
Abstract
Aims: This study aimed to investigate the anatomical attributes determining myocardial territory of diagonal branches and to develop prediction models for clinically relevant branches using myocardial perfusion imaging (MPI) and coronary CT angiography (CCTA).
Methods and results: The amount of ischaemia and subtended myocardial mass of diagonal branches was quantified using MPI by percent ischaemic myocardium (%ischaemia) and CCTA by percent fractional myocardial mass (%FMM), respectively. In 49 patients with isolated diagonal branch disease, the mean %ischaemia by MPI was 6.8±4.0%, whereas in patients with total occlusion or severe disease of all diagonal branches it was 8.4±3.3%. %ischaemia was different according to the presence of non-diseased diagonal branches and dominant left circumflex artery (LCx). In the CCTA cohort (306 patients, 564 diagonal branches), mean %FMM was 5.9±4.4% and 86 branches (15.2%) had %FMM ≥10%. %FMM was different according to LCx dominance, number of branches, vessel size, and relative dominance between two diagonal branches. The diagnostic accuracy of prediction models for %FMM ≥10% based on logistic regression and decision tree was 0.92 (95% CI: 0.85-0.96) and 0.91 (95% CI: 0.84-0.96), respectively. There was no difference in the diagnostic performance of models with and without size criterion.
Conclusions: LCx dominance, number of branches, vessel size, and dominance among diagonal branches determined the myocardial territory of diagonal branches. Clinical application of prediction models based on these anatomical attributes can help to determine the clinically relevant diagonal branches in the cardiac catheterisation laboratory. Clinical trial registration: NCT03935542
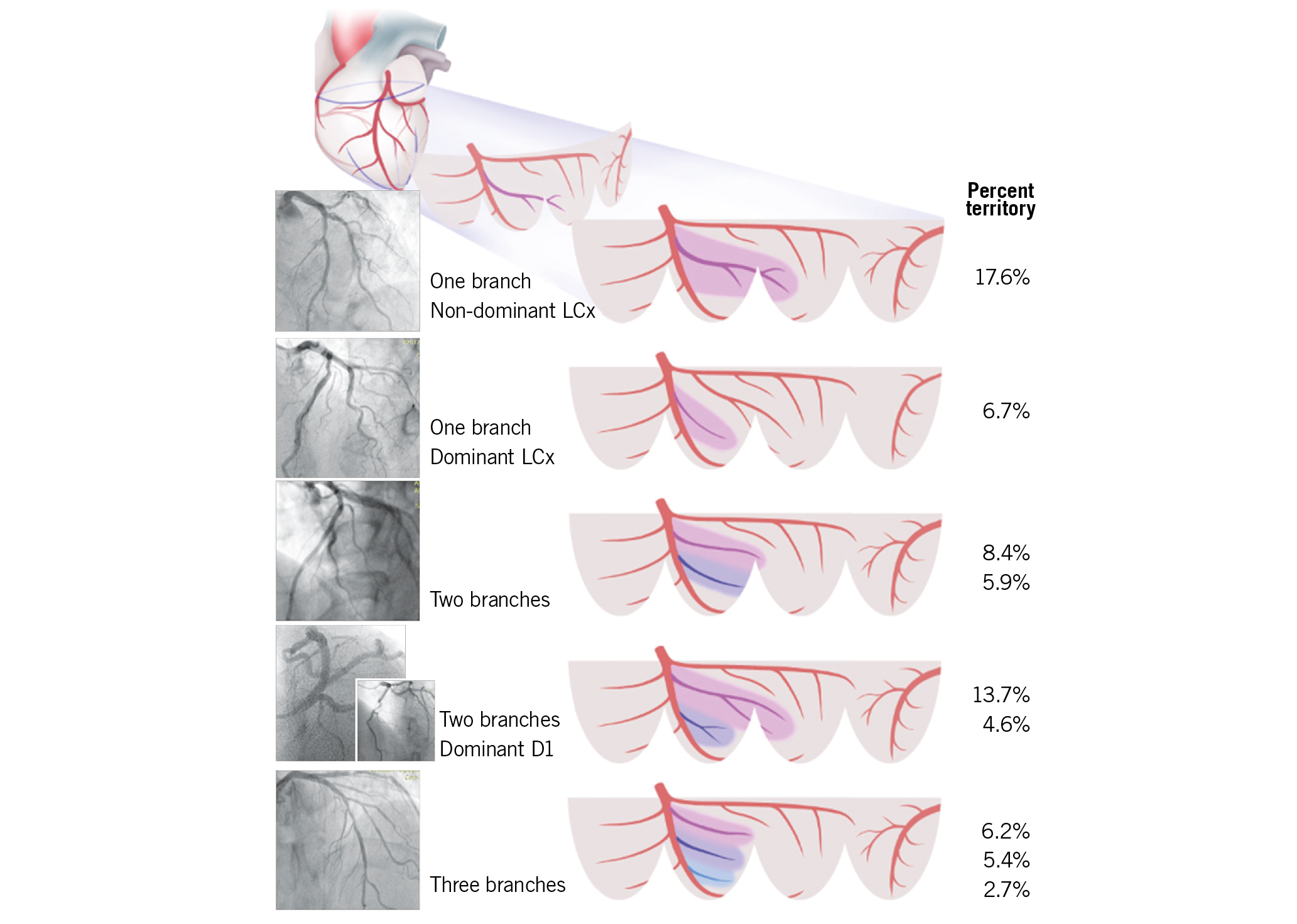
Visual summary. Myocardial territory of a diagonal branch is determined by LCx dominance, number of branches and dominance among branches.
Introduction
Bifurcation lesions are one of the most challenging lesion subsets in the field of percutaneous coronary intervention (PCI). Despite recent advances in PCI techniques and stent technology, most randomised studies have failed to prove the superiority of a routine two-stent strategy compared with a provisional side branch intervention strategy1.
A certain amount of ischaemic burden is required to achieve the benefit of revascularisation over medical treatment2,3. Compared with main branches, side branches are smaller, more variable in anatomy, supplying less myocardium and are less clinically relevant4,5. Therefore, it is important to assess the myocardial mass at risk of side branches to determine the appropriate treatment strategy for bifurcation lesions. However, the method to identify the clinically relevant side branches associated with benefit from revascularisation in the cardiac catheterisation laboratory is not well defined.
We performed this study to investigate anatomical attributes that determine ischaemic burden and myocardial territory of diagonal branches, and to develop a prediction model for clinically relevant diagonal branches using myocardial perfusion imaging (MPI) and coronary computed tomography angiography (CCTA).
Methods
STUDY POPULATION
Two different modalities, MPI and CCTA, were used to quantify the amount of ischaemia and myocardial territory of a diagonal branch, respectively. For the MPI cohort, patients with severe jailed diagonal branch disease with available MPI within three months of invasive coronary angiography were selected from the Seoul National University Hospital Cardiac Catheterization and MPI database. Severe stenosis of a diagonal branch was defined as ≥90% angiographic stenosis or fractional flow reserve <0.75. Patients with >50% stenosis in the left anterior descending coronary artery (LAD) or left circumflex artery (LCx), or regional wall motion abnormality at the LAD territory were excluded (Figure 1). For the CCTA cohort, patients from a previous multicentre prospective registry were retrospectively reviewed for a post hoc analysis4. Patients with an available fractional myocardial mass (FMM) value of diagonal branches were included and those with diffuse diagonal branch disease were excluded from the analysis (Figure 1). The study protocol was approved by the institutional review board of each centre.
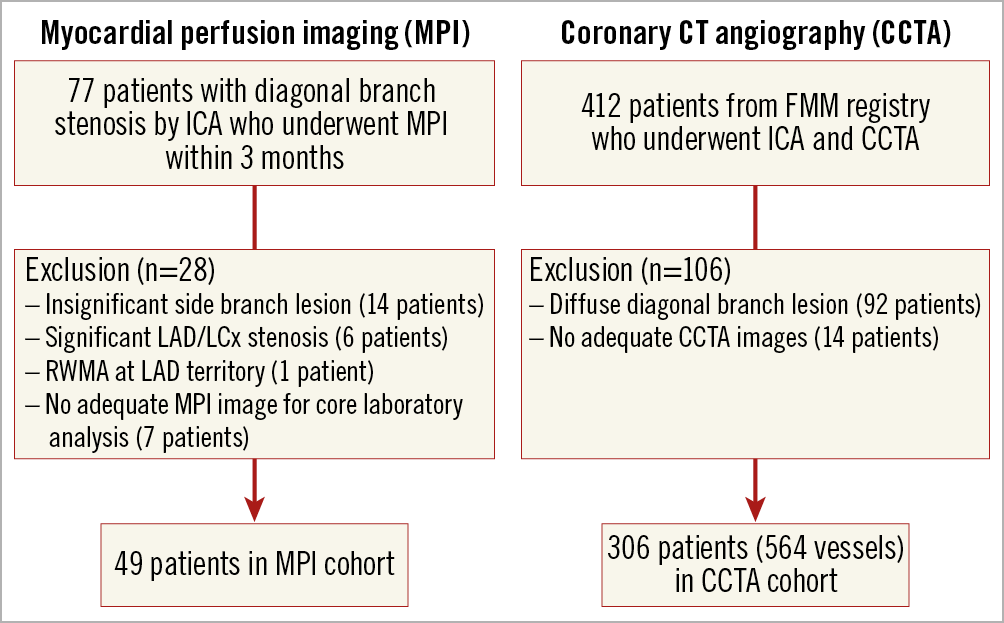
Figure 1. Study flow. CCTA: coronary CT angiography; CT: computed tomography; FMM: fractional myocardial mass; ICA: invasive coronary angiography; LAD: left anterior descending coronary artery; LCx: left circumflex artery; MPI: myocardial perfusion imaging; RWMA: regional wall motion abnormality
MPI PROTOCOLS AND PARAMETERS
For the myocardial perfusion positron emission tomography (PET)/CT image acquisition, one-day stress and rest protocol was conducted using a Biograph™ 40 TruePoint PET/CT (Siemens Medical Solutions, Knoxville, TN, USA). Rest images were acquired in 3D list-mode and stress images were acquired with adenosine infusion. Both images were acquired after administering 370 MBq of 13N-NH3. For the myocardial perfusion single-photon emission CT (SPECT) image acquisition, the dual isotope protocol was conducted using 99mTc–sestamethoxyisobutylisonitrile and 201Tl for stress and rest images, respectively.
All MPI images were screened and assessed by an independent MPI core laboratory at Seoul National University Hospital. Perfusion parameters were evaluated automatically using Quantitative Perfusion SPECT (QPS; Cedars-Sinai Medical Center, Los Angeles, CA, USA). Myocardium was divided into 20 segments, and summed rest score (SRS), summed stress score (SSS), and summed difference score (SDS) were scored in each segment according to a five-grade system (0-4) for the assessment of perfusion status. SSS and SDS of diagonal segments were converted to percent of myocardial ischaemia (%ischaemia) of diagonal territory by dividing summed scores by 80 and multiplying by 100 (Figure 2, Supplementary Figure 1),2. PET flow, relative flow reserve and coronary flow reserve were assessed as previously described6,7.
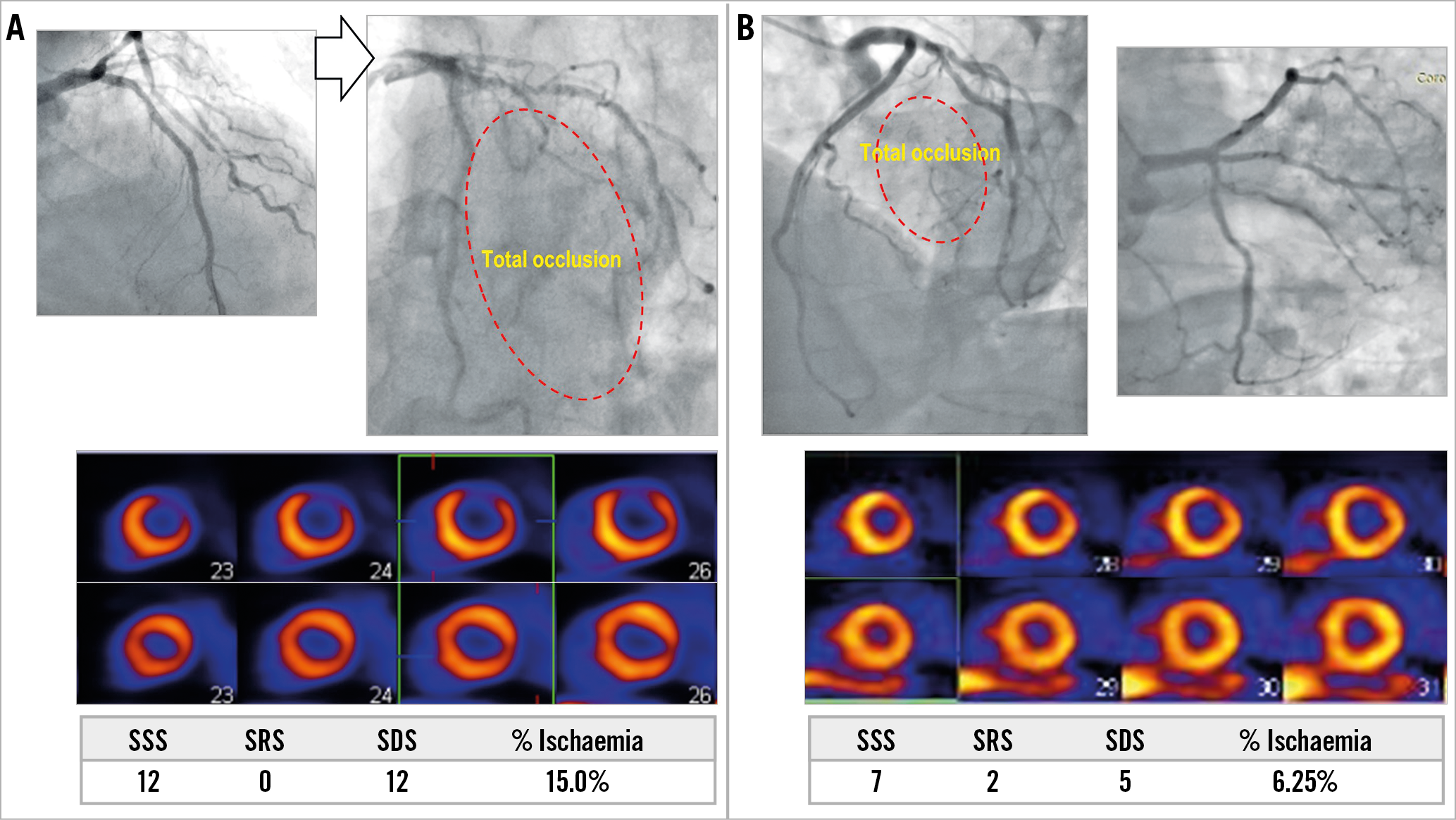
Figure 2. Myocardial ischaemic territory caused by diagonal branch occlusion. A) Occlusion of a solitary diagonal branch caused %ischaemia of 15%. B) However, %ischaemia due to first diagonal branch occlusion was 6.25% in a case of residual diagonal branch and large ramus intermedius. SDS: summed difference score; SRS: summed rest score; SSS: summed stress score
CORONARY CT ANGIOGRAPHY AND FRACTIONAL MYOCARDIAL MASS
CCTA was performed with at least 64-slice CT scanners (Aquilion ONE™ or Aquilion 64 [Toshiba Medical Systems Corporation, Tochigi, Japan]; SOMATOM® Definition [Siemens Medical Solutions]; LightSpeed VCT [GE Healthcare, Chicago, IL, USA]). Image data were reconstructed to a 3D coronary arterial tree model by a dedicated system (iNtuition; TeraRecon, Durham, NC, USA). CCTA analysis and FMM calculation were performed in an independent CCTA core laboratory at Samsung Medical Center. FMM of each diagonal branch was converted to percent FMM (%FMM) of diagonal branch by dividing each FMM by left ventricular myocardial mass. CCTA data were used to train and validate the prediction models for %FMM ≥10%.
DIAGONAL BRANCH SPECIFIC ANGIOGRAPHIC ATTRIBUTES
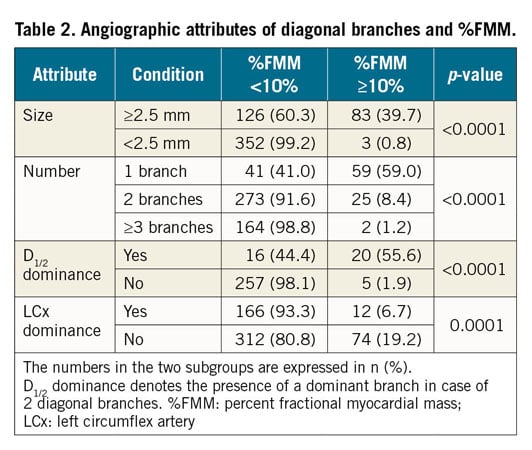
Coronary angiography was performed using standard techniques. Quantitative coronary angiography (QCA) was performed in an independent core laboratory with a validated software (CAAS 5.9; Pie Medical Imaging, Maastricht, the Netherlands). Angiographic attributes for diagonal branches >1.5 mm in diameter were visually defined as follows. Size was a binary attribute of vessel diameter ≥2.5 mm or <2.5 mm. Number was counted as one, two, and three or more diagonal branches. Dominance in patients with two diagonal branches (D1/2 dominance) was a binary attribute for one of two diagonal branches whose diameter was more than two times greater than its smaller counterpart. As part of the diagonal branch territory is shared with obtuse marginal branches, the total diagonal branch territory is influenced by the relative dominance of the LCx8. Therefore, the component of LCx dominance was defined as a left-dominant system or the presence of an obtuse marginal branch originating within the proximal 1/3 of the LCx and crossing the LAD at a right anterior oblique caudal view. The intermediate branch was classified as one of the obtuse marginal branches. Clinically relevant diagonal branches were defined with %FMM ≥10% in this study. Two independent observers assessed the angiographic attributes in a blinded fashion. In cases with disagreement between observers, consensus was reached through discussion between the two observers.
STATISTICAL ANALYSIS
Continuous variables are presented as means with standard deviations. Categorical variables are presented as frequencies and percentages. Baseline characteristics, %ischaemia and %FMM of the total diagonal territory are presented per patient. Per-vessel analysis was performed for the prediction of %FMM of an individual diagonal branch. The Student’s t-test and chi-square test were used to compare continuous variables and categorical variables between groups, respectively.
To train and validate models to predict %FMM ≥10%, the entire CCTA data set was split into training and validation sets (4:1). A binomial logistic regression model with tenfold cross-validation was used to train the prediction model. To build a decision tree model, the training and validation sets were used for recursive partitioning with tenfold cross-validation. Information gain was used to select attributes for higher nodes.
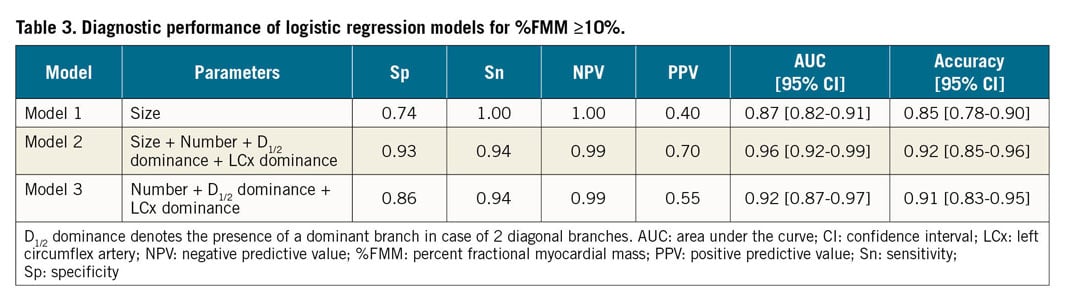
The performance was compared in three different models: model 1 – size criterion only; model 2 – size, number, LCx dominance and D1/2 dominance; model 3 – number, D1/2 dominance and LCx dominance. The discrimination ability of a model was analysed by comparing the area under the receiver operating characteristic (ROC) curve with a bootstrap method. The diagnostic performance of each model was assessed with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. All probability values were two-sided, with p<0.05 considered statistically significant. All statistical analyses were conducted using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
BASELINE CHARACTERISTICS
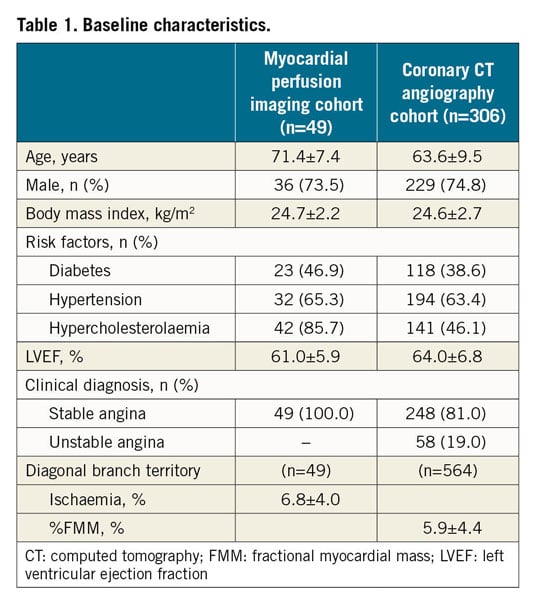
In the MPI cohort, 49 patients were included in the analysis after exclusion of 28 patients. Among 412 patients in the CCTA cohort, those with poor image quality and diffuse diagonal disease were excluded, resulting in 306 patients (564 diagonal branches) being included in this study (Figure 1). Baseline characteristics of patients in the MPI and CCTA cohorts are presented in Table 1.
ISCHAEMIC TERRITORY OF DIAGONAL BRANCHES
Among 49 patients assessed by MPI, 12 patients (24.5%) had %ischaemia ≥10%. In 29 patients with severe disease in all diagonal branches, %ischaemia was 8.4±3.3%, with 10 patients having %ischaemia ≥10%. LCx dominance was present in 28 patients and their %ischaemia was smaller than those without (5.6±3.8% vs 8.4±3.7%, p=0.012). Patients with non-diseased diagonal branches had lower %ischaemia than those without LCx dominance (4.6±3.8% vs 8.4±3.3%, p=0.001). In 32 patients who underwent 13NH3-PET, mean hyperaemic blood flow, coronary flow reserve, and relative flow ratio of ischaemic diagonal territory were 1.66±0.35 g/mL/min, 1.83±0.57, and 0.66±0.10, respectively.
MYOCARDIAL TERRITORY ACCORDING TO ANATOMICAL ATTRIBUTES
Among 564 diagonal branches, 86 branches (15.2%) showed %FMM ≥10%. In patients with LCx dominance, the %FMM of total diagonal territory was smaller than in those without LCx dominance (8.9±3.1% vs 12.0±4.5%, p<0.0001) (Figure 3). For the total number of diagonal branches, a solitary diagonal branch had the highest %FMM (11.7%±5.1%) (Figure 3). Among the patients with three or more diagonal branches, only 1.2% showed %FMM ≥10% (Table 2). When there were two diagonal branches, the dominant branch had a greater chance of having %FMM ≥10% (Figure 3, Table 2).
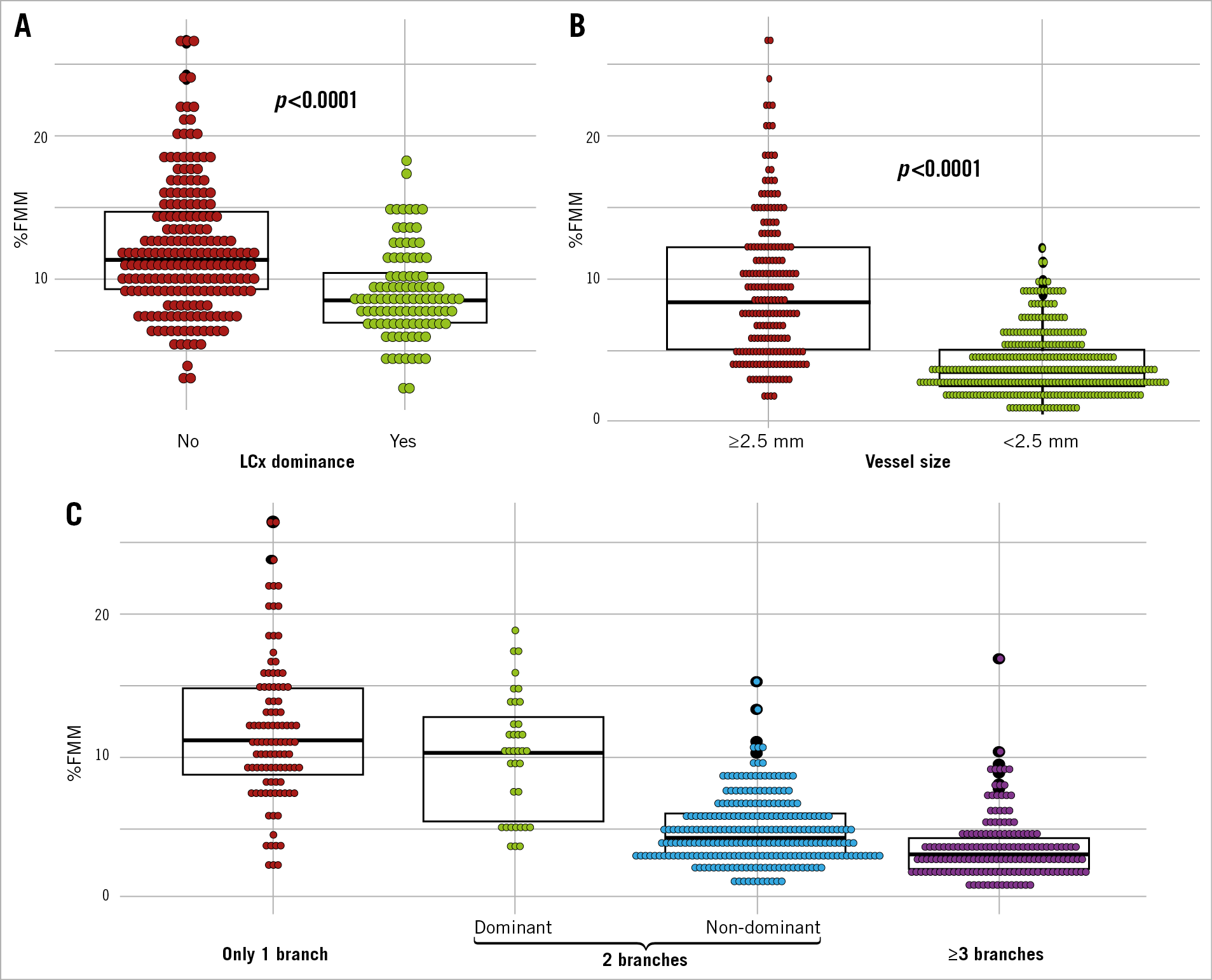
Figure 3. Diagonal branch territory according to dominance of left circumflex artery, vessel size and number of branches. Stacked dot plots represent the distribution of fractional myocardial mass (%FMM) of entire diagonal branches (A) and an individual diagonal branch (B). Branches are categorised into single diagonal branch, dominant and non-dominant branches of the two diagonal branches, and the branch of more than three diagonal branches (C). The overlaid box plot represents the median, first and third quartile of %FMM of the diagonal branch of each group.
DIAGNOSTIC PERFORMANCE OF PREDICTION MODELS BASED ON ANATOMICAL ATTRIBUTES
The models based on logistic regression were built to predict %FMM ≥10% with anatomical attributes of diagonal branches (Table 3). The model with the criterion of size ≥2.5 mm only had excellent negative predictive value. However, its positive predictive value was low (0.40). When the number of branches and dominance (LCx dominance and D1/2 dominance) were added (model 2), specificity and positive predictive value were improved with higher area under the curve (AUC) (0.96 vs 0.87, p<0.001). The diagnostic performance of models with and without size criterion (model 2 vs model 3) was not different (AUC 0.96 vs. 0.92, p=0.06) (Table 3). In addition, the diagnostic performance was similar, whether the vessel size was assessed by QCA or by visual estimation (Supplementary Figure 2, Supplementary Table 1).
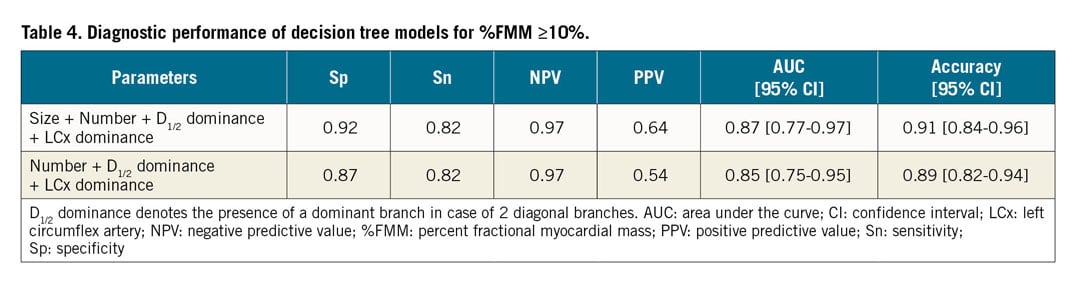
To facilitate instant decision making at the time of evaluation, a decision tree model was developed to predict %FMM ≥10% (Figure 4). Its diagnostic performance is described in Table 4.
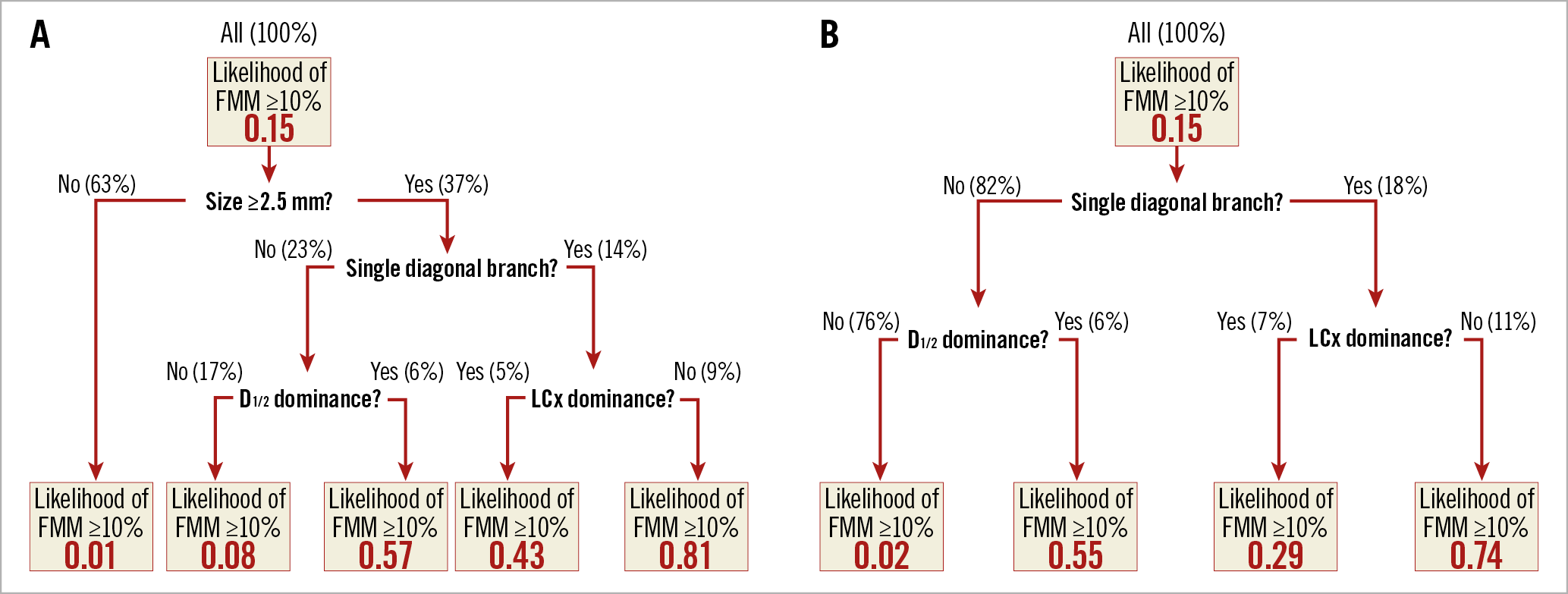
Figure 4. Decision trees to predict the likelihood of %FMM of a diagonal branch ≥10%. Each tree represents a decision algorithm for a case with (A) or without (B) information on the size of a diagonal branch. The top boxes are the root nodes containing the entire branches of the training set (100%). They are partitioned into one of two nodes by specific attribute in the next layer. FMM: fractional myocardial mass
Discussion
This study was performed to assess the ischaemic burden and myocardial territory of diagonal branches using MPI and CCTA, and to develop models with anatomical attributes that can define the clinical relevance of diagonal branches. The major findings of this study were as follows. 1) The ischaemic territory of diagonal branches was variable and most diagonal branches did not cause %ischaemia >10%. The presence of non-diseased diagonal branches and LCx dominance were the anatomical attributes that determined the ischaemic territory. 2) The anatomical attributes of myocardial territory assessed by CCTA were vessel size, number of branches and relative dominance. 3) Combining these anatomical attributes improved the diagnostic performance of a prediction model for clinically relevant diagonal branches compared with the model of vessel size only. 4) Vessel size information did not significantly improve the diagnostic performance of the prediction model. These results suggest that these concepts and prediction models could help clinicians to select the clinically relevant diagonal branches and determine the appropriate treatment strategy for LAD-diagonal bifurcation lesions.
CLINICAL RELEVANCE OF SIDE BRANCHES
The benefit of revascularisation is dependent not only on the presence of ischaemia but also on its extent. Revascularisation for lesions that can cause ischaemic burden >10% of the left ventricle is regarded as having prognostic benefit compared with medical treatment2,3. Compared with the main branches, side branches supply less myocardium and have less clinical and prognostic relevance5,8,9,10. This can be one of the main reasons why routine side branch stent implantation could not show benefit over the provisional strategy1, and FFR-guided revascularisation for jailed side branches did not improve outcomes compared with angiography-guided intervention11,12. In our study, %ischaemia in patients with severe disease in all diagonal branches was only 8.4±3.3%, and 65.5% of them did not have %ischaemia ≥10%. Therefore, assessment of the ischaemic territory of a side branch should be one of the key steps in the process of selecting the appropriate treatment strategy for coronary bifurcation lesions.
ANATOMICAL DETERMINANTS OF MYOCARDIAL TERRITORY
Myocardial territory of a secondary branch is determined by the entire territory of a primary vessel and the partition of that specific branch among the secondary branches. The LAD generally supplies 40-45% of the left ventricle, and less than half of that territory is supplied by diagonal branches4,13,14. Diagonal branches mainly supply the anterior wall and part of the anterolateral wall, which is also supplied by branches from the LCx. Therefore, anatomical variations influence the myocardial territory of diagonal branches, and the number and territory of diagonal branches was reported to be smaller in cases with a dominant LCx or large obtuse marginal branch8. In this study, we defined LCx dominance as a left-dominant system or presence of an obtuse marginal branch originating within the proximal 1/3 of the LCx and crossing the LAD in a right anterior oblique caudal view. It was used as one of the anatomical attributes that can influence total diagonal branch territory in anterior and anterolateral walls. Our study results showed that patients with LCx dominance had smaller %ischaemia (5.6±3.8% vs 8.4±3.7%, p=0.012) and %FMM (8.9±3.1% vs 12.0±4.5%, p<0.0001) than those without LCx dominance. In addition, the presence of LCx dominance was the most important attribute in predicting the likelihood of %FMM ≥10% in patients with a single diagonal branch.
It is natural that each branch’s territory decreases according to the number of branches. When the association between the total number of diagonal branches and %FMM of an individual diagonal branch was assessed, the %FMM of a solitary diagonal branch was the highest (11.7±5.1%) followed by the two diagonal branches (5.3±3.1%). Among 166 branches of patients with ≥3 diagonal branches, only two branches showed %FMM ≥10%. In cases of two branches, relative dominance (D1/2 dominance) can determine the territory of that branch. In our study, the dominant branch had a greater %FMM (10.1±4.3% vs 4.6±2.3%) than the non-dominant branch.
CLINICALLY APPLICABLE PREDICTION MODELS
Recent studies showed that CCTA can be used to estimate the relative and absolute amount of myocardial territory of the specific vessel4,13,14,15. However, these methodologies require ad hoc CCTA analysis and cannot be applied to patients without CCTA. In addition, this estimation can be inaccurate for branches with diffuse and severe disease which are potential targets for revascularisation. In previous studies and during daily practice, vessel size has been the most commonly used anatomical attribute in order to estimate the myocardial territory or clinical importance of a side branch16,17. However, neither vessel size nor disease severity based on angiography had an influence on the outcomes according to the treatment strategy for bifurcation lesions17,18. In addition, the limitations of QCA for bifurcation lesions19 are well known. Therefore, a simple and practical approach that can incorporate anatomical attributes for the myocardial territory is needed. In our study, the diagnostic performance of predictive models was not different between the models with and without vessel size, and vessel diameter by QCA did not improve the diagnostic performance compared with visual assessment. When the dominance of the LCx and between two diagonals and the number of diagonal branches were used, the accuracy of a logistic regression model and a decision tree was 0.91 and 0.89, respectively. Therefore, these models can be used easily in daily practice regardless of the availability of vessel size data.
Limitations
This study has several limitations. First, the number of cases in the MPI arm was small. In addition, the burden of inducible ischaemia assessed by MPI depends not only on vessel territory, but also on other factors such as stenosis severity, presence of non-viable myocardium, or inadequate hyperaemia. Second, the clinical importance of side branches becomes greater in patients with left ventricular dysfunction or multivessel disease. Third, ischaemic or infarction territory is smaller than myocardial territory at risk and %FMM may be larger than the ischaemic territory of that vessel. In addition, it should be recognised that %FMM by CCTA is not an exact measurement but an estimation for the territory supplied by a diagonal branch. Fourth, this study could not include all variables that can be used to define myocardial territory, such as vessel length, lumen area and blood flow. In this study, we selected simple angiographic attributes that can be used in daily practice from the results of previous studies and scientific reasoning. Finally, the prediction model was trained and validated in two different subgroups in the same study population. External validation could have increased the value of this model.
Conclusions
LCx dominance, number of branches, vessel size, and dominance among diagonal branches determine the myocardial territory of diagonal branches. Clinical application of prediction models based on these anatomical attributes can help to determine the clinically relevant diagonal branches in the cardiac catheterisation laboratory.
|
Impact on daily practice Defining a clinically relevant side branch should be the first step of bifurcation lesion intervention as most side branches cannot cause myocardial ischaemia ≥10%. Myocardial territory of diagonal branches can be estimated by LCx dominance, number of branches, relative dominance, and vessel size. Clinical application of prediction models based on these anatomical attributes can help to determine the clinically relevant diagonal branches in the cardiac catheterisation laboratory. |
Appendix. Study collaborators
Jinlong Zhang, MD; Jeehoon Kang, MD; Jung-Kyu Han, MD, PhD; Han-Mo Yang, MD, PhD; Kyung Woo Park, MD, PhD; Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
Funding
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1277).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

