS) study which was selectively conducted in patients with type 2 diabetes mellitus and stable coronary artery disease (58% of total population had undergone prior PCI)91. The study randomised 19,220 patients and showed that ticagrelor 60 mg/bid plus aspirin 75-150 mg/od, as compared with aspirin plus placebo, reduced MACE by 10%, but significantly increased major bleeding at 40 months91. The net clinical benefit was more favourable among patients with a history of PCI compared to those without118. A relevant aspect in both the PEGASUS-TIMI 54 and THEMIS studies is the consistent efficacy of low-dose ticagrelor over time, as the benefit was present irrespective of time from most recent MI and PCI118119.
Finally, the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial was the first study to assess whether adding a vascular dose regimen of rivaroxaban (2.5 mg/bid) to low-dose aspirin, a strategy known as DPI, could reduce ischaemic events in patients with stable atherosclerotic disease at risk for recurrences120. The trial randomised 27,395 patients to receive either rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg once daily), rivaroxaban (5 mg twice daily), or aspirin (100 mg once daily). Rivaroxaban 2.5 mg plus aspirin, but not rivaroxaban 5 mg twice daily, reduced MACE by 24% compared to aspirin alone120. This occurred at the expense of a significant increase in major bleeding.
Overall, these above-mentioned trials led to the most recent changes in guideline recommendations for long-term intensified antithrombotic regimens by means of DAPT or DPI among high and moderate ischaemic risk patients in the absence of high bleeding risk4041. The guidelines, however, do not provide recommendations for choosing between the 2 strategies (DAPT vs DPI) nor as to which P2Y12 inhibitor to consider if a DAPT strategy is chosen.
Strategies focused on reducing bleeding events
The introduction of stent platforms with low thrombogenicity, together with the fact that thrombotic risk is highest during the first months after PCI and decreases thereafter, while bleeding risk remains stable over time (Figure 3), has prompted investigations focused on the reduction of bleeding events55. Importantly, both major and minor bleeding have important prognostic implications. In particular, major bleeding has shown to have prognostic relevance (i.e., mortality) similar to or greater than that of a major ischaemic event; minor bleeding can result in an abrupt suspension of antiplatelet treatment, potentially resulting in higher ischaemic events35. We next discuss the major strategies tested in RCTs proposed to reduce bleeding, in hopes of providing a more favourable trade-off between bleeding and ischaemic risk over time. These strategies include shortening DAPT, the use of P2Y12 monotherapy and de-escalation of P2Y12 inhibitors (Central illustration). The vast majority of them were not designed to reduce MACE (Table 3). Thus, meta-analyses play an important role in increasing statistical power with respect to defining the impact on hard ischaemic endpoints in this setting.
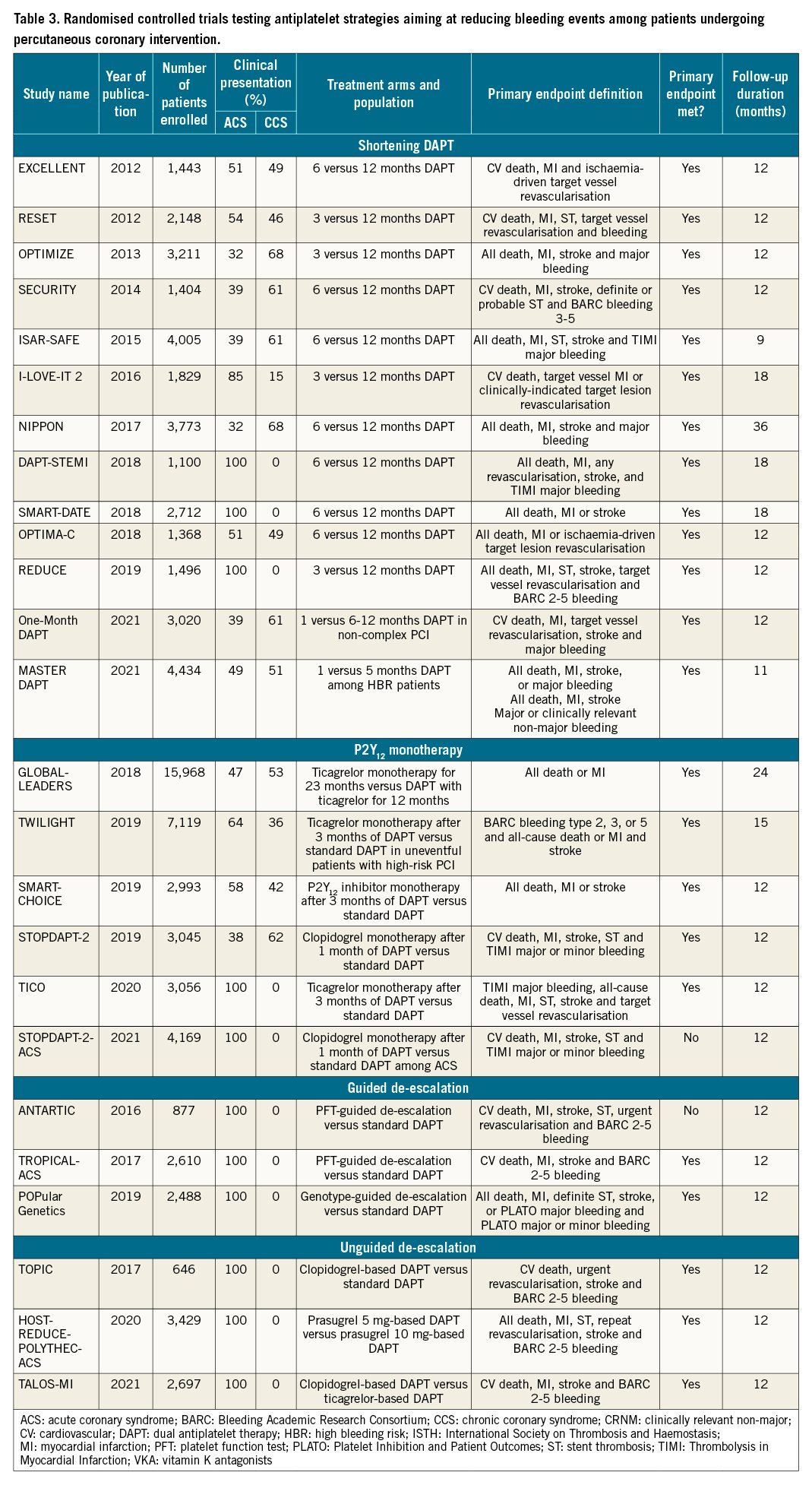
Shortening DAPT
Shortening the duration of DAPT as a strategy to reduce bleeding events has been the most broadly explored approach in 13 published RCTs (Table 3). The shortening of DAPT traditionally consists of the withdrawal of the P2Y12 inhibitor before the recommended standard period of DAPT, usually 3 or 6 months after PCI. Alternatively, shortened DAPT can also occur due to the discontinuation of aspirin while maintaining P2Y12 inhibitor monotherapy, described in greater detail in the aspirin-free approach section below. Seven trials included shortened DAPT, the discontinuation of a P2Y12 inhibitor while maintaining aspirin, and compared 6-month versus 12-month DAPT. Four trials included 3-month versus 12-month DAPT and 2 trials a 1-month versus 6- and 12-month DAPT121122123124125126127128129130131. Although a number of registries with different stent platforms conducted in high bleeding risk (HBR) patients have evaluated the safety and efficacy of short DAPT, the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation with an Abbreviated versus Standard DAPT Regimen (MASTER-DAPT) was the first RCT to selectively enrol HBR patients130132133. Overall, results of the individual studies, as well as pooled analyses of RCTs, showed the early withdrawal of P2Y12 inhibitor reduced bleeding, including major bleeding without any significant increase of thrombotic events112134. However, it has been argued that many of the studies enrolled patients at low ischaemic risk or that the studies were not powered for hard ischaemic endpoints; whether a trade-off in thrombotic complication can be ruled out in high-risk settings remains to be fully defined. The recent ARC-HBR trade-off model may represent a useful tool to balance bleeding and ischaemic risks135. In fact, patients with ACS had an increase in MI with 6 months compared with 12 months of DAPT127. Overall, these considerations are the reason for which shortened DAPT durations should be reserved for HBR patients for whom the benefits are more likely to outweigh the risks. Further studies are warranted to support shortened DAPT among high-risk populations for both bleeding and thrombotic events.
P2Y12 monotherapy
For decades, aspirin has represented the backbone therapy for all novel antithrombotic regimens34. Accordingly, the relative benefit of new drugs has remained elusive given that treatment regimens were developed with aspirin as an integral component. Hence, with the development of new therapies, often with enhanced antithrombotic efficacy, the stacking on of treatment consistently showed an increase in bleeding, offsetting any ischaemic benefit134136. These considerations in conjunction with the established gastrointestinal toxicity associated with aspirin have prompted investigations evaluating the safety and efficacy of P2Y12 monotherapy regimens137.
The first therapeutic area exploring this concept was in patients requiring OAC, such as those with atrial fibrillation, undergoing PCI50. These patients have the theoretical need for triple antithrombotic therapy (TAT, defined as DAPT+OAC), which, however, is associated with prohibitively high rates of bleeding. In light of the pivotal role of P2Y12 mediated signalling on arterial thrombotic complications in patients undergoing PCI, a strategy of dropping aspirin as an attempt to reduce (mostly gastrointestinal) bleeding, and maintaining DAPT with a P2Y12 inhibitor and an OAC, was explored. Importantly, pharmacodynamic studies have shown a synergism in antithrombotic effects with treatment with an OAC and a P2Y12 inhibitor138139140. A seminal investigation demonstrating that stented patients who were also treated with a vitamin K antagonist had a significant reduction in bleeding without any increase in thrombotic complications by discontinuing aspirin and maintaining a P2Y12 inhibitor141, prompted subsequent RCTs to test all 4 commercially available agents (dabigatran, rivaroxaban, apixaban, and edoxoban)142143144145. The results of these trials support overall the concept that after a brief period of DAPT, aspirin can be safely discontinued without any trade-off in ischaemic complications in most patients. While the optimal timing of aspirin discontinuation for patients on an OAC is a topic of controversy (between hospital discharge up to 1 week versus 1 month), it is now commonly accepted that this should not be prolonged beyond 30 days4151. It is, however, important to note that these studies are of limited scope, due to their sample size, to rule out any increase in hard ischaemic events. To this extent, several meta-analyses have been performed146. Some, but not all, showed early discontinuation of aspirin to be associated with a significant increase in risk of thrombotic complications, suggesting that aspirin be maintained for at least 30 days in high-risk subjects147148149150151. Moreover, given that the recommended antiplatelet agent to be used in these patients is clopidogrel, the potential risk deriving from aspirin withdrawal among patients non-responsive to clopidogrel, as well as the impact of potent P2Y12 inhibitors in this setting, requires further investigations3233103.
The favourable safety findings observed with aspirin discontinuation in patients undergoing PCI who also require treatment with an OAC has stimulated interest in investigations evaluating dropping aspirin even among patients not requiring OAC in the presence of effective P2Y12 inhibition. Of note, in vitro and ex vivo PD investigations have suggested that aspirin provides limited antithrombotic effects in addition to potent P2Y12 blockade, and have represented the rationale for the use of P2Y12 monotherapy approaches138152153154155156. GLOBAL-LEADERS was the first study to clinically test this strategy, comparing 1-month DAPT followed by 23 months of ticagrelor 90 mg/bid monotherapy versus standard DAPT for 12 months followed by aspirin among 15,968 patients undergoing PCI. Although at a follow-up of 24 months, there was no significant difference in the primary ischaemic endpoint of all-cause death and MI between arms, there were no safety (i.e., bleeding) concerns that emerged from this study157. GLOBAL-LEADERS was however followed by a number of RCTs that consistently showed that discontinuing aspirin after 1-3 months and maintaining P2Y12 inhibitor monotherapy markedly reduced bleeding without any increase in thrombotic events144158159160.
The Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial was a double-blind, placebo-controlled design trial including 7,119 patients with at least 1 clinical and 1 angiographic feature associated with a high risk of ischaemic or bleeding events, who were randomised after 3 months of uneventful DAPT with ticagrelor plus aspirin to take ticagrelor and receive aspirin or a placebo for 1 year144. There was a significant 44% reduction of the primary endpoint of Bleeding Academic Research Consortium (BARC) 2-5 bleeding, favouring ticagrelor monotherapy and no difference in MACE between groups144. Importantly, MACE results were consistent among subgroups of high-risk patients such as those with diabetes mellitus and ACS and those undergoing complex PCI161162163. The use of prasugrel monotherapy in the setting of P2Y12 monotherapy strategies is limited, and thus far has only been tested in a pilot study including 201 patients with CCS undergoing low-risk PCI164. Overall, pooled analyses showed a very short DAPT followed by a P2Y12 inhibitor monotherapy reduced bleeding, including major bleeding, without a trade-off in ischaemic events134136.
It is important to note that while some of the above-mentioned studies were conducted with clopidogrel, these were in low-risk patients and thus question whether the lack of increase of thrombotic complications would be preserved in ACS patients. The ShorT and OPtimal Duration of Dual AntiPlatelet Therapy-2 ACS study (STOPDAPT-2 ACS), including a total of 4,169 patients, failed to meet non-inferiority for the primary endpoint of net adverse clinical events (NACE) with clopidogrel monotherapy after a 1-month DAPT versus the standard 12-month DAPT, mainly driven by an increase of MI (H W. STOPDAPT-2 ACS: 1-month dual antiplatelet therapy followed by clopidogrel monotherapy in acute coronary syndrome. ESC Congress 2021). These findings call for caution on dropping aspirin too early among high-risk patients, such as those with ACS, when a non-potent P2Y12 inhibitor is used as monotherapy.
De-escalation of P2Y12 inhibitors
De-escalation of P2Y12 inhibiting therapy consists in switching from more potent (i.e., prasugrel or ticagrelor) to less potent (i.e., clopidogrel) agents, and aims at reducing bleeding without any trade-off in ischaemic events165. Accordingly, the strategy of de-escalation typically applies to the setting of ACS, in which more potent P2Y12 inhibitors are recommended as the standard of care. De-escalation can be guided or un-guided (Table 3). A guided approach implies the use of platelet function or genetic tests that rule out clopidogrel-HPR or the presence of CYP2C19 LoF alleles which are known to be associated with an increased risk of thrombotic complications post-PCI. An unguided approach consists in de-escalation without the aid of platelet function or genetic testing. A guided approach allows for de-escalation early after PCI. On the contrary, an unguided de-escalation early after PCI has been associated with an increase in thrombotic complications166. Accordingly, waiting for the highest risk period of thrombotic complications post-PCI to elapse (e.g., 1 month) prior to de-escalation, represents a safer time frame for considering unguided de-escalation (Figure 3).
Guided de-escalation
A guided de-escalation approach has been tested in 3 RCTs, all in patients with ACS, using either platelet function testing (n=2) or genetic testing (n=1) (Table 3)167168169. Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment For Acute Coronary Syndromes Trial (TROPICAL-ACS) is the largest of the studies using platelet function testing, the results of which had an impact on guideline recommendations167. In particular, among 2,610 ACS patients undergoing PCI, guided de-escalation was non-inferior for the primary composite endpoint of NACE as compared to standard of care, with a trend towards reduced bleeding at 12 months compared to the standard group167.
The Cost-effectiveness of Genotype Guided Treatment With Antiplatelet Drugs in ST-segment elevation MI (STEMI) Patients: Optimization of Treatment (POPular Genetics) used genetic testing to guide de-escalation and was conducted in 2,488 STEMI patients undergoing primary PCI who were randomised to either a genotype-guided de-escalation or a standard of care selection (mostly ticagrelor, 91%) of oral P2Y12 inhibitors169. The genotype-guided strategy was found to be non-inferior for NACE and superior in terms of PLATO major or minor bleeding, as compared to standard of care at 12-month follow-up169. The observation that platelet function testing-guided de-escalation was not associated with significantly reduced bleeding as compared to standard therapy can be explained by the fact that some patients who de-escalate to clopidogrel are found to have HPR and need to escalate to more potent P2Y12 inhibition, which enhances the risk of bleeding. It is also important to note that 7-14 days of maintenance treatment with clopidogrel is needed after de-escalation before assessing platelet responsiveness, a time frame during which patients who have HPR are at increased risk of thrombotic events66166. Overall, it may be argued that the individual RCTs were of limited power to assess with granularity the ischaemic and bleeding endpoints. A recent meta-analysis overcoming such limitations found that a strategy of guided de-escalation of antiplatelet therapy is associated with a 19% reduction of any bleeding, driven by a reduction of minor bleeding, without any trade-off in efficacy, as compared to standard selection of antiplatelet therapy among patients undergoing PCI33. Moreover, there were no differences between the use of genetic or platelet tests33. Finally, a network meta-analysis focusing on ACS has shown that, compared with routine selection of potent P2Y12 inhibiting therapy (prasugrel or ticagrelor), a guided selection of P2Y12 inhibiting therapy is associated with the most favourable balance between safety and efficacy170.
Unguided de-escalation
The Timing of Platelet Inhibition After Acute Coronary Syndrome (TOPIC) was a single-centre RCT with 646 patients comparing standard versus unguided de-escalation from a potent platelet inhibitor to clopidogrel 1 month after ACS171. There was a significant reduction of both the primary endpoint of NACE and bleeding171. However, it should be noted that MI was not included in the primary composite endpoint (CV death, urgent revascularisation, stroke and BARC bleeding 2-5) and this population was mostly composed of patients undergoing non-complex PCI. Prasugrel-based de-escalation of DAPT after PCI in patients with ACS (HOST-REDUCE-POLYTECH-ACS), compared standard versus unguided de-escalation of antiplatelet therapy by reducing prasugrel dosage (from 10 mg to 5 mg daily) versus standard prasugrel dosing (10 mg daily) 1 month after ACS among East Asian patients undergoing PCI (n=2,338) and showed that de-escalation was non-inferior to standard therapy for the primary endpoint of MACE172. Finally, the TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-MI), comparing de-escalation from ticagrelor- to clopidogrel 1 month after PCI versus standard ticagrelor-based DAPT among 2,697 East Asian ACS patients, showed that the primary endpoint of NACE, as well of BARC bleeding 2-5, was significantly reduced in the de-escalation arm173. It is important to note that in these trials the de-escalation of P2Y12 inhibitors was performed 1 month after PCI - the period in which the risk of ischaemic events is highest - while among trials using a guided de-escalation this was performed earlier (0-14 days) after PCI. A recent meta-analysis has shown that both guided and unguided de-escalation reduce bleeding without any trade-off in ischaemic events174.
Future perspectives
Future directions in the field of antiplatelet therapy among PCI patients include the development of new antiplatelet agents, the implementation of new antiplatelet strategies with available agents and the conduct of further studies in support of current antiplatelet strategies (Table 4). Several new antiplatelet agents intended for use among patients undergoing PCI are under clinical development. Agents include selatogrel (a reversible non-thienopyridine P2Y12 receptor antagonist administered subcutaneously), RUC-4 (a GPIIb/IIIa inhibitor with a novel mechanism of action for intramuscular administration) and revacept (an inhibitor of GPVI, the major platelet collagen receptor, administered intravenously) (Figure 1)175176177. A detailed description of these agents intended for acute use goes beyond the scope of this manuscript.
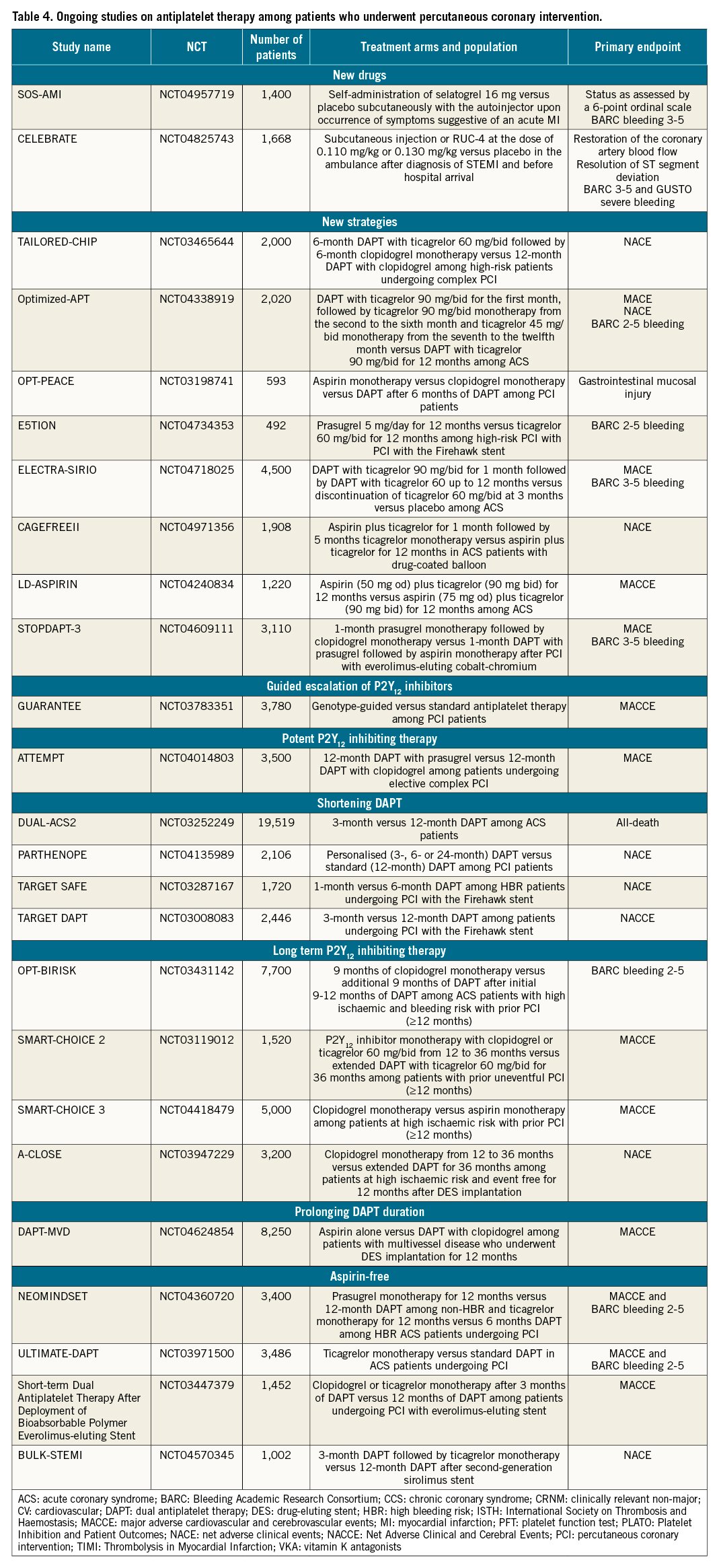
Although prolonging intensified antithrombotic therapy by adding a P2Y12 inhibitor (i.e., DAPT) or rivaroxaban 2.5 mg bid (i.e., DPI) to aspirin reduces the risk of ischaemic recurrences in high-risk patients with CCS compared to aspirin alone, the concerns surrounding the increased risk of bleeding has stimulated research on identified treatments with a better trade-off between ischaemic and bleeding events. Compared with aspirin, rivaroxaban 5 mg/bid did not significantly reduce ischaemic events in patients with stable vascular disease, underscoring the importance of antiplatelet therapy for patients with CAD120. Although the strategy of P2Y12 inhibitor monotherapy and discontinuing aspirin after a brief period of DAPT has shown to reduce bleeding complications without any trade-off in ischaemic events, the available evidence is limited to up to 12 months of therapy after which most patients resumed aspirin monotherapy. Whether P2Y12 inhibitor monotherapy is superior to aspirin monotherapy in patients who have completed required DAPT after DES implantation was tested in the Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis- EXtended Antiplatelet Monotherapy (HOST-EXAM) trial178. The trial randomised 5,530 patients from South Korea without clinical events for 6-18 months after PCI to either aspirin 100 mg/od or clopidogrel 75 mg/od monotherapy. At 24 months, the primary endpoint of MACE as well as BARC bleeding type 3-5 were reduced with clopidogrel compared to aspirin178. These results are consistent with those from the CAPRIE trial, published 20 years earlier, which enrolled 19,185 patients with atherosclerotic disease, showing clopidogrel to be associated with a reduction, albeit of marginal statistical significance, of ischaemic events with better gastrointestinal tolerability. It is important to note that in CAPRIE, a 325 mg dose regimen of aspirin was used179.
Ultimately, new formulations of aspirin designed to reduce gastrointestinal toxicity while maintaining adequate absorption and antiplatelet effects are currently being designed. Among these, a liquid formulation of a novel pharmaceutical lipid-aspirin complex (PL-ASA) has been approved. In particular, PL-ASA has pharmacokinetic and pharmacodynamic profiles similar to immediate-release aspirin and provides more reliable drug absorption than enteric-coated aspirin formulations known to be more delayed and erratic180181. Moreover, its formulation and release properties mitigate disruption of the protective phospholipid bilayer of the gastrointestinal mucosa, resulting in less acute gastric injury (erosions and/or ulcers)182. How PL-ASA compares with standard enteric-coated aspirin and its long-term gastrointestinal safety remain unknown.
Conclusions
Antiplatelet therapy represents the cornerstone of treatment for the prevention of local and systemic ischaemic complications in patients with CAD undergoing PCI. Over the past decades different antiplatelet regimens using aspirin and P2Y12 inhibitors have been developed and implemented in clinical practice. A better understanding of the ischaemic and bleeding risk profile, as well as individual responsiveness to antiplatelet agents, has been instrumental in defining the optimal regimen for the individual patient. In particular, the intensity and the duration of aspirin and P2Y12 inhibiting therapy should be adjusted to reduce the risk of ischaemic complications while minimising the risk of bleeding. Strategies developed to mitigate the risk of bleeding include shortening DAPT duration, P2Y12 inhibitor monotherapy and de-escalation. In the absence of high bleeding risk, patients at increased ischaemic risk may consider prolonging intensified antithrombotic therapy either by means of DAPT or DPI. The use of platelet function and genetic testing can indeed be of aid in the selection of P2Y12 inhibitor therapy. An integrated approach, including scores/definitions to define ischaemic and bleeding risk, procedural characteristics, and tools to help assess drug response, represents the most promising approach for a personalised selection of antiplatelet agents among patients undergoing PCI.
Acknowledgments
BioRender platform and templates were used for creating figures.
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. J.P Collet has received financial support/sponsorship for research support, consultation or speaker fees from the following companies: AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Lead-Up, Medtronic, WebMD and Sanofi Aventis. M.L. O'Donoghue declares that she has received grants via Brigham and Women’s Hospital from Amgen, Novartis, AstraZeneca, Janssen, Intarcia, Merck, and Pfizer and honararia from Novartis, AstraZeneca, Amgen, and Janssen. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

