
Abstract
Aims: Durable fluoropolymer-coated everolimus-eluting stents (FP-EES) have shown lower rates of stent thrombosis (ST) versus bare metal stents (BMS) and first-generation bioabsorbable polymer (BP) DES. The aim of the study was to evaluate the specific role of the FP in thromboresistance.
Methods and results: A total of 57 stents were assessed in three separate ex vivo swine arteriovenous shunt model experiments (first shunt experiment, custom-made fluoropolymer-coated BMS [FP-only] vs. BMS [n=8 each]; second shunt experiment, FP-EES vs. abluminally coated biodegradable polymer sirolimus-eluting stents [BP-SES] vs. BMS [n=8 each]; and third shunt experiment, FP-EES vs. polymer-free Biolimus A9-coated stents [PF-BCS] vs. BMS [n=6 each]). After one hour of circulation, stents were bisected, and each half was dual-immunostained using a platelet cocktail and a marker for inflammation. Antibody staining was visualised by confocal microscopy. In addition, stents were evaluated by scanning electron microscopy. FP-only stents showed significantly lower platelet adherence compared with BMS (% fluorescence-positive area: FP-only=1.8%, BMS=5.6%, p=0.047) with similar inflammatory cell density. FP-EES also demonstrated the lowest platelet adherence compared with BP-SES (p=0.056), PF-BCS (p=0.013) and BMS (p=0.003) with the significantly lowest inflammatory cell density.
Conclusions: Fluoropolymer coating imparts greater thromboresistance relative to BMS and to polymer-free DES designs, which reflects an unique phenomenon known as fluoropassivation, representing one proposed mechanism for clinically observed low ST rates in FP-EES.
Abbreviations
BMS: bare metal stents
BP-SES: biodegradable polymer sirolimus-eluting stents
FP-EES: fluoropolymer-coated everolimus-eluting stents
FP-only: fluoropolymer-coated stents
PF-BCS: polymer-free Biolimus A9-coated stents
ST: stent thrombosis
Introduction
Recent comprehensive meta-analyses of clinical trials established that durable fluoropolymer (poly[vinylidene fluoride-co-hexafluoropropylene] [PVDF-HFP])-coated everolimus-eluting stents (FP-EES) have lower rates of stent thrombosis (ST) and target vessel revascularisation than bare metal stents (BMS) or thick-strut biodegradable polymer drug-eluting stents (BP-DES)1,2. A previous preclinical arteriovenous swine shunt study demonstrated superior acute thromboresistance for FP-EES relative to BMS and BP-DES3,4, which supports the prior clinical observations regarding lower ST for FP-EES. However, it still remains uncertain whether other components of FP-EES besides the polymer itself might be responsible for these findings. Moreover, stent designs have continued to evolve with many newer stent designs having thinner struts, while some do not contain polymers. These design differences raise the question of whether FP-EES still hold a thromboresistance advantage versus newer stent designs.
More recently developed BP-DES have demonstrated non-inferiority to FP-EES for the composite primary endpoint of safety and efficacy in several randomised controlled trials5,6. Polymer-free Biolimus A9-coated stents (PF-BCS), another new concept in DES design, have demonstrated superior clinical outcomes compared to early paclitaxel-eluting stents7. The common concept of most newer DES is based on the idea that polymers are “harmful”. The idea has been raised from the unfavourable clinical results of the first-generation DES due to the durable polymers that have been associated with inflammatory responses and hypersensitivity reactions8-10.
Here we specifically tested the hypothesis that the FP on EES is the most important component of its design with respect to thromboresistance by comparing stents of similar design with and without FP coating (i.e., no drug) in a swine ex vivo shunt model. The acute thrombogenicity of FP-EES was also compared with thin-strut abluminally coated BP-DES and PF-DES to examine whether FP-EES still hold an advantage with respect to acute thrombogenicity versus these newer stent designs.
Methods
SWINE EX VIVO ARTERIOVENOUS SHUNT MODEL
The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee and met the ARRIVE guidelines. For each procedure, pigs were anaesthetised with ketamine/xylazine, intubated and maintained under general anaesthesia with isofluorane. A swine ex vivo carotid to jugular arteriovenous shunt model was established to study the extent of platelet adherence, thrombus formation, and acute inflammation, as previously described3,11 (Supplementary Appendix 1). Three separate shunt studies were performed. In the first shunt study, custom-made fluoropolymer-only coated MULTI-LINK 8™ BMS (Abbott Vascular, Santa Clara, CA, USA) without everolimus (FP-only [n=8]) and MULTI-LINK 8 BMS (no polymer [n=7]) were compared. In the second shunt study, XIENCE Alpine™ EES (Abbott Vascular) with durable fluoropolymer (FP-EES [n=8]), Ultimaster® (Terumo Corp., Tokyo, Japan) abluminally coated biodegradable polymer sirolimus-eluting stents (BP-SES [n=8]), and MULTI-LINK VISION® (Abbott Vascular) BMS (n=8) were compared. In the third shunt study, FP-EES (n=6), the polymer-free Biolimus A9™-coated stent (BioFreedom™; Biosensors International, Singapore) (PF-BCS [n=6]), and the MULTI-LINK VISION BMS (n=6) were compared (Supplementary Figure 1). In summary, a total of 57 stents were deployed in 19 shunts from 10 swine for the assessment of acute thrombogenicity.
The extent of platelet aggregation to struts was studied after exposure to circulating blood for one hour through an established arteriovenous carotid to jugular shunt. Blood activated clotting times (ACT) were measured every 20 minutes and targeted to be between 150 s and 190 s using intravenous heparin (100 IU/kg) without antiplatelet agents.
ASSESSMENT OF PLATELET AGGREGATION AND INFLAMMATION
CONFOCAL MICROSCOPY
Stent halves were incubated overnight in an antibody cocktail directed against specific platelet markers – a platelet cocktail of CD61 and CD42b. Each half was processed for immunostaining using an inflammatory marker for neutrophils (PM-1) or monocytes (CD14). The positive area of platelet staining was analysed by proprietary software within the entire bisected segment of the stented artery, which was maintained for all examined samples and reported as absolute positive area (mm2) of the device and percentage of positive area.
SCANNING ELECTRON MICROSCOPY (SEM)
After confocal microscopy, stent halves were processed for SEM. Low-power images of the entire luminal surface were collected to assess the extent of thrombus attached to the strut surfaces. The percentage of thrombus coverage was estimated visually for each row of struts corresponding to a ring.
QUANTIFICATION OF INFLAMMATORY CELLS
Images for quantification of inflammatory cells were acquired at every other strut in regions relatively free of platelet thrombus. The number of inflammatory cells was manually counted and expressed as cell density (cells/mm2) relative to the strut surface area.
STATISTICAL ANALYSIS
Nested generalised linear mixed models (GLMM) with Dunnett’s correction for multiple testing were employed in order to investigate group differences in consideration of multiple measurements per individual. Within these models, stent type was considered as fixed effect, while the experimental factor variables animal, shunt number and linear position were considered as nested random effects. Values are expressed as estimated mean with 95% confidence interval (CI). To assess the consistency of the three shunt experiments, thrombus-occupied area and inflammatory cell density of BMS in three shunt experiments were also compared using GLMM with Dunnett’s correction for multiple testing. The analyses were performed with SPSS Advanced Statistics, Version 22 (IBM Corp., Armonk, NY, USA). The statistical tests were two-tailed and a value of p<0.05 was considered to indicate statistical significance12.
Results
BLOOD COAGULATION AND PLATELET FUNCTION
There was no evidence of blood coagulation or platelet function abnormalities in any of the animals studied. The mean ACT was 174.7±17.2 seconds during the first shunt and 167.2±17.6 seconds during the second shunt for the 10 swine included in this study (Supplementary Table 1, Supplementary Table 2).
SHUNT EXPERIMENT 1 (FP-ONLY VS. BMS)
FP-only stents demonstrated percent positive fluorescence areas for CD42b/CD61 corresponding to aggregated platelets which were less compared with BMS (FP-only=1.8%, BMS=15.6%, p=0.047) (Figure 1, Figure 2, Table 1). Percent thrombus areas assessed by SEM were also significantly less in FP-only compared with BMS (FP-only=2.4%, BMS=21.3%, p=0.019) (Figure 2, Figure 3).
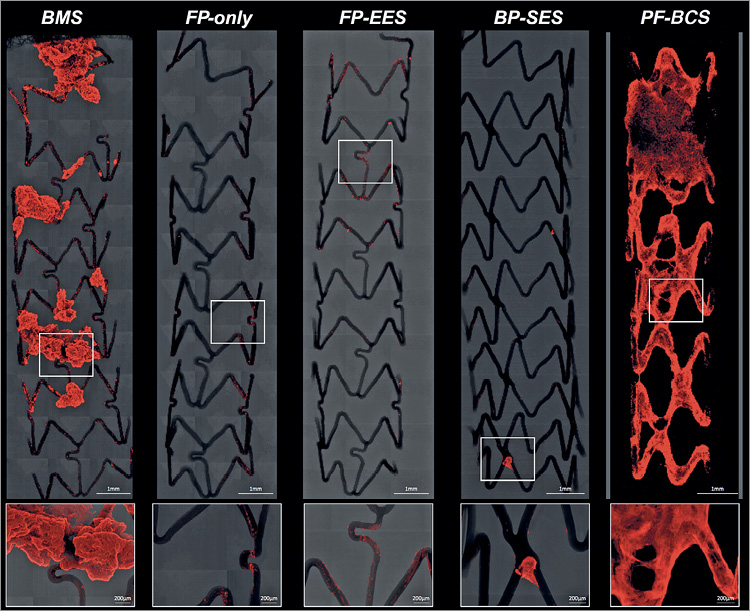
Figure 1. Representative confocal microscopic images of BMS, FP-only, FP-EES, BP-SES, and PF-BCS with immunofluorescent staining against dual platelet markers (CD61/CD42b) in a swine shunt model. Low- and high-power confocal microscopic images showing the least thrombus-occupied area in stents with fluoropolymer (FP-only and FP-EES) as compared with the other stents. Note minimal thrombus is only observed in the link portion of FP-only and FP-EES, whereas large thrombus covers almost all the struts in PF-BES.
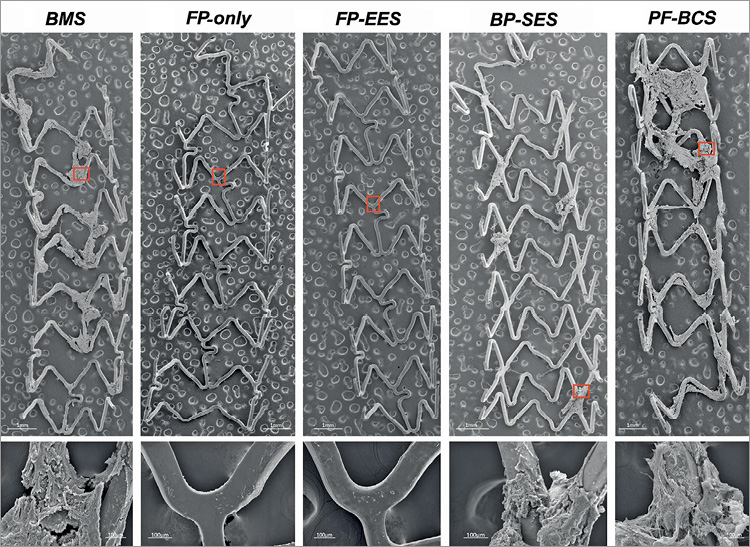
Figure 2. Representative scanning electron microscopic images of BMS, FP-only, FP-EES, BP-SES, and PF-BCS in a swine shunt model. Low- (×15) and high- (×200) power images of scanning electron microscopy showing least platelet aggregation clot formation (red boxes) on stents with fluoropolymer (FP-only and FP-EES) as compared with the other stents.
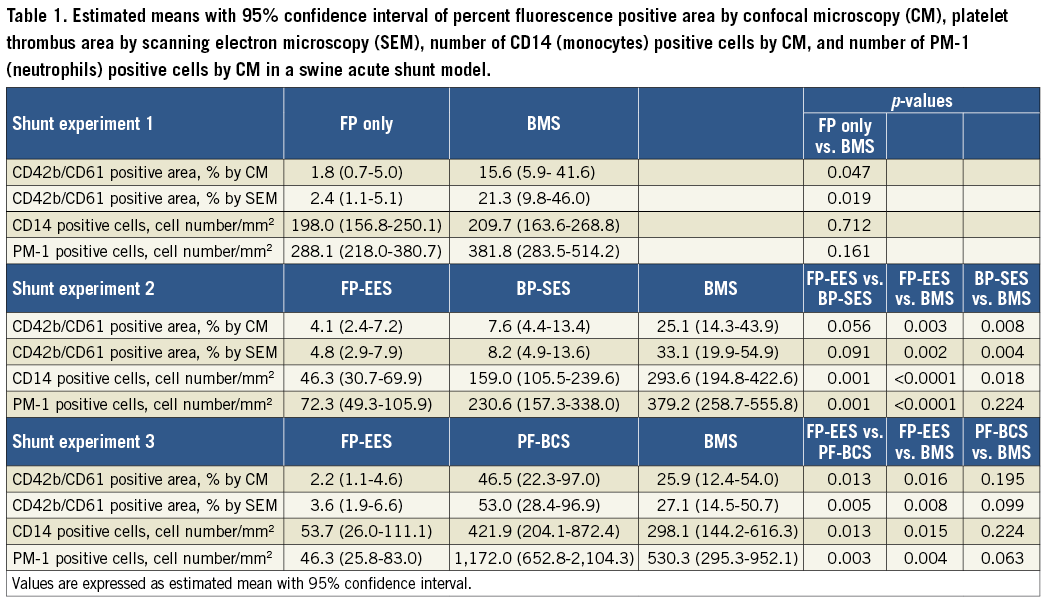
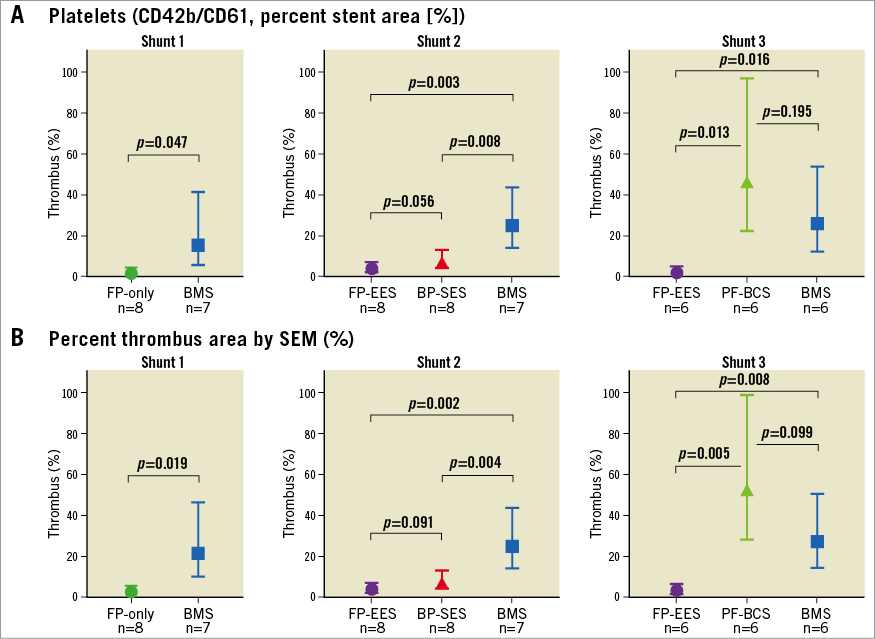
Figure 3. Comparisons of acute thrombogenicity on the stent surface in a swine shunt model. Graph with 95% confidence interval showing percent fluorescent positive area (percent area occupied by thrombus) assessed by confocal microscopy (A) and SEM (B) from three shunt models (first shunt experiment, FP-only vs. BMS; second shunt experiment, FP-EES vs. BP-SES vs. BMS; and third shunt experiment, FP-EES vs. PF-BCS vs. BMS).
On the other hand, cell density of monoytes (CD14) and neutrophils (PM-1) on strut surfaces was similar in FP-only and BMS (monocytes [CD14]: FP-only=198.0 cells/mm2 vs. BMS=209.7 cells/mm2, p=0.712; neutrophils [PM-1]: FP-only=288.1 cells/mm2 vs. BMS=381.8 cells/mm2, p=0.161) (Figure 4, Figure 5).
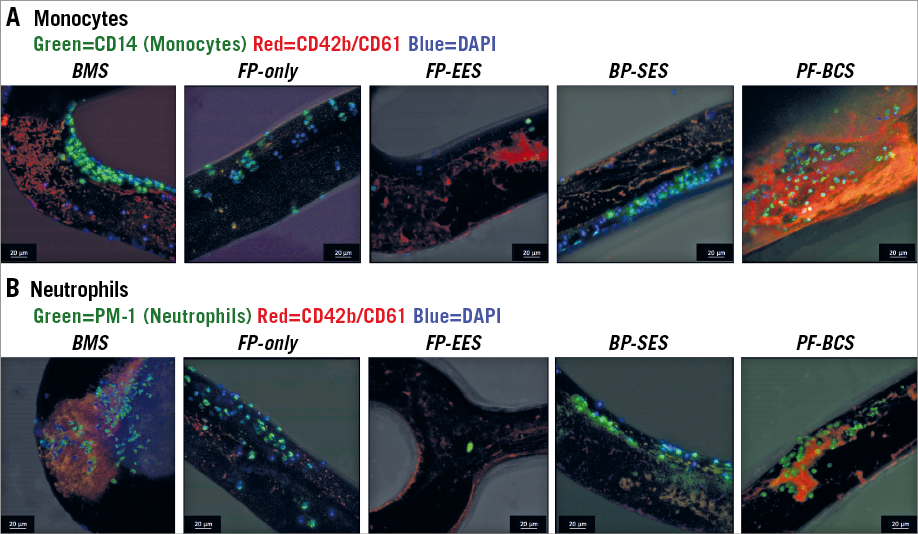
Figure 4. Representative confocal images of each stent with inflammatory cells in a swine shunt model. CD14 stained nuclei represent adherent monocytes (A), whereas PM-1 stained nuclei represent adherent neutrophils (B).
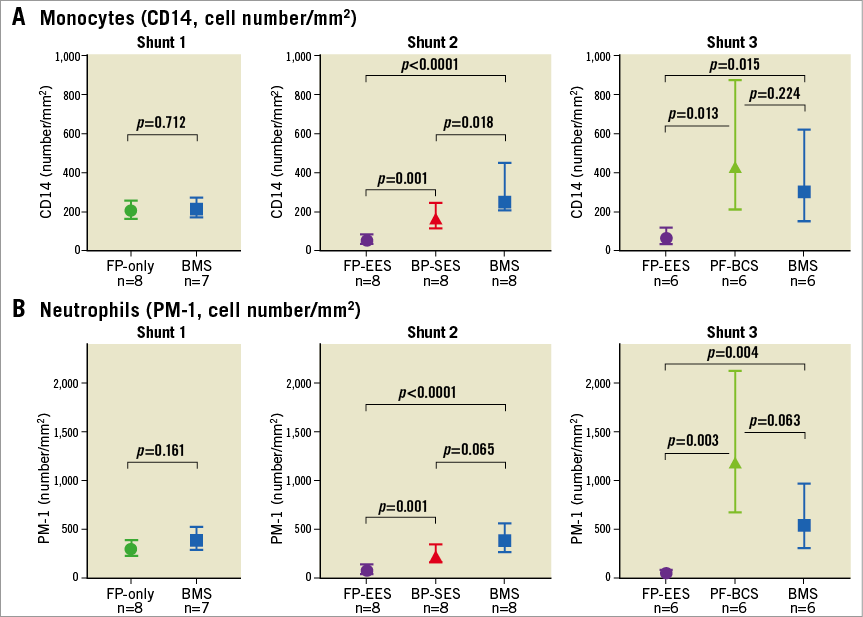
Figure 5. Comparisons of inflammatory cell attachment on the stent surface in a swine shunt model. Graph with 95% confidence interval showing number of monocytes (CD14) (A) and neutrophils (PM-1) (B) assessed by confocal microscopy from three shunt models (first shunt experiment, FP-only vs. BMS; second shunt experiment, FP-EES vs. BP-SES vs. BMS; and third shunt experiment, FP-EES vs. PF-BCS vs. BMS).
SHUNT EXPERIMENT 2 (FP-EES VS. BP-SES VS. BMS)
FP-EES demonstrated the least percent positive fluorescence areas for CD42b/CD61 compared with BP-SES and BMS (FP-EES=4.1%, BP-SES=7.6%, BMS=25.1%) (Figure 1, Figure 2, Table 1); however, the difference between FP-EES and BP-SES did not reach statistical significance by linear mixed-effects model (p=0.056), whereas the difference between FP-EES and BMS reached statistical significance (p=0.003) (Figure 2). Percent thrombus areas assessed by SEM also demonstrated similar results as shown in confocal assessment (Figure 2, Figure 3).
Cell density of monocytes (CD14) and neutrophils (PM-1) on strut surfaces was significantly less in FP-EES compared with BP-SES (monocytes [CD14]: FP-EES=46.3 cells/mm2 vs. BP-SES=159.0 cells/mm2, p=0.001; neutrophils [PM-1]: FP-EES=72.3 cells/mm2 vs. BP-SES=230.6 cells/mm2, p=0.001) and BMS (monocytes [CD14]: BMS=293.6 cells/mm2, p<0.001; neutrophils [PM-1]: 379.2 cells/mm2, p=0.004) (Figure 4, Figure 5).
SHUNT EXPERIMENT 3 (FP-EES VS. PF-BCS VS. BMS)
PF-BCS demonstrated the highest percent positive fluorescence areas for CD42b/CD61 compared with FP-EES (PF-BCS=46.5%, FP-EES=2.2%, BMS=25.9%) (Figure 1, Figure 2, Table 1). The linear mixed-effects model demonstrated statistical significance between PF-BCS and FP-EES (p=0.013), and BMS and FP-EES (p=0.016), whereas the difference of PF-BCS and BMS did not reach statistical significance (p=0.195) (Figure 2). Percent thrombus areas assessed by SEM also demonstrated similar results as shown in confocal assessment (Figure 2, Figure 3).
Cell density of monocytes (CD14) and neutrophils (PM-1) on strut surfaces was significantly higher in PF-BCS compared with FP-EES (monocytes [CD14]: PF-BCS=421.9 cells/mm2 vs. FP-EES=53.7 cells/mm2, p=0.013; neutrophils [PM-1]: PF-BCS=1,172.0 cells/mm2 vs. FP-EES=46.3 cells/mm2, p=0.003). There was no difference in either inflammatory cell type between PF-BCS compared with BMS (monocytes [CD14]: BMS=298.1 cells/mm2, p=0.22; neutrophils [PM-1]: BMS=530.3 cells/mm2, p=0.063) (Figure 4, Figure 5).
PLATELET AGGREGATION AND INFLAMMATORY CELL ADHESION OF BMS IN THREE SHUNT EXPERIMENTS
There were no significant differences among the three BMS used in the three shunt experiments, respectively, in positive fluorescence areas for CD42b/CD61, percent thrombus areas, and cell density of both monocytes and neutrophils (Supplementary Table 3).
Discussion
FP-EES have been associated with a lower risk of ST when compared to BMS and early thick-strut BP-DES, but which component of the stent is responsible for this lower ST risk as well as its performance relative to contemporary DES remains uncertain. The current study examined acute thrombogenicity with respect to platelet aggregation and inflammatory cell adhesion of various stents including custom fluoropolymer-only stents without everolimus using an established ex vivo swine arteriovenous shunt model. The principal findings in the current preclinical study are as follows. 1) Custom fluoropolymer-only XIENCE stents without everolimus demonstrated a significantly lower percentage of thrombus-occupied area than BMS (p=0.047) with similar inflammatory cell density. 2) FP-EES demonstrated the least percentage of thrombus-occupied area compared with BP-SES (p=0.056), PF-BCS (p=0.013) and BMS (shunt experiment 2, p=0.003; shunt experiment 3, p=0.016) with the lowest inflammatory cell density. 3) BMS demonstrated similar thrombus-occupied area and inflammatory cell density in all three shunt experiments, suggesting consistency among the shunt runs.
ROLE OF FLUOROPOLYMER
This study is the first study to have evaluated the thromboresistance of the FP-coated stent itself using custom FP-only stents without everolimus and matched BMS in an acute ex vivo shunt model. The fluoropolymer coating alone imparts greater thromboresistance as compared to a BMS with the same stent platform.
Fluoropolymers have known ability to reduce platelet adhesion and activation compared to controls. Platelet adhesion and activation were more strongly suppressed as the amount of fluorine dosing was increased13,14. A recent in vitro study performed by our group demonstrated a significantly higher antithrombogenic effect of the fluoropolymer compared with the various polymers used in the early durable polymer DES13. Fluoropassivation is a blood contact phenomenon by which fluorinated surfaces elicit a decreased local thrombotic response15-18. Unlike hydrophobic polymers that showed thromboresistance in an in vivo rabbit model19, fluoropassivation is thought to result from preferential affinity for albumin and retention of albumin. This albumin-rich protein layer modulates host-material interface interactions and competitively inhibits the adhesion of prothrombotic proteins, such as fibrinogen13,20. This mechanism of albumin retention and spreading depends on surface hydrophobicity21. PVDF-HPF surface coating on CoCr-EES/Pt-Cr-EES has a carbon backbone of which more than 50% is saturated with fluorine to form a hydrophobic surface. The protein spreading rate increases with substrate hydrophobicity ranging from 0.02 to 0.16 nm2/molecule/s for albumin21: fluoropolymers possess an extremely high degree of hydrophobicity (for example, PVDF homopolymer has an equilibrium water adsorption in a range from 0.01% [w/w] to <0.04% [w/w]). Additionally, high bond strength, low polarisability and a high degree of fluorination also contribute to fluoropolymer interaction with culprit proteins such as fibrinogen and vWF.
On the other hand, anti-inflammatory effects were similar between FP-only stents and BMS, whereas fluoropolymer-coated everolimus-eluting stents with the same stent platform (FP-EES) demonstrated significantly lower inflammatory cell adhesion compared with BMS. The result suggests that there are two mechanisms of superior clinical outcomes of FP-EES: thromboresistance owing to the fluoropolymer, with an additional local anti-inflammatory effect owing to the drug (everolimus) release from a circumferential coating that covers all strut surfaces. Further studies are needed to reveal the difference between fluoropolymer and other durable polymers of contemporary DES (e.g., BioLinx polymer [a mixture of C10/C19, and polyvinylpyrrolidine polymers] in the Resolute Onyx™ zotarolimus-eluting stent [Medtronic, Minneapolis, MN, USA]22,23). Although not specifically examined here, we have also shown recently in human autopsy cases minimal inflammatory reaction surrounding stents coated with fluoropolymer surfaces (i.e., CoCr-EES) versus bare metal surfaces implanted for greater than one year24. Thus the debate about whether polymer exposure should be minimised depends upon the type of polymer used.
DIFFERENCE BETWEEN CIRCUMFERENTIALLY COATED DURABLE FLUOROPOLYMER AND ABLUMINALLY COATED BIODEGRADABLE POLYMER
The BP-SES (Ultimaster) used in the current study is abluminally coated with a biodegradable polymer in which the luminal surface of the stent remains a bare metal surface. One of the proposed goals of abluminally coated polymer is to induce faster endothelial coverage23. However, abluminally coated BP-SES showed numerically greater platelet aggregation (p=0.056) compared with FP-EES, and the result is consistent with our previous preclinical study3 that demonstrated significantly greater platelet aggregation in the CE-marked abluminally coated BP-DES (SYNERGY™; Boston Scientific, Marlborough, MA, USA). These results suggest that the “bare metal” surface might be one of the causes of acute thrombogenicity, and thrombus could aggregate on the bare surface of the luminal side in the abluminally coated BP-DES. Kolandaivelu et al also measured greater thrombogenicity for BMS compared to polymer-coated DES in an in vitro model4. On the other hand, BP-SES showed superior thromboresistance (p=0.008) compared with BMS. As both BP-SES and BMS have bare metal luminal surfaces, this difference may be due to the presence of the drug. Some data suggest that BP-DES that are coated only abluminally lack coating integrity upon deployment and also during acute time points (zero to two months)25,26. The FP-EES coating integrity and fluoropassivation phenomena may combine to contribute to its greater thromboresistance relative to BP-DES. The significantly lower inflammatory cell adhesion of FP-EES compared with BP-SES may be attributed to the elution of everolimus from the luminal and strut sidewall surfaces.
CIRCUMFERENTIALLY COATED FP-EES VS. POLYMER-FREE BCS
PF-BCS (BioFreedom) demonstrated higher platelet adherence compared with FP-EES. We assume that the reason for the higher acute thrombogenicity of PF-BCS could be that the abluminal surface of the PF-BCS is selectively microstructured which allows adhesion of the antiproliferative agent (Biolimus A9) to the abluminal surface of the stent without a polymer or binder27. Although the rough microstructured surface may contribute to faster endothelialisation27, it also increases platelet adherence compared with smooth fluoropolymer or metallic surface in the very acute phase.
Biolimus A9 is coated on the abluminal surface of PF-BCS; however, the anti-inflammatory effect of PF-BCS was significantly less than FP-EES, and was similar to that of BMS. Not only is it the bare metal surface of the luminal side that causes inflammatory cell adherence as previously described, but also aggregated thrombus might be a reason for the current result because platelet aggregation on the surface of stent struts is known to be a trigger to recruit circulating leukocytes consisting mainly of neutrophils and monocytes28.
CLINICAL IMPLICATIONS
In the first-generation DES era, durable polymers employed in these devices were associated with ST, in part due to inflammation and hypersensitivity reactions9. Clinical trials also demonstrated a higher incidence of ST compared with BMS. These results suggested untoward effects of durable polymers and helped to spur the development of biodegradable polymer DES and polymer-free DES that leave a bare metal surface behind in a few months after the stent implantation. On the other hand, the durable fluoropolymer DES have demonstrated lower ST rates in several clinical trials and meta-analyses, and the result is consistent with the current preclinical study. Our study suggests important antithrombotic benefits of FP-EES which continue to compare favourably versus other stents.
Limitations
There are some limitations in the current study. First, this study was performed with a swine ex vivo shunt model without antiplatelet agents, so that applying preclinical findings to human diseased coronary arteries with dual antiplatelet therapy is more complex. Although this model is relatively simplistic in order to allow direct cross comparison of stents between shunts, this simple model cannot account for all the potential variables than can contribute to acute thrombogenicity in diseased human coronary arteries. Second, although systemic effects of the coated drug in DES are low and transient, becoming undetectable quickly, the drug eluted from a DES may affect inflammatory cell adhesion of the adjacent stent in this model. However, we believe that the established ex vivo shunt model in the current study could account for a number of covariates influencing acute platelet aggregation, thrombus formation, and leukocyte adhesion, and would be more likely to resemble flow conditions which occur in humans in vivo compared with an in vitro flow loop model.
Conclusions
Our data suggest that the choice of surface covering of metallic stents is an important determinant of the relative thromboresistance profile of different DES. Fluoropolymer coating imparts greater thromboresistance relative to BMS and to polymer-free DES designs, which reflects an unique phenomenon known as fluoropassivation, representing one proposed mechanism for clinically observed low ST rates in FP-EES.
| Impact on daily practice In this study, fluoropolymer-only stents showed significantly lower platelet adherence compared with BMS with similar inflammatory cell density. FP-EES also demonstrated the lowest platelet adherence compared with BP-SES, PF-BCS and BMS with the lowest inflammatory cell density. The results reflect an unique phenomenon known as fluoropassivation, representing an important mechanism for clinically observed low stent thrombosis rates in FP-EES. |
Acknowledgements
We are grateful to Dr Fuh-Wei Tang and Darry Valdemoro of Abbott Vascular for their work on fabrication of the fluoropolymer-only coated stents.
Funding
This study was funded by Abbott Vascular, Santa Clara, CA, USA. CVPath Institute, Inc., Gaithersburg, MD, USA provided full support for this work.
Conflict of interest statement
S. Torii receives honoraria from Abbott Vascular Japan and Terumo Corporation, and research grants from SUNRISE lab. R. Virmani and A. Finn have received institutional research support from Abbott Vascular, Biosensors International, Biotronik, Boston Scientific, Medtronic, MicroPort Medical, OrbusNeich Medical, SINO Medical Technology, and Terumo Corporation. R. Virmani has speaking engagements with Merck, receives honoraria from Abbott Vascular, Boston Scientific, Lutonix, Medtronic, and Terumo Corporation, and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. L. Perkins, S. Hossainy, and S. Pacetti are full-time employees of Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Table 1A. Summary of the mean values from all animal blood coagulation (PT, PPT), platelet quantification (platelet counts, platelet estimates [EST]), activated platelet factor (PF4) as assessed by ELISA and activated clotting time (ACT), flow and shear rate in a swine acute shunt model.
Supplementary Table 1B. Second shunt experiment (FP-EES vs. BP-SES vs. BMS).
Supplementary Table 1C. Third shunt experiment (FP-EES vs. PF-BCS vs. BMS).
Supplementary Table 2A. Shunt matrix, first shunt experiment (FP-only vs. BMS).
Supplementary Table 2B. Shunt matrix, second shunt experiment (FP-EES vs. BP-SES vs. BMS).
Supplementary Table 2C. Shunt matrix, third shunt experiment (FP-EES vs. PF-BCS vs. BMS).
Supplementary Table 3. Estimated means with 95% confidence interval of percent fluorescence positive area by confocal microscopy (CM), platelet thrombus area by scanning electron microscopy (SEM), number of CD14 (monocytes) positive cells by CM, and number of PM-1 (neutrophils) positive cells by CM in a swine acute shunt model.
Supplementary Figure 1. Overview of stent profiles used in the current study.
To read the full content of this article, please download the PDF.

