Abstract
This consensus document, a summary of the views of an expert panel organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), appraises the importance of ischaemia with non-obstructive coronary arteries (INOCA). Angina pectoris affects approximately 112 million people globally. Up to 70% of patients undergoing invasive angiography do not have obstructive coronary artery disease, more common in women than in men, and a large proportion have INOCA as a cause of their symptoms. INOCA patients present with a wide spectrum of symptoms and signs that are often misdiagnosed as non-cardiac leading to under-diagnosis/investigation and under-treatment. INOCA can result from heterogeneous mechanism including coronary vasospasm and microvascular dysfunction and is not a benign condition. Compared to asymptomatic individuals, INOCA is associated with increased incidence of cardiovascular events, repeated hospital admissions, as well as impaired quality of life and associated increased health care costs. This consensus document provides a definition of INOCA and guidance to the community on the diagnostic approach and management of INOCA based on existing evidence from research and best available clinical practice; noting gaps in knowledge and potential areas for further investigation.
Introduction
Angina pectoris, the most common symptom of ischaemic heart disease (IHD), affects approximately 112 million people globally.1 The 2019 ESC guidelines provides guidance on the diagnosis and management of patients with chronic coronary syndromes (CCS).2 A large proportion of patients (up to 70%) undergoing coronary angiography because of angina and evidence of myocardial ischaemia do not have obstructive coronary arteries but have demonstrable ischaemia.2,3 Studies carried out in the past two decades have highlighted that coronary microvascular dysfunction (CMD) and epicardial vascular dysfunction are additional pathophysiologic mechanisms of IHD.4 Coronary microvascular dysfunction and epicardial vasospasm, alone or in combination with coronary artery disease (CAD), are adjunctive mechanisms of myocardial ischaemia. However, these conditions are rarely correctly diagnosed and, therefore, no tailored therapy is prescribed for these patients. As a consequence, these patients continue to experience recurrent angina with impaired quality of life, leading to repeated hospitalizations, unnecessary coronary angiography and adverse cardiovascular outcomes in the short-and long term.5,6 This consensus document provides a definition of ischaemia with non-obstructive coronary arteries (INOCA) and guidance to the clinical community on the diagnostic approach and management of INOCA based on existing evidence and best current practices. In addition, having a universal definition of INOCA and identifying gaps in knowledge will serve to encourage research to improve outcomes for this patient population. Discussion of angina caused by CMD in the context of cardiomyopathy (hypertrophic, dilated), myocarditis, aortic stenosis, infiltrative diseases of the heart, percutaneous/surgical interventions, and other possible mechanisms7 (Figure 1) such as inflammation, systemic inflammatory or autoimmune disease (lupus, rheumatoid arthritis), platelet/coagulation disorders, primary metabolic abnormalities, as well as by myocardial bridging, is beyond the scope of this consensus document. A failure to diagnose epicardial CAD in a patient with documented angina/ischaemia should promote a subsequent search pathway to elucidate INOCA endotypes before a search for non-cardiac causes of chest discomfort is explored.
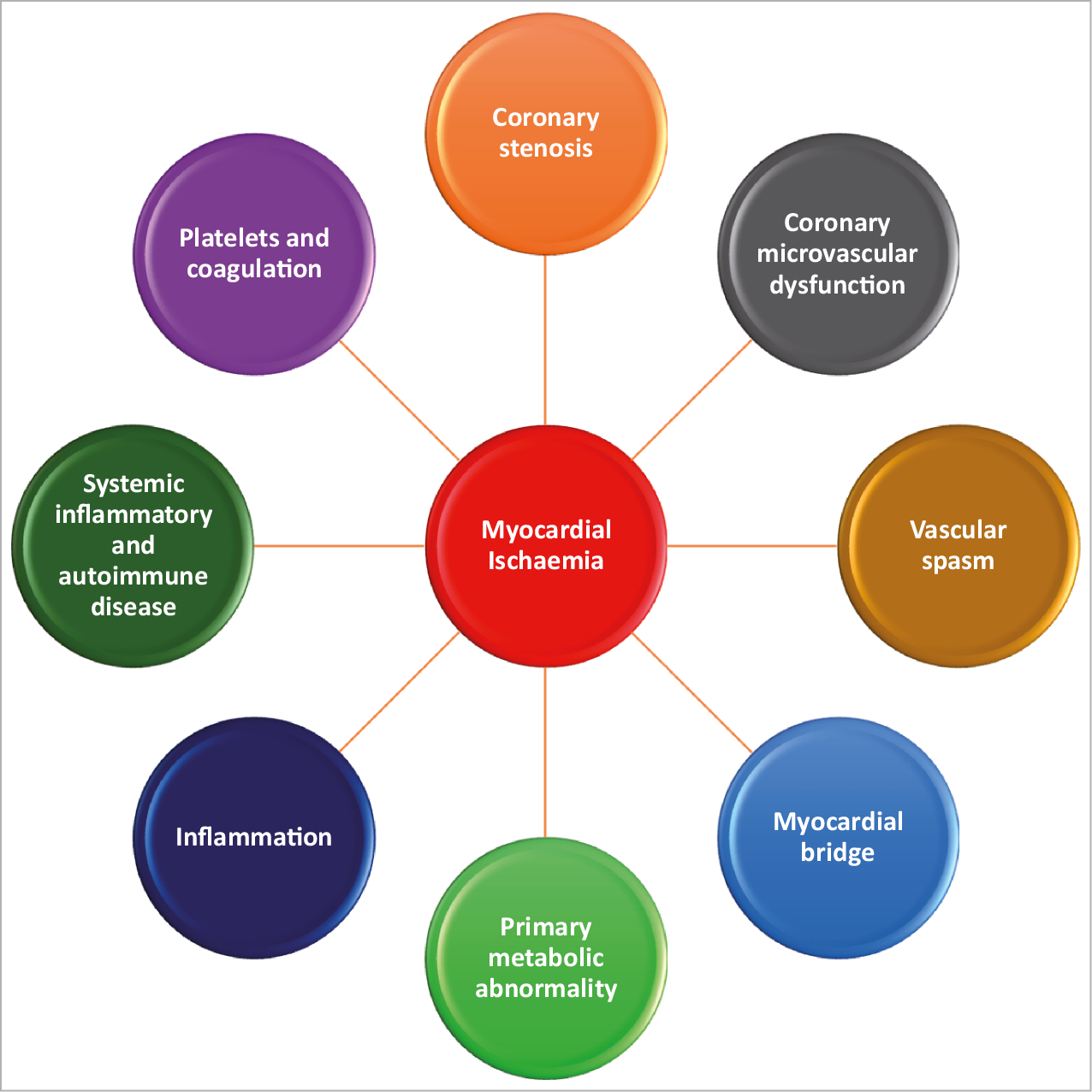
Figure 1. Mechanisms of myocardial ischaemia.
INOCA endotypes
In the setting of CCS, a mismatch of demand-supply of coronary artery blood flow may lead to transient or recurrent cardiac chest pain related to myocardial ischaemia due to inadequate cellular availability of adenosine-50-triphosphate.8 Although obstructive CAD is a frequent and well-acknowledged cause of myocardial ischaemia, many stenoses judged as severe on visual assessment, are not flow-limiting. Functional misclassification of obstructive lesions frequently occurs in the range of 40-80% stenosis severity, being particularly high in case of patients with multiple coronary lesions.9,10,11 The most recent ESC guidelines recommend the use of myocardial fractional flow reserve (FFR) or instantaneous wave-free ratio to identify patients at high event risk who will benefit from revascularisation.2 Cardiac ischaemia may also be caused by vascular dysfunction without obstructive CAD, a condition recently termed INOCA. In INOCA, the mismatch between blood supply and myocardial oxygen demands may be caused by CMD and/or epicardial coronary artery spasm, typically in the setting of non-obstructive coronary atherosclerosis.12 Figure 2,13,14 shows the mechanisms of INOCA. Of note, these mechanisms may also cause ischaemia in patients with concomitant obstructive CAD and atherosclerosis with outward remodelling but these cases are not included in INOCA by definition.
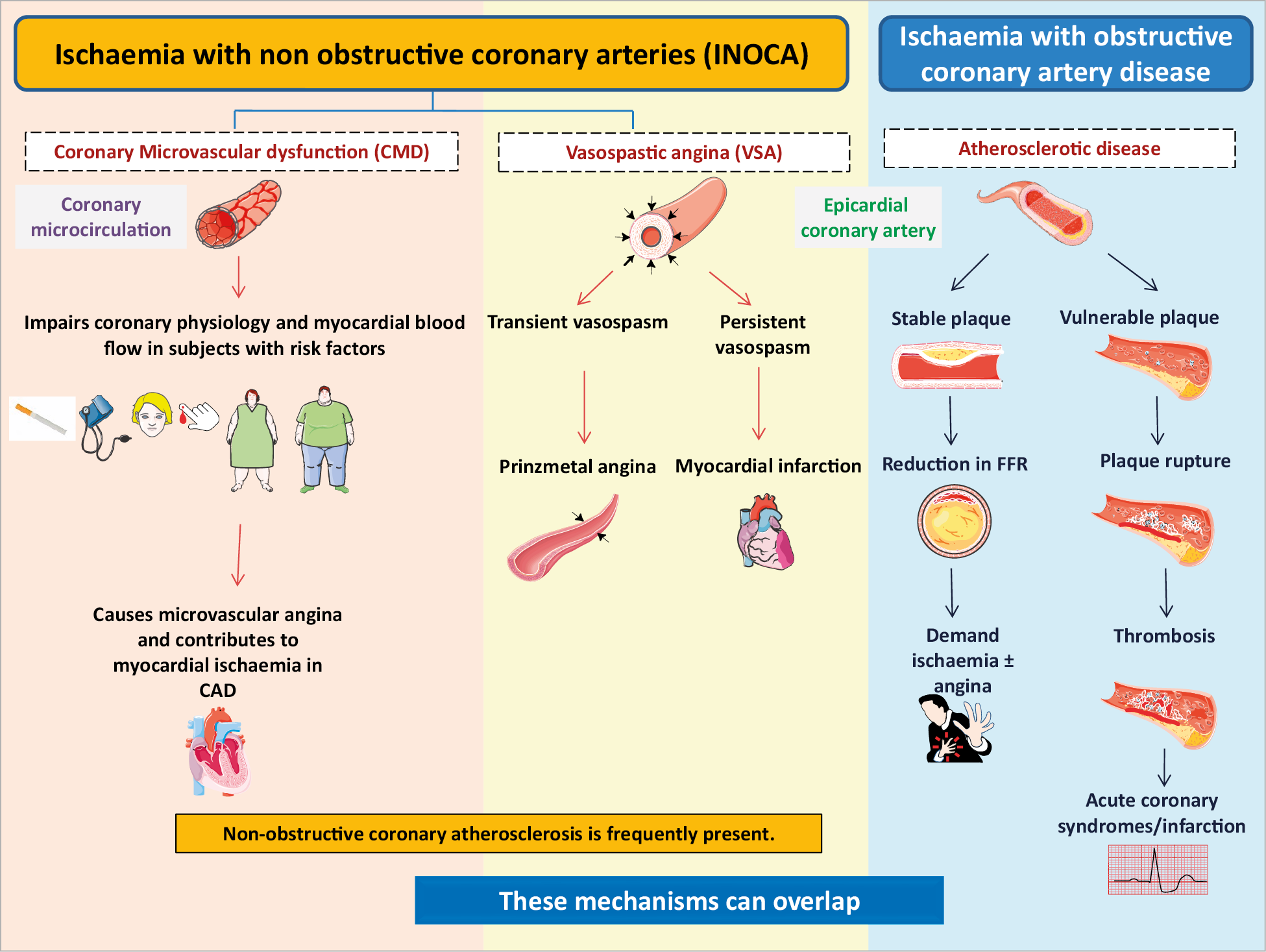
Figure 2. Mechanisms of myocardial ischaemia in INOCA and obstructive coronary artery disease. CAD: coronary artery disease; FFR: fractional flow reserve.
MICROVASCULAR ANGINA
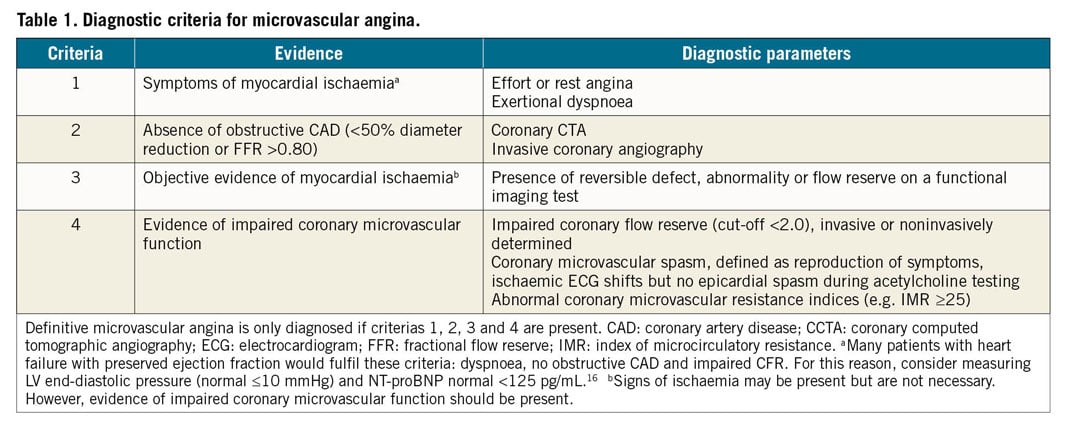
Microvascular angina (MVA) is the clinical manifestation of myocardial ischaemia caused by CMD. In this clinical entity, myocardial ischaemia may result from structural remodelling of the microvasculature (leading to fixed reduced microcirculatory conductance) or vasomotor disorders affecting the coronary arterioles (causing dynamic arteriolar obstruction).15,16 Both vascular dysfunction mechanisms may co-exist and contribute to MVA. An updated standardization of criteria for MVA in patients presenting with angina pectoris or ischaemia-like symptoms in the absence of flow-limiting CAD has been proposed by the COVADIS group15 (Table 1).
EPICARDIAL VASOSPASTIC ANGINA
Vasospastic angina (VSA) is the clinical manifestation of myocardial ischaemia caused by dynamic epicardial coronary obstruction caused by a vasomotor disorder. In 1959, Prinzmetal described the clinical and electrocardiographic manifestations (transient ST-segment elevation) of a disorder thought to be due to epicardial coronary artery spasm.17 Subsequently, other forms of vasomotor disorders causing chest pain with transient ST-segment depression or T-wave inversion were described. Overall, these clinical entities caused by epicardial vessel spasm were grouped under the term VSA. A standardization of diagnostic criteria for VSA has been previously described by the COVADIS group (Supplementary material online, Table S1).18 Microvascular angina and epicardial VSA can co-exist which is associated with worse prognosis.19
Epidemiology
PREVALENCE IN THE GENERAL POPULATION AND ACCORDING TO SEX AND AGE
The majority of patients referred for assessment for angina do not have obstructive coronary arteries. In unselected populations referred for assessment less than 10% have obstructive CAD.3,20 In all studies, there is a strong female preponderance for the condition. A large US multicentre study showed that nearly 39% of the patients selected for coronary angiography because of suspected angina and/or positive stress test have non-obstructive CAD.21 This frequency is higher among women (approximately 50-70%), compared to men (30-50%). In a retrospective registry from Eastern Denmark including 11 223 patients with angina referred for coronary angiography between 1998 and 2009, 65% of women vs. 33% of men had non-obstructive CAD, with an increasing rate over the 10-year study period in both sexes, reaching up to 73% among women in 2009.5 Similarly, almost two-thirds (62%) of women referred for coronary angiography and enrolled in the National Heart, Lung, and Blood Institute sponsored Women’s Ischaemia Syndrome Evaluation (WISE), did not have a significant obstructive stenosis. Women with non-obstructive CAD were younger than those with obstructive CAD.22
PREVALENCE OF CORONARY MICROVASCULAR DYSFUNCTION
The prevalence of CMD in patients with angina and no obstructive CAD undergoing invasive angiography depends on the methods and cut-off applied. In the iPower study, 26% of 963 symptomatic women with no obstructive CAD had coronary flow velocity reserve (CFVR) below two when assessed by transthoracic Doppler echo.23 However, these studies should be interpreted in the context that non-invasive estimation of CFVR has several limitations.24,25
Other studies assessing CMD invasively or by positron emission tomography with different cut-offs have found 39-54% have CMD.21,26 In a large study with invasive assessment of CMD in 1439 men and women with chest pain and no obstructive CAD included over a period of 19 years, 30% had abnormal CFVR in response to adenosine.27
The association between traditional cardiovascular risk factors and INOCA is not well established. Smoking has been associated with CMD.28 Age, diabetes, hypertension, and dyslipidaemia were associated with impaired CMD both in the iPower study and WISE study.21,23 Other studies have shown that diabetes was uncommon among patients presenting with angina and non-obstructive CAD, while hypertension and dyslipidaemia were relatively more prevalent.27,29
Coronary microvascular dysfunction is associated with proinflammatory markers in women with INOCA.30,31 In the WISE cohort, novel risk variables like those associated with inflammation seemed to play a role in CMD.32 For instance, systematic lupus erythematosus and rheumatoid arthritis are associated with CMD and are frequently encountered in patients with angina and CMD.33,34 After menopause, inflammatory diseases occur more often in women compared to men, which may contribute to sex-differences in CMD.35 Although large studies are lacking, there is increasing evidence that psychosocial stress is more involved in coronary vasomotor disorders and variant manifestations of IHD compared to obstructive CAD.36 These seem to affect men and women differently.37 Women have elevated levels of high sensitive C reactive protein (hsCRP), and a lower monocyte and eosinophil count than men. A significant positive association between Beck Depression Inventory cognitive symptoms with elevated hsCRP level is observed in men, but not in women.37
PREVALENCE OF CORONARY ARTERY SPASM
The Japanese population has a higher prevalence of angina related to coronary vasomotor disorders38 compared with western populations. In addition, the frequencies of multiple coronary spasm (≥2 spastic arteries) by provocative testing in Japanese (24.3%)39 and Taiwanese populations (19.3%)40 are markedly higher than those in Caucasians (7.5%).41 Interestingly, VSA is more prevalent among men than women.40 Most patients with VSA are between 40 and 70 years of age, and the prevalence tends to decrease after the age of 70 years.40 Previous Asian studies of patients with non-obstructive CAD have shown that the prevalence of coronary vasomotor disorders is around 50% in patients with angina.42,43 European studies have also shown a high prevalence of epicardial vasospasm when systematically tested.44,45 However, due to differences in stress protocols and definitions applied, the studies are not directly comparable. Female patients were more sensitive to acetylcholine with vasomotor dysfunction occurring at lower acetylcholine doses compared with male patients. Smoking is a risk factor for VSA, unlike diabetes and hypertension, and the relationship with dyslipidaemia is unclear.46,47
Pathophysiology and endotypes
MICROVASCULAR ANGINA AND EPICARDIAL CORONARY ARTERY SPASM
In the absence of flow-limiting coronary artery disease, myocardial ischaemia can result from specific pathways of microcirculatory dysfunction.16 Two microcirculatory dysfunction endotypes account for most cases of MVA: structural microcirculatory remodelling and functional arteriolar dysregulation. In other words, microvascular dyfsunction may be structural, functional or both.16,48
(i) Structural remodelling of the coronary microvasculature is associated with a decrease in microcirculatory conductance and impaired oxygen delivery capacity.49 This is typically caused by inward remodelling of coronary arterioles, with an increase in wall to lumen ratio, loss of myocardial capillary density (capillary rarefaction) or both.50 Remodelling may occur as a result of cardiovascular risk factors, atherosclerosis, left ventricular hypertrophy, or cardiomyopathies.50 A direct consequence of these pathological changes is a reduction of the vasodilatory range of the coronary microcirculation, limiting maximal blood and oxygen supply to the myocardium. Furthermore, remodelled arterioles are hypersensitive to vasoconstricting stimuli.51 The haemodynamic correlates of structural microcirculatory remodelling in response to a non-endothelium-dependent vasodilator, like adenosine, are (i) a reduced coronary flow reserve (CFR) and (ii) an increase in minimal (hyperaemic) microcirculatory resistance.
(ii) Functional arteriolar dysregulation typically takes place in medium and large size arterioles, in which flow-mediated vasodilation is predominant.16 Under physiological conditions, an increase in myocardial oxygen consumption generates an upstream vasodilatory cascade in coronary resistance vessels. This is initiated by metabolically triggered vasodilation of distal arterioles, that are particularly sensitive to certain metabolites, and it is followed by flow-mediated (endothelium-dependent) vasodilation of larger arterioles located up-stream, as well as epicardial vessels.52 In the presence of endothelial dysfunction, dysregulation of the described upstream vasodilatory cascade occurs. Thus, endothelial dysfunction is associated with impaired vasodilation and even paradoxical vasoconstriction of upstream arteries and arterioles when myocardial oxygen demands increase which may be the result of hypersensitivity to vasoconstrictor stimuli.53 Some of the haemodynamic correlates of arteriolar dysregulation, observed during intracoronary acetylcholine challenge, are (i) a limited vasodilatory response to the drug (less than 1.5 times resting flow), (ii) a marked reduction in blood flow, equivalent to the no-reflow phenomenon, without epicardial vessel spasm –denoting arteriolar spasm– and (iii) the development of diffuse narrowing of distal epicardial vessels without focal, tight coronary spasm. The above-mentioned changes frequently run along the development of anginal symptoms and ischaemic electrocardiogram changes, which confirm the ischaemia-generating potential of this endotype of micro-circulatory dysfunction. Effects of fluctuating oestrogen levels on epicardial vessel and arteriolar vasomotion have been postulated as explanations for a higher frequency of symptoms in premenopausal women without obstructive CAD.54
Epicardial vessel spasm typically has an origin in a hyper-reactive epicardial coronary segment that undergoes maximal contraction when exposed to a vasoconstrictor stimulus.55 Among such triggering stimuli are smoking, drugs, peaks in blood pressure (BP), cold exposure, emotional stress, and hyperventilation. Severe coronary vasospasm may also occur in the context of allergic reactions (Kounis syndrome). Coronary segments adjacent to implanted drug-eluting stents may also become prone to undergo coronary spasm.56 The substrate of coronary spasm can be found in abnormal function of both vascular smooth muscle and endothelial cells. A primary and non-specific hyper-reactivity of coronary vascular smooth muscle cells has been consistently demonstrated in patients with variant angina and appears to be a key component of epicardial vessel spasm. Available evidence suggests that endothelial dysfunction facilitates the induction of spasm in predisposed coronary segments.57
Clinical presentation
Patients with INOCA present with a wide spectrum of symptoms and signs that are often misdiagnosed as of non-cardiac origin, leading to under-investigation and under-treatment (Supplementary material online, Table S2). Patients with INOCA may present with symptoms similar to angina occurring with obstructive CAD.58,59 INOCA, like obstructive CAD, can also present with other symptoms such as breathlessness, pain between the shoulder blades, indigestion, nausea, extreme fatigue, weakness, vomiting, and/or sleep disturbances.
It is important to recognize that there is gender variation in the clinical manifestation of both obstructive and non-obstructive CAD.60,61,62 These differences in presentation are of particular relevance in young and middle-aged women and also men2,63 who do not present with classical anginal symptoms.64,65 With the same symptoms, women are much less likely to have obstructive CAD and much more likely to have CMD as a cause of their symptoms. In addition, because symptoms may be uncharacteristic, many cases of CMD may go undiagnosed.
Importantly, INOCA is associated with a wide variation in clinical presentation and symptom burden may vary over time. These symptoms should not be automatically classified as non-cardiac in origin, particularly given the fact that women have a much higher prevalence of INOCA than men.66
Short- and long-term prognosis
The prognosis of patients with INOCA is far from benign. Angina with no obstructive CAD is associated with impaired quality of life for patients,6,67 higher risk of disability,68 as well as a higher incidence of adverse events5 including increases in mortality, morbidity, and healthcare costs with higher recurrence rates of hospital readmissions and higher rates of repeated coronary angiograms.69,70,71,72,73,74 In the WISE study, persistent chest pain, smoking, CAD severity, diabetes, and increased QTc interval were significant independent predictors of cardiovascular events defined as cardiovascular death, myocardial infarction (MI), congestive heart failure, or stroke.75 In a meta-analysis,74 incidence of all-cause death and non-fatal MI in patients with non-obstructive atherosclerosis was much higher (1.32/100 person-years) than in those with angiographically normal epicardial vessels (0.52/100 person-years). Proven myocardial ischaemia by non-invasive imaging techniques (stress echocardiography or nuclear imaging) was associated with a higher incidence of events (1.52/100 person-years) compared to ischaemia detected by exercise electro-cardiographic stress testing 0.56/100 person-years.
It must be noted, the condition is heterogeneous and not all patients with angina and no obstructive CAD have ischaemia as a cause of their symptoms. However, when ischaemia is documented through CMD or endothelial dysfunction the prognosis is further impaired. Meta-analyses have shown a two-to four-fold higher risk of adverse cardiovascular outcome for patients with CMD diagnosed by positron emission tomography (PET) or transthoracic Doppler echocardiography and a two-fold higher risk in patients with epicardial endothelial-dependent dysfunction.67 Vasospastic angina is associated with major adverse events including sudden cardiac death, acute MI, and syncope which may unfortunately occur before the diagnosis is established.76,77,78
Should the possibility of non-obstructive causes of ischaemia not be considered by the treating physician, a coronary angiogram showing no obstructive disease may be followed by incorrect interpretation of the patient’s symptoms, avoidance of further diagnostic evaluation, and lack of adequate treatment. Indeed, coronary angiography in INOCA showing non-obstructive coronary arteries may result in inappropriate discontinuation of medical therapy, paradoxical reassurance by the treating physician and potentially, the physician may even refute the underlying symptoms. This approach is not patient-centred, as many will continue to have symptoms that will lead to rehospitalization, repeated diagnostic testing and inappropriate treatment.
Diagnosis
NON-INVASIVE METHODS TO DETECT ISCHAEMIA
Functional or structural abnormalities of the coronary microcirculation can be responsible for impairment of myocardial perfusion and ischaemia, even in the absence of large epicardial coronary arteries stenosis.13,14,79 Common non-invasive techniques assessing ischaemia rely on detection of relatively large regional differences in left ventricular perfusion and/or wall motion in epicardial perfusion territories (i.e. myocardial single-photon emission computed tomography or dobutamine stress echocardiography). These techniques are ineffective if ischaemia affects the whole left ventricle as in patients with CMD.80,81 Currently, no technique allows a direct anatomical visualization of the coronary microcirculation in vivo in humans. Therefore, its assessment relies on the measurement of parameters which reflect its functional status, such as myocardial blood flow and CFR.
Coronary flow reserve is the ratio of hyperaemic blood flow in response to various vasoactive stimuli divided by resting blood flow. Coronary flow reserve is an integrated measure of flow through both the large epicardial arteries and the coronary microcirculation, but once severe obstructive disease of the epicardial arteries is ruled out, reduced CFR is a marker of CMD. The maximal vasodilatation and hyperaemia necessary to calculate the CFR is usually achieved through intravenous administration of endothelium-independent vasodilators such as adenosine, or regadenoson.21
In the diagnostic pathway for patients assessed for angina recommended in the ESC CCS 2019 guideline,2 first line of testing is non-invasive. In patients with no obstructive CAD on their coronary computed tomographic angiography and/or no regional reversible ischaemia on functional testing, CMD or VSA may be the cause of their symptoms and in patients with a significant burden of disease, further testing through non-invasive and invasive techniques should be considered. While non-endothelial dependent dysfunction may be assessed non-invasively, acetylcholine can only be administered during invasive testing. Thus, a full diagnostic assessment for INOCA currently requires invasive angiography. Several non-invasive techniques allow assessment of CFR (Figure 3, Supplementary material online, Table S3).
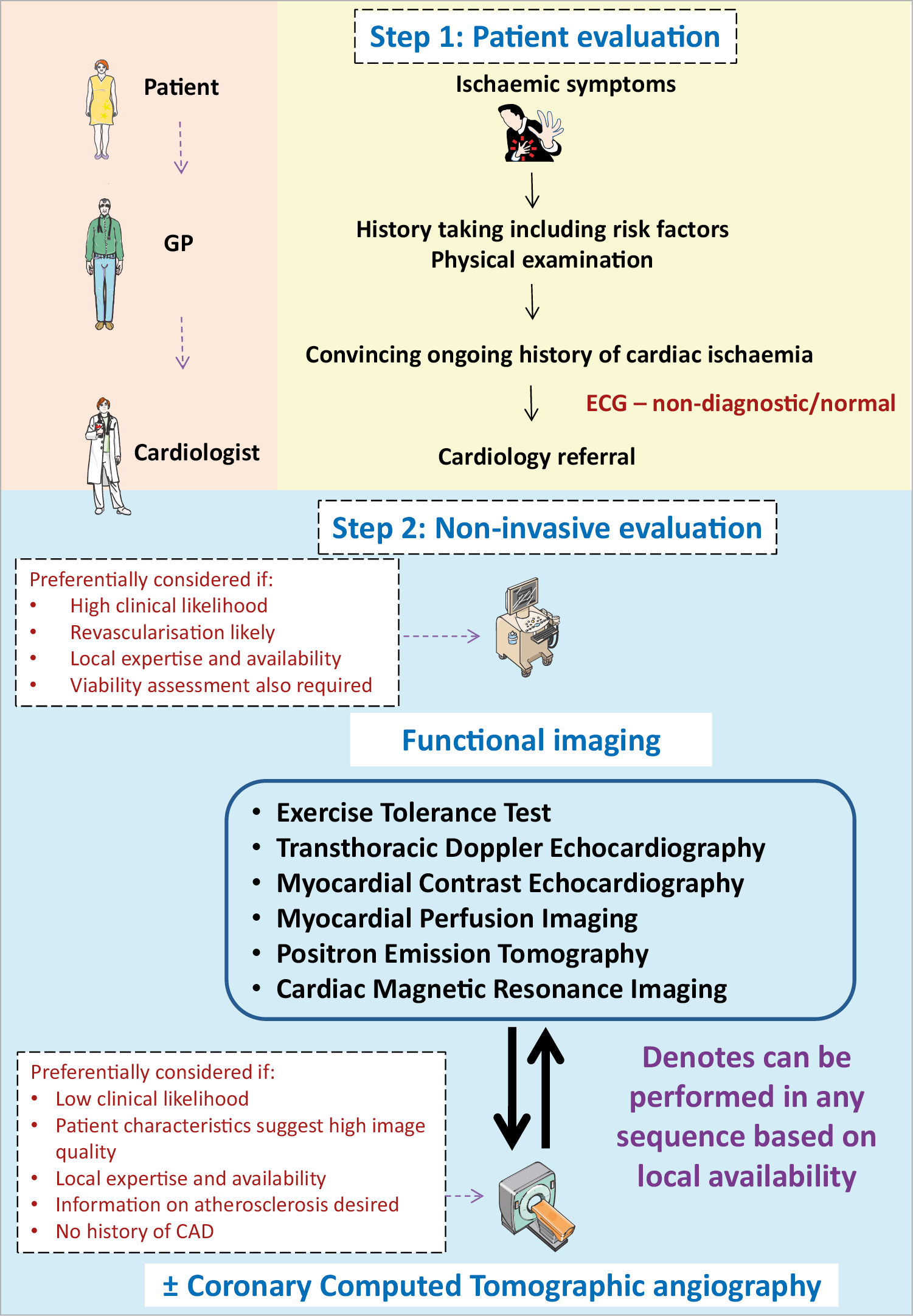
Figure 3. Non-invasive evaluation of INOCA. GP: general practitioner
INVASIVE DIAGNOSIS IN THE CATHETERIZATION LABORATORY
The 2019 ESC CCS guidelines2 have given a IIa recommendation (‘should be considered’) for guidewire-based measurement of CFR and/or microcirculatory resistance measurements in patients with persistent symptoms, but coronary arteries that are either angiographically normal or have moderate stenoses with non-flow-limiting disease. Intracoronary acetylcholine (ACH) testing is supported by a IIb recommendation ‘may be considered’ to assess coronary microvascular spasm and for patients in whom VSA is considered, a IIa recommendation to clarify both endothelium-dependent as well as endothelium-independent pathobiological mechanisms of CMD.
Diagnostic testing provides information on coronary vascular dysfunction, including a functional disorder, i.e. impaired vasodilatation, or vasospasm, and/or structural problem, i.e. an increase in minimal vascular resistance. Relevant endotypes include (i) MVA, (ii) VSA, (iii) both, (iv) none, i.e. non-cardiac chest pain, and (v) non-flow-limiting CAD, e.g. diffuse atherosclerosis, <50% stenosis severity by visual assessment. A clinical diagnosis may be according to expert consensus criteria.15 The diagnostic criteria are shown in Table 2. Catheter-based measurements of absolute coronary blood flow and microvascular resistance have also been previously described which requires further evaluation in INOCA patients.82
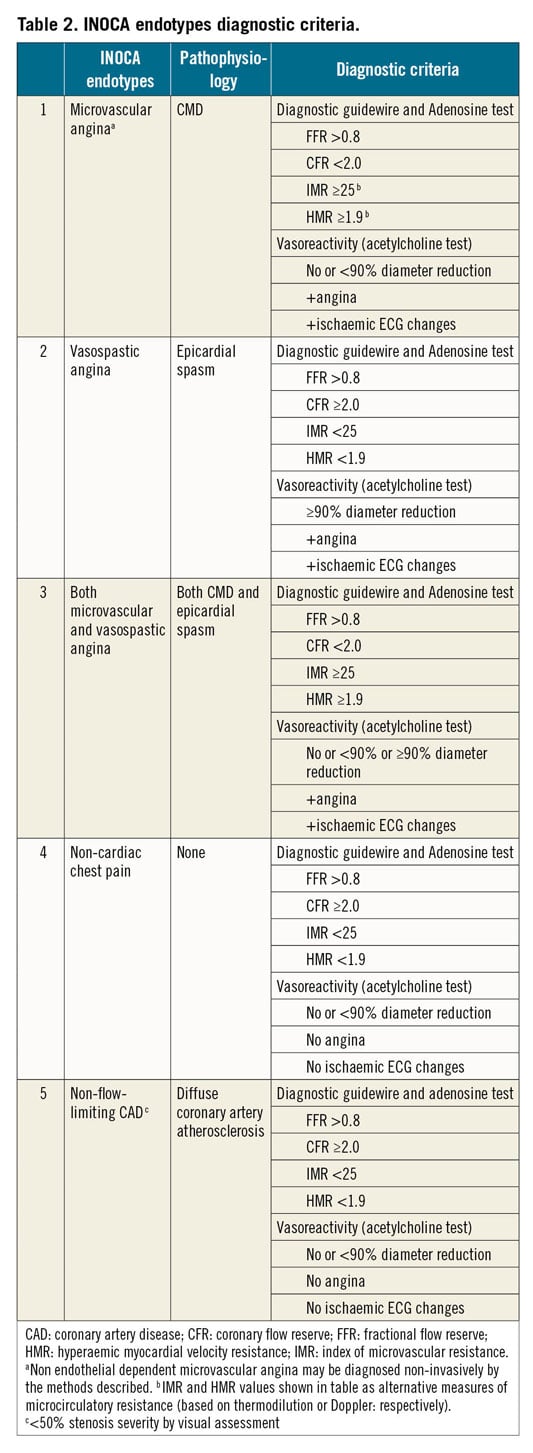
CORONARY ANGIOGRAPHY
Glyceryl trinitrate (GTN) has a short half-life and is preferred during coronary angiography. A corrected thrombolysis in myocardial infarction frame count >27 (images acquired at 30 frames/s)83 in the presence of GTN suggests MVA due to impaired resting flow (coronary slow-flow phenomenon).15 Slow-flow points to an increase in vascular resistance under resting conditions.
INVASIVE FUNCTIONAL CORONARY ANGIOGRAPHY
Invasive functional coronary angiography (FCA) is a combinatory technique involving direct invasive measurements of coronary vasomotor function initially with a diagnostic guidewire in combination with pharmacological reactivity testing (Figure 4).84 Different approaches may slightly vary according to local experience and preference.55,84,85,86,87
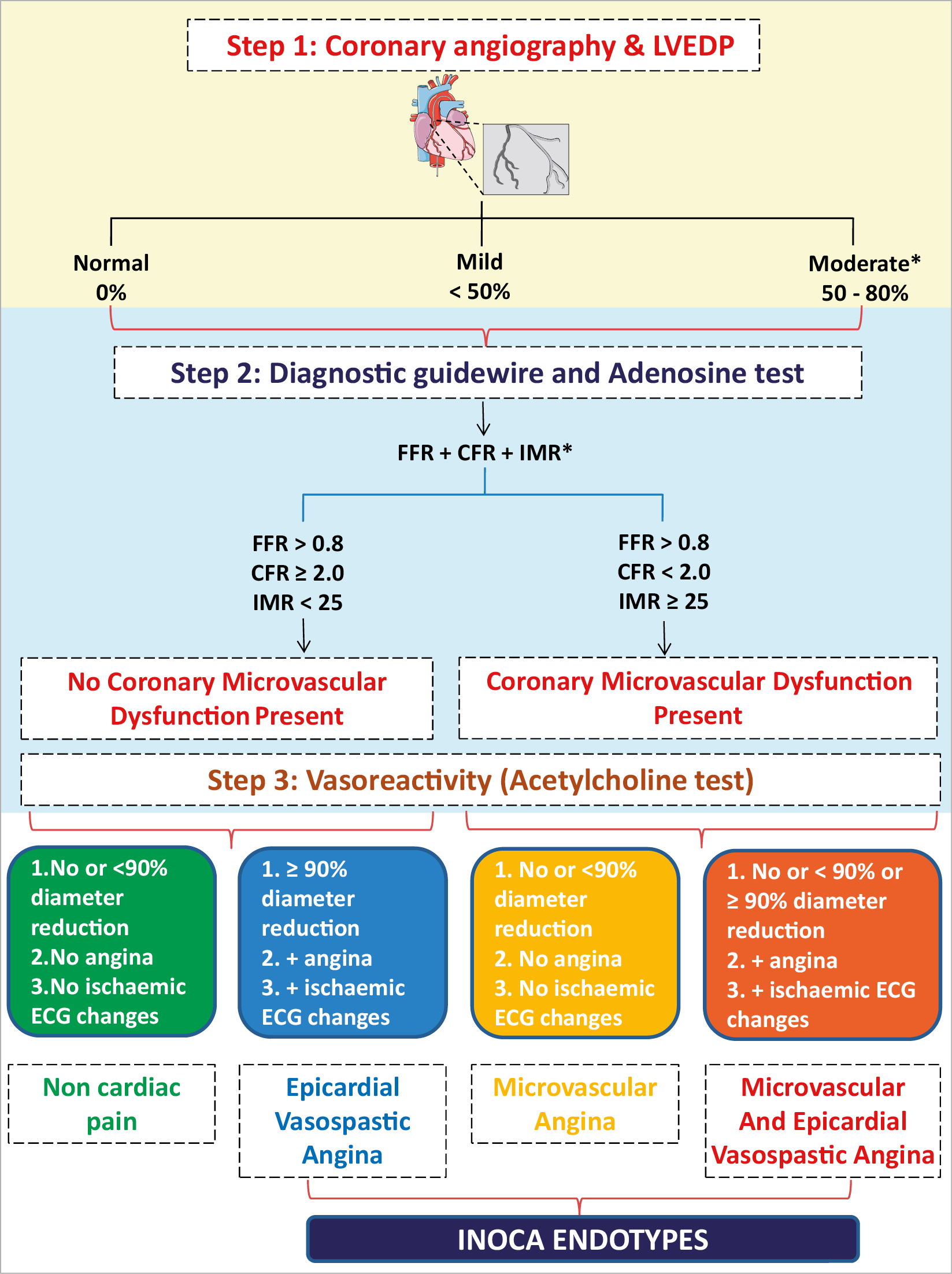
Figure 4. Invasive evaluation of INOCA. CFR: coronary flow reserve; FCA: functional coronary angiography; FFR: fractional flow reserve; IMR: index of microvascular resistance; LVEDP: left ventricular end-diastolic pressure. aAnd negative non-invasive or invasive testing for epicardial ischaemia. bCombo wire is an alternative option to measure FFR, CFR and IMR.
DIAGNOSTIC GUIDEWIRE
Coronary function testing using a diagnostic guidewire is performed as an adjunct to coronary angiography. The left anterior descending coronary artery is usually preferred as the pre-specified target vessel reflecting its subtended myocardial mass and coronary dominance. Additional studies in other coronary arteries may be appropriate if the initial tests are negative and clinical suspicion is high. Intravenous heparin (50-70 U/kg) should be administered to achieve therapeutic anticoagulation (activated clotting time ~250 s). Diagnostic options include coronary thermodilution using a pressure–temperature sensor guidewire (PressureWire X™, Abbott Vascular, Santa Clara, CA, USA) or a Doppler technique (ComboWire XT or Flowire, Philips Volcano Corporation, San Diego, CA, USA). The ComboWire XT connects to the ComboMap system (Philips, Eindhoven). The usual approach to inducing steady-state hyperaemia is by use of intravenous adenosine (140 µg/kg/min) to achieve endothelium-independent vasodilation.88 Intracoronary bolus injection of adenosine (up to 200 µg) is an alternative option to assess endothelium-independent vasodilatation.
Coronary flow reserve can be calculated using thermodilution (as resting mean transit time divided by hyperaemic mean transit time)89,90 or Doppler flow velocity (hyperaemic flow velocity divided by resting flow velocity).91 Overall, most studies demonstrating the prognostic value of thermodilution-based CFR have used a cut-off value of 2.0,92,93 while studies showing a prognostic impact of CFR based on Doppler have used a CFR cut-off of 2.5 or lower.27,94,95
Microcirculatory resistance can be calculated by combining pressure and flow measurements (either thermodilution- or Doppler-based). The index of microvascular resistance (IMR) is calculated as the product of distal coronary pressure at maximal hyperaemia multiplied by the hyperaemic mean transit time.96 Increased IMR (≥25) is representative of microvascular dysfunction.97 The hyperaemic myocardial velocity resistance (HMR) index is a Doppler-based index, calculated by dividing intracoronary pressure by hyperaemic flow velocity. In a previous study of patients with angina and non-obstructed coronary arteries, HMR>1.9 [odds ratio: 15.6 (95% confidence interval 2.1-114.0), P=0.007] was an independent predictor of recurrent chest pain.98 Other studies have suggested that a cut-off of ≥2.5 mmHg/cm/s provides the optimal sensitivity and specificity for predicting CMD, as judged with PET.99 Further studies are required to determine the optimal HMR index that would predict CMD.
Flow-limiting obstructive CAD may be assessed using FFR which is the ratio of mean distal coronary pressure to mean aortic pressure at maximal hyperaemia – abnormal FFR is defined as ≤0.80100 or a non-hyperaemic pressure ratio ≤0.89.100,101,102 The binary thresholds of continuous data should be viewed within the context of the patient. Coronary flow reserve, IMR, and FFR have prognostic significance across the diagnostic range of their values. Thus, in this invasive evaluation it is possible to determine endothelium-independent CMD (CFR, IMR); endothelium-dependent CMD (microvascular response to ACH) and vasospastic response (epicardial artery response to ACH) as well as an assessment of low-grade stenoses (FFR).
PHARMACOLOGICAL INVASIVE FUNCTIONAL CORONARY ANGIOGRAPHY
The most established approach for vasoreactivity testing is by intracoronary infusion of acetylcholine,55,84,85,86,87,103,104,105,106,107,108 which influences coronary vascular tone via muscarinic receptors on endothelial and vascular smooth muscle cells. The use of intracoronary acetylcholine for the diagnosis of MVA and VSA is recommended by the 2019 ESC CCS clinical practice guidelines2 on the grounds of its demonstrated safety and efficacy.109 A pragmatic approach for FCA according to whichever protocol works best in individual centres might be implemented. A standard approach involves sequential infusion of acetylcholine at concentrations approximating 10–6, 10–5, and 10–4 mol/L, respectively (Supplementary material online, Table S4). A clinical diagnosis to rule-in or rule-out MVA and/or VSA due to vasospasm is made according to established criteria.15,55 Figure 4 shows the steps in the invasive evaluation of INOCA. Based on current practice, Steps 1, 2, 3 as shown in Figure 4 are suggested though some institutions might prefer Steps 1, 3, 2 in the invasive evaluation of INOCA. Further studies are warranted to determine the best sequence of invasive evaluation in the diagnosis of INOCA. The complications and risks of invasive coronary procedures are previously well described.110,111The potential risk of the invasive assessment should be weighed against the benefit of the diagnosis for the patient, acknowledging that so far it has not been studied whether management based on information gathered by invasive diagnostics may influence prognosis while only one pilot trial (CorMicA) has found a benefit in terms of symptoms.
Management of INOCA
Management should be patient-centred with a multidisciplinary care approach might be helpful to the patient. Unfortunately, studies on therapy to improve CMD are small and heterogeneous in design and methodology and currently there is no evidence-based treatment of CMD.112 There is a strong need for well-designed clinical trials to guide future research and clinical recommendations. Figure 5 provides an algorithm for the management of INOCA.
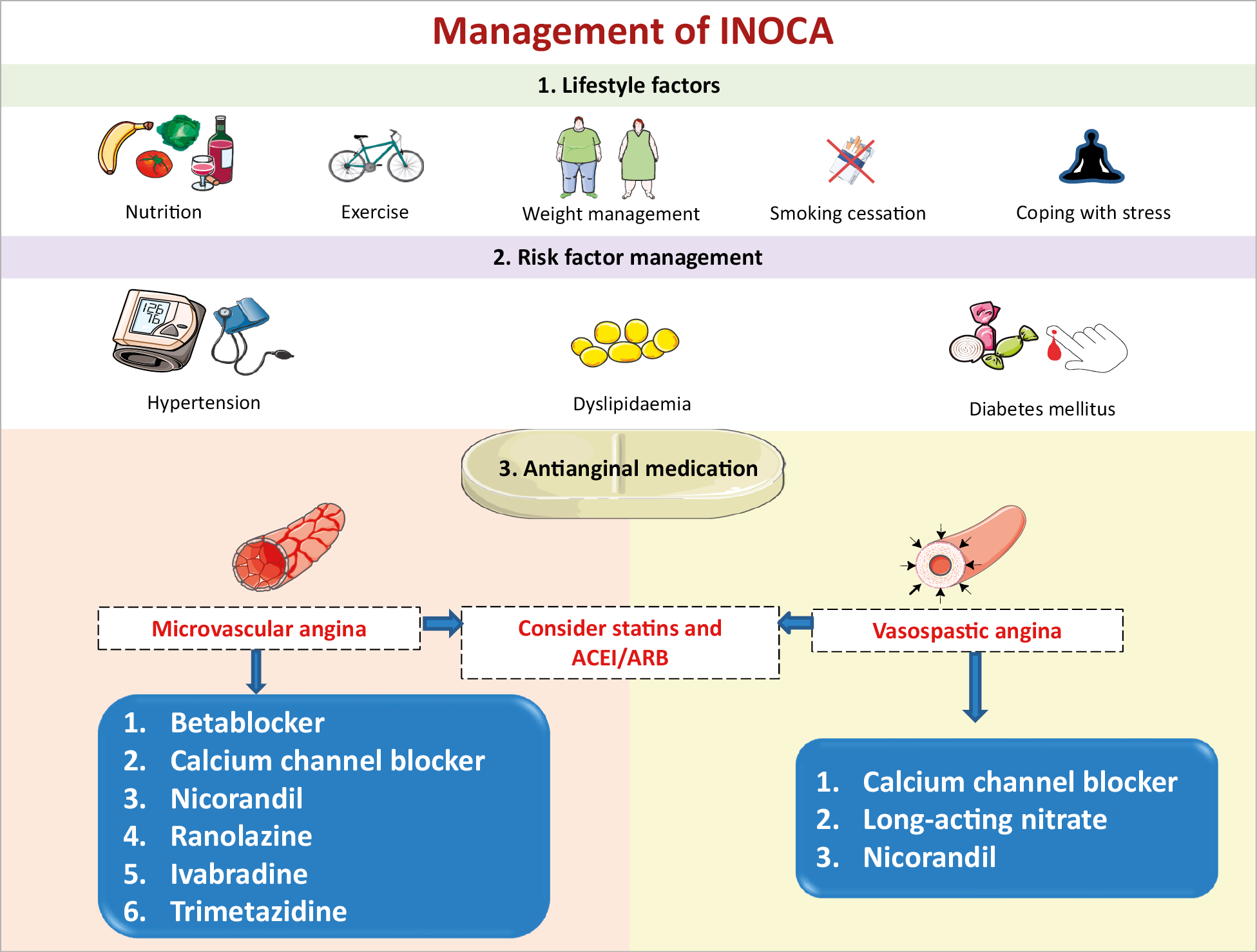
Figure 5. Management of INOCA. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
LIFESTYLE FACTORS
In all patients with established INOCA due to the frequent presence of coronary atherosclerosis and endothelial dysfunction,12,113 tailored counselling on lifestyle factors is warranted to address risk factors, reduce symptoms and improve quality of life and prognosis. Behavioural interventions can be supported by nurse practitioners, experts in nutrition, psychologists, exercise physiotherapists, sports medicine, and so on. Adequate lifestyle support is comparable to other cardiovascular disease (CVD) prevention guidelines and preventive strategies in patients with stable CAD.59,114 The ability of specific diets, such as anti-inflammatory, vegan, or Mediterranean, to improve symptomatic coronary vascular dysfunction is unknown. However, obesity should be addressed. Coping with stress, the chronic and recurrent nature of symptoms may need extra attention, as they may have an important impact on working abilities in this often relatively young patient group.
RISK FACTOR MANAGEMENT
The traditional CVD risk factors hypertension, dyslipidaemia, smoking, and diabetes may all contribute to the pathology of coronary microvascular and vasospastic dysfunction and structural remodelling of the circulation. The main therapeutic objective of strict control of BP is to prevent progression of microvascular changes and to reduce the frequency and intensity of anginal symptoms.115 Best choice of (combined) BP medications depends on the predominant mechanism of anginal symptoms, e.g. vasospastic and/or MVA. The use of angiotensin-converting enzyme inhibitors (ACEis) improves CFR in CMD116 and ACEi/angiotensin receptor blockade (ARB) can be easily combined with both calcium antagonists and beta-blockers.59,108,117,118 Statins are beneficial in patients with non-obstructive CAD, and their anti-inflammatory properties may also be effective in those patients with reduced CFR and vascular spasm.119,120,121
ANTIANGINAL MEDICATION
Treatment of anginal symptoms in patients with INOCA is challenging as the patients represent a heterogeneous group and randomized trials are lacking. Standard pharmacological anti-ischaemic treatment often achieves disappointing results.122 The efficacy of short-acting nitrates may vary and often needs to be repeated. Long-acting nitrates are frequently ineffective, poorly tolerated and may aggravate symptoms in patients with MVA due to a stealing effect.59,123 In patients with evidence of either epicardial or microvascular spasm following acetylcholine testing, calcium antagonists should be considered as first-line therapy. In patients with severe VSA it may be needed to give unusual high dosages of calcium antagonist (2×200 mg diltiazem daily), or even a combination of hydropyridine (such as diltiazem) with dihydropyridine calcium blockers (such as amlodipine), Table 3. Inpatients with MVA and reduced CFR and/or increased IMR (that may reflect arteriolar remodelling) beta-blockers, calcium channel blockers, and ACEi are used.124 ACEi have been demonstrated to improve hyperaemic myocardial blood flow in hypertensive MVA patients,125 and in women with CMD with improved CFR and angina frequency.116 In the CorMicA trial, a stratification based medical therapy was used, taking into account the measurements at coronary testing and the approach was shown to improve angina control and quality of life in patients with no obstructive CAD at 6 months and at 1 year.84,126
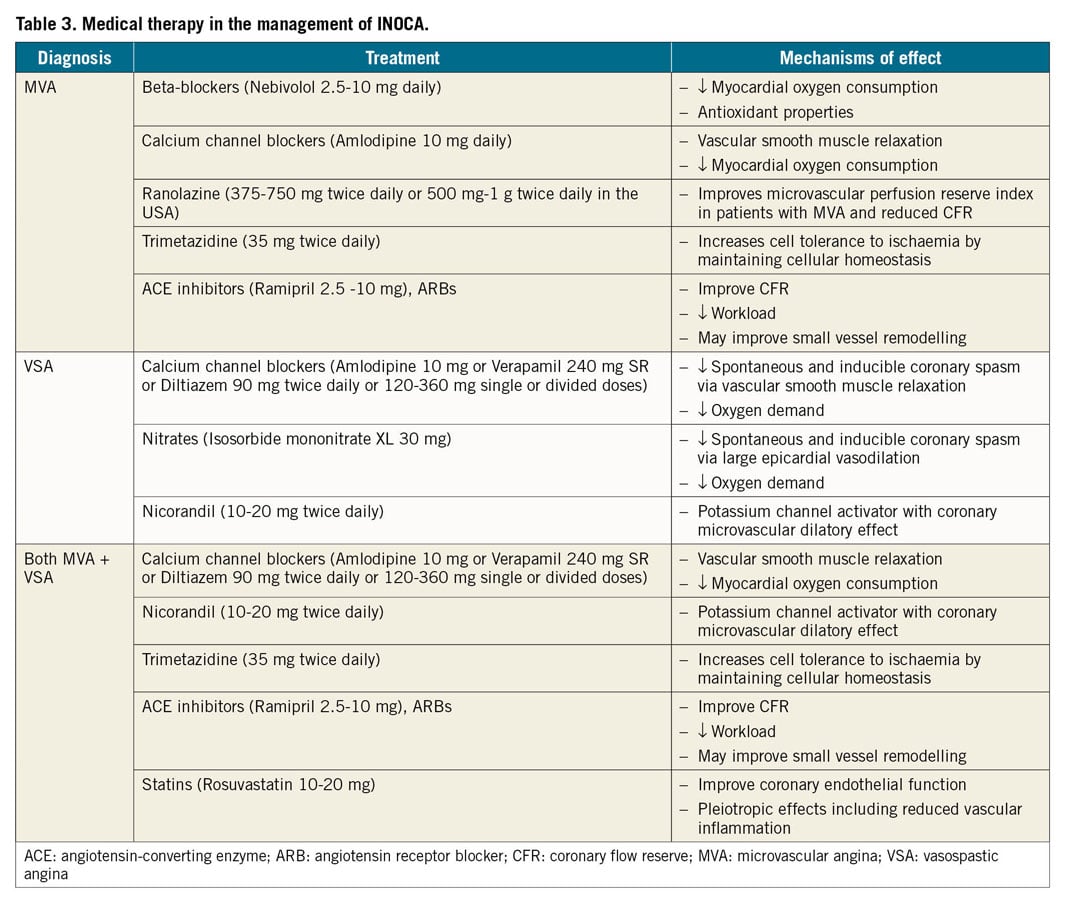
In perimenopausal women without obstructive CAD, a combined regimen of a low-dose alpha beta-blocker or selective beta-blocker (nebivolol, bisoprolol) and calcium antagonist (diltiazem) can be highly effective in reducing anginal symptoms, as the loss of oestrogens often induces autonomic dysfunction with a fast rise in heart rate during exercise.127
The use of nicorandil, a combinatorial vasodilator agent acting via nitrate and potassium channel activation, may be an effective alternative although side effects are often reported.128 First-line therapy can also be combined with the use of ranolazine, an anti-anginal agent which improves myocyte relaxation and ventricular compliance by decreasing sodium and calcium overload.129 In patients with MVA mixed beneficial results of ranolazine have been published, demonstrating benefit in patients with low CFR.130,131 Some patients with persistent anginal symptoms may benefit from the use of ivabradine, which decreases heart rate both at rest and during exercise without affecting left ventricular contractility. However, its efficacy in MVA is poorly investigated and still controversial.132,133 Rho kinase inhibitors reduce contractility in the vascular wall and are currently under investigation for reducing coronary vasoreactivity.134 The use of low-dose tricyclic antidepressants, such as imipramine, may be helpful to reduce the intensity of symptoms.108,117,118 However, it should be noted that there is currently no evidence-based medication for INOCA and aggravated nociception.112 Therefore we recommend antianginals as currently stipulated in the updated 2019 ESC CCS guidelines which provides a stepwise strategy for antianginal drug therapy. The CCS guidelines also recommend trimetazidine as a second-line drug in patients with CCS whose symptoms are not adequately controlled by, or who are intolerant to, other medicines for angina pectoris.2 In about 25% of patients, symptoms are refractory to these treatment options. Enhanced external counterpulsation might be used as an adjunctive treatment for INOCA only in CCS patients who are refractory to both traditional antianginal drugs (beta blockers, calcium channel blockers, nitrates, etc.) as well as more novel interventions such as ranolazine, trimetazidine, and ivabradine.135
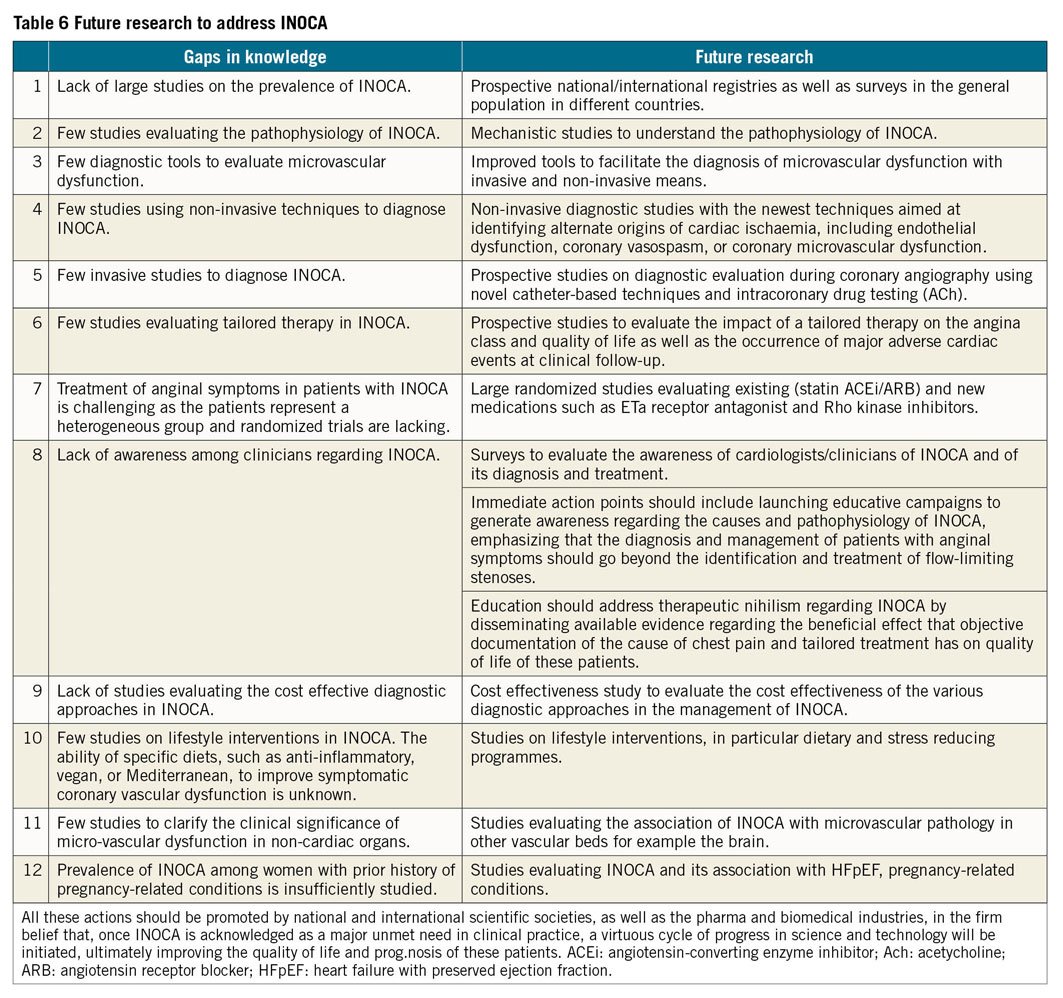
Gaps in knowledge and future studies
The key messages are shown in Table 4 and Visual summary and Figure 6. It is evident that INOCA is not often correctly diagnosed and that, as a consequence, no tailored therapy is prescribed for these patients who are often dismissed as ‘false positive’. Consequently, these patients will continue to experience recurrent angina with poor quality of life, leading to repeated hospitalizations and unnecessary coronary angiography,21,136 as well as poor clinical outcome. There is an urgent need of large studies designed to address this problem as shown in Tables 5 and 6. The CorCTCA trial (NCT03477890) is ongoing and will help clarify the prevalence and clinical significance of INOCA when standard care is based on coronary computed tomography angiography.137 To date, there are no disease-modifying therapies specific to INOCA. The Women’s IschemiA Trial to Reduce Events in Non-ObstRuctIve CORonary Artery Disease is currently enrolling subjects (WARRIOR: NCT03417388) in a multicentre, prospective, randomized blinded outcome evaluation, to evaluate intensive statin and ACEI/ARB therapy (IMT) and usual care (UC) on major adverse cardiovascular events in symptomatic women with INOCA. The Precision Medicine With Zibotentan in Microvascular Angina (PRIZE) trial holds future promise (ClinicalTrials.gov Identifier: NCT04097314). Zibotentan is an oral, endothelin A receptor antagonist that may provide benefit by opposing the reported increase in vasoconstrictor response of coronary microvessels to endothelin.53
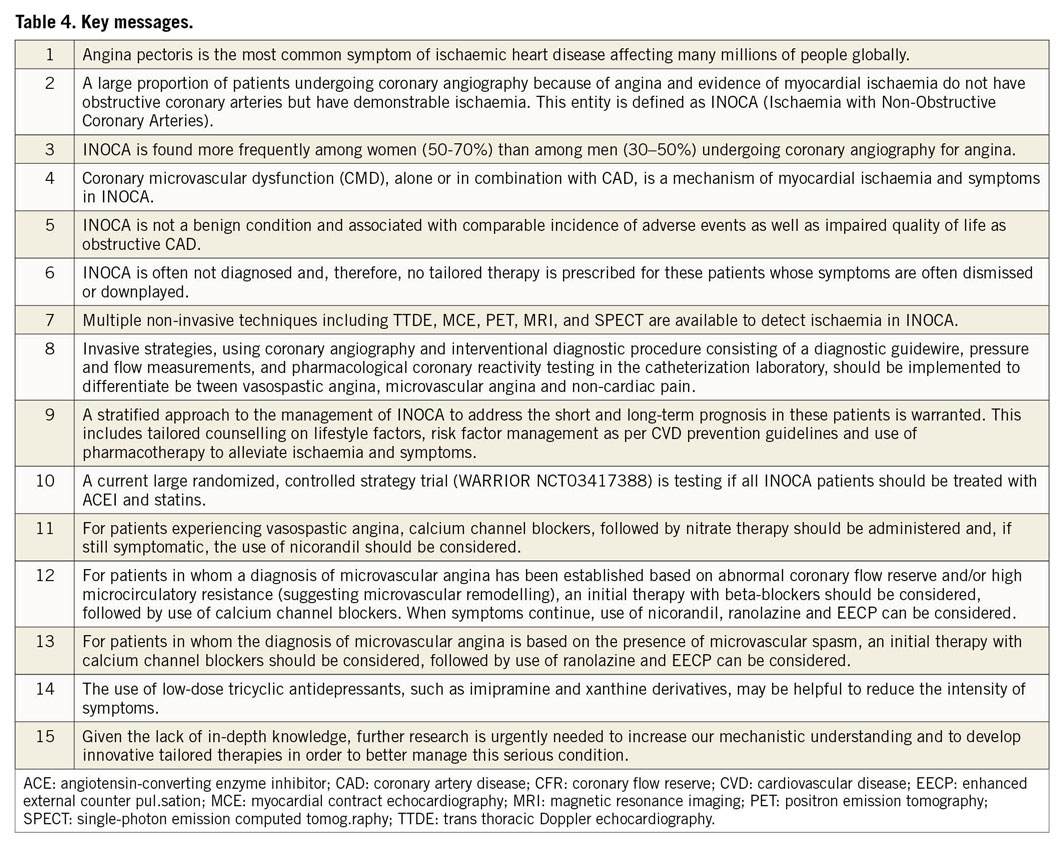
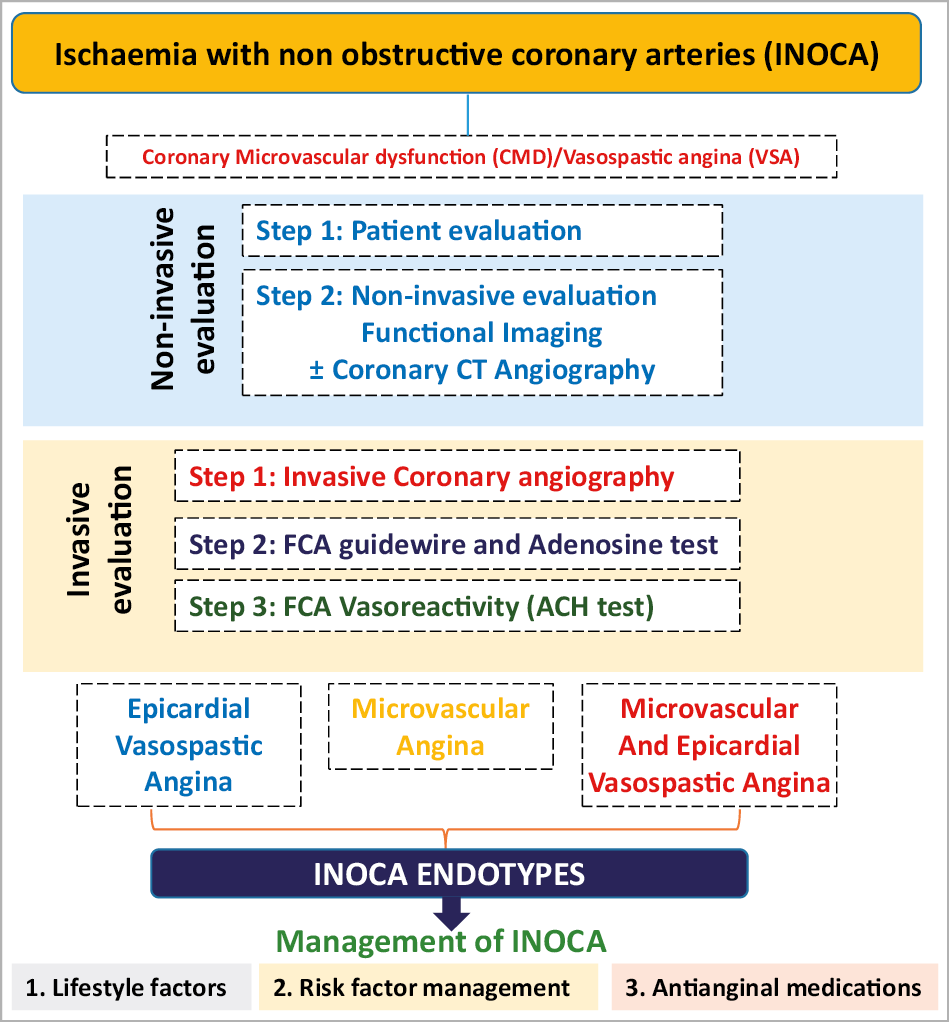
Visual summary.
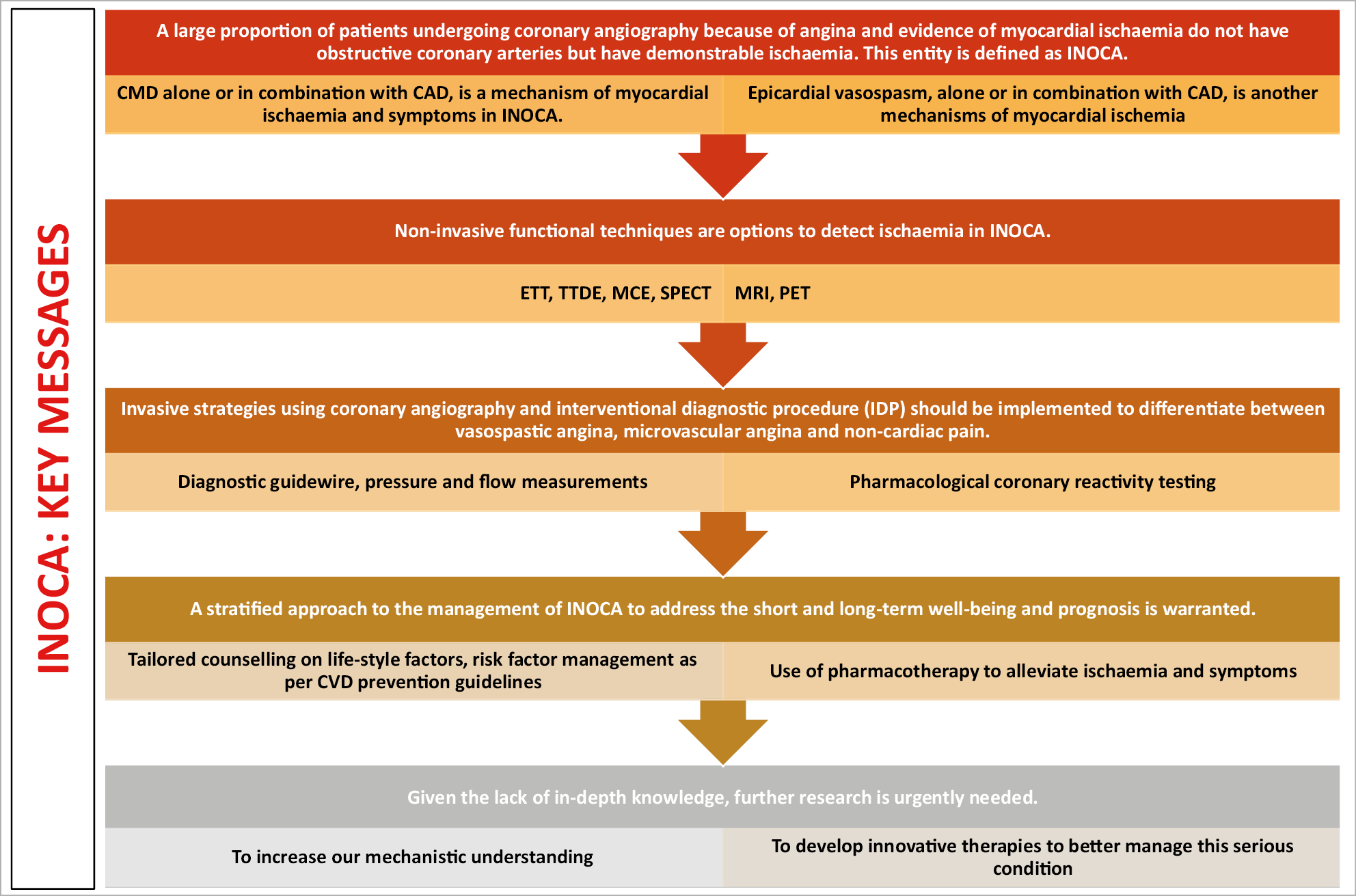
Figure 6. Key messages.

Conclusions
INOCA, a major health problem, is associated with under-diagnosis, under-treatment and poor prognosis. This consensus document provides the treating clinician/interventional cardiologist guidance regarding the recommended diagnostic/investigational approach and the management of INOCA based on the existing evidence and the best available current practice. Future prospective well-designed ongoing research is required to address a number of unanswered questions in the diagnosis and management of these patients.
Acknowledgements
COVADIS Steering Committee: C. Noel Bairey Merz (USA); John Beltrame (AU); Colin Berry (UK); Paolo Camici (IT); Filippo Crea (IT); Juan Carlos Kaski (UK); Peter Ong (DE); Carl Pepine (US); Udo Sechtem (DE); Hiroaki Shimokawa (JP). Dr Phyo Khaing NIHR Academic Clinical Fellow in Cardiology Newcastle University, UK and Dr Novalia Sidik, BHF Clinical PhD Fellow University of Glasgow, UK for their contribution to the Figures in this document. The EAPCI INOCA consensus document was proposed by the EAPCI Women’s Committee and its members. Marielle de la Torre and Marion Diebold from the ESC/EAPCI office for their valuable help and support in the co-ordination of the writing committee.
Conflict of interest
Vijay Kunadian reports other from Bayer, other from Amgen, other from Abbott, other from Astra Zeneca, other from Daiichi Sankyo, outside the submitted work; and Vijay Kunadian is supported by an external research grant from Astra Zeneca (funder reference number ISSBRIL0303). Vijay Kunadian is also supported/funded by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Vijay Kunadian also supported by the British Heart Foundation Clinical Study Grant CS/15/7/31679 for the British Heart Foundation older patients with non-ST SEgmeNt elevatIOn myocaRdial infarction Randomised Interventional TreAtment Trial. Alaide Chieffo reports personal fees from Abiomed, personal fees from Biosensor, personal fees from Abbott, personal fees from Cardinal Health, personal fees from Magenta, outside the submitted work. Paolo G. Camici reports personal fees from Servier, during the conduct of the study. Colin Berry reports grants, non-financial support and other from Abbott Vascular, grants, non-financial support and other from AstraZeneca, non-financial support from Boehringer Ingelheim, grants and non-financial support from GSK, grants, non-financial support and other from HeartFlow, non-financial support and other from Opsens, grants, non-financial support and other from Novartis, non-financial support from Siemens Healthcare, outside the submitted work; and Colin Berry acknowledges research support from the British Heart Foundation (PG/17/ 2532884; FS/17/26/32744; RE/18/6134217) and Medical Research Council (MR/S005714/1). Javier Escaned reports personal fees from Abbott, personal fees from Philips, outside the submitted work. Angela H.E.M. Maas has nothing to disclose. Eva Prescott has nothing to disclose. Nicole Karam has nothing to disclose. Yolande Appelman has nothing to disclose. Chiara Fraccaro has nothing to disclose. Gill Louise Buchanan reports grants and personal fees from Bayer, grants and personal fees from Pfizer, grants and personal fees from Daichii-Sanyo, grants from Menarini, outside the submitted work. Stephane Manzo-Silberman has nothing to disclose. Rasha Al-Lamee reports other from Philips Volcano, other from Menarini, outside the submitted work. Evelyn Regar has nothing to dis-close. Alexandra Lansky has nothing to disclose. J. Dawn Abbott has nothing to disclose. Lina Badimon reports grants from AstraZeneca, other from Sanofi, grants from A-Biotics, other from Lilly, other from Astra-Zeneca, other from Research Forum on Beer and Lyfestyle, other from Research Forum on Beer and Lyfestyle, other from Pfizer, outside the submitted work. Dirk J. Duncker reports grants from Dutch Heart Foundation, outside the submitted work. Roxana Mehran reports grants from Abbott Laboratories, grants from AstraZeneca, grants from Bayer, grants from Beth Israel Deaconess, grants from BMS, grants from CSL Behring, grants from DSI, grants from Medtronic, grants from Novartis Pharmaceuticals, grants from OrbusNeich, personal fees from Abbott Laboratories, other from Abbott Laboratories, other from Abiomed, other from The Medicines Company, personal fees from Boston Scientific, personal fees from Medscape/WebMD, personal fees from Siemens Medical Solutions, personal fees from PLx Opco Inc/dba PLx Pharma Inc, non-financial support and other from Regeneron Pharmaceuticals, personal fees from Roivant Sciences, other from Spectranetics/Philips/Volcano Corp, personal fees from Sanofi, personal fees from Medtelligence (Janssen Scientific Affairs), personal fees from Janssen Scientific Affairs, other from Bristol Myers Squibb, other from Watermark Research Partners, other from Claret Medical, other from Elixir Medical, outside the submitted work. Davide Capodanno has nothing to disclose. Andreas Baumbach has nothing to disclose.
Supplementary data
To read the full content of this article, please download the PDF.

