Abstract
Umbilical cord blood (UCB) cells may offer a promising, off-the-shelf, therapy for the treatment of myocardial infarction. In this review, the current use of UCB as an alternative to bone marrow transplantation is described. In addition, preclinical studies using umbilical cord blood derived cells for cardiac regeneration are discussed including our own experience in a porcine model of reperfused myocardial infarction.
Introduction
Myocardial infarction (MI) is a major cause of heart failure, and thus forms one of the main targets for cardiac regeneration by cell transplantation. The treatment of MI with stem cell therapy seems promising, however the cellular source with the highest potential for cardiac regeneration remains unclear. To optimally treat MI patients with stem cell therapy, a widely available source of stem cells would be of great use1. Many candidates have been proposed in clinical and experimental cell transplantation, e.g. bone marrow mononuclear cells, mesenchymal adult progenitor cells, cardiac progenitor cells and skeletal myoblasts1. Umbilical cord blood (UCB) derived cells have the advantage of being easy to obtain in large numbers, which is especially important for the sick and elderly population because they may have impaired stem cell numbers and their cells may have a decreased capacity for proliferation and differentiation2. Together with their presumed hypo-immunogenic properties, UCB holds great potential to facilitate cardiac regeneration after myocardial infarction.
UCB in routine clinical use
Allogeneic haematopoetic stem cell transplantation has been firmly established as an important treatment modality for advanced haematological diseases. However, allogeneic transplantation has been limited by the availability of suitable related and unrelated donors. Haematopoietic progenitor cells can be harvested from the donor bone marrow, from mobilised peripheral blood, and such progenitors can also be found in UCB. Although the first transplantation was reported more than a decade ago, UCB is still considered a relatively novel source of haematopoetic progenitor cells. The first clinical application of UCB was reported in 1972 when American scientists transfused foetal cord blood cells into a 16-year old boy with acute lymphoblastic anaemia. He received UCB units from 8 different donors from which one unit engrafted successfully and lasted 38 hours3. The first successful transplantation had to wait until 1989 in the treatment of a child with Fanconi’s anemia4. Since then, regeneration of haematopoiesis by UCB is considered as a valuable alternative source for bone marrow derived stem cells, especially in paediatric patients. The relatively low number of progenitor cells in UCB has long limited the use mainly to paediatric patients, but the development of UCB transplantation in adults with haematological disorders is now rapidly evolving. Recent comparative studies have suggested that outcome after UCB transplantation compares well to outcome after volunteer unrelated donor transplantation5,6. However, major challenges of UCB transplantation in adults still include better approaches to improve engraftment, reduce the incidence of graft versus host disease and improve immune reconstitution7. Apart from the presence of haematopoetic progenitor cells, UCB may contain other progenitor cells, including mesenchymal, endothelial stem cells, and neuronal precursor cells. The combination of haematopoetic and mesenchymal stem cells as well as endothelial progenitors and the presumed hypo-immunogenicity of UCB sparks hope for the future of stem cell mediated tissue regeneration. To date clinical trials on the use of UCB in non haematopoetic disorders are scarce, but experimental data hold promises for the future. In neurology, UCB have been identified as candidates for tissue regeneration in a range of neurodegenerative disorders8. In comparison, in the field of cardiology, recent evidence for possible tissue regeneration in vitro of several cardiac cell types like heart valves9,10, blood vessels9 and cardiomyocytes11 have lead to an increased interest in UCB.
Studies in animal models
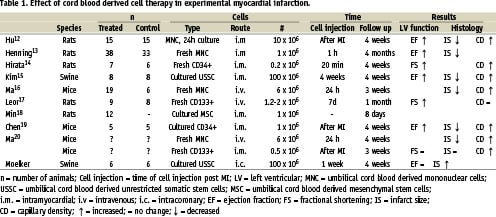
In the last three years, nine preclinical studies12-20 have been published in which UCB derived cells were studied in the heart (Table 1). All but one18 evaluated the effect of UCB derived cell injection in a MI model. MI was induced by a permanent occlusion of the left anterior descending coronary artery12-17,19,20 or by cryoinfarction20. Cells were administered intramyocardially12-15,18-20 or intravenously16,17,20. Table 1 shows that studies used different derived umbilical cord blood cells and injected different numbers of cells at various time points. The variety of experimental models can explain the equivocal effects of cell therapy after MI. In only two studies15,17, a baseline measurement of left ventricular (LV) function after MI, but before stem cell injection, was performed. In previous studies in mice and rats, a significant decrease in infarct size was observed in cell treated animals12,13,16,19,20. Furthermore, a significant improvement in ejection fraction12,13,19 or fractional shortening14,17, which was measured with echo, was observed in UCB derived cell treatment in rodents in all but one study20. Only one study14 reported a positive effect of UCB on LV remodelling. In several studies, histology showed a higher capillary density12,14,16,19,20 in cell treated animals compared to controls, however this finding was not observed by Leor et al17.
In our laboratory MI was induced in 12 swine by proximal PTCA balloon occlusion of the left circumflex coronary artery for two hours followed by reperfusion21. Five additional swine were used as healthy controls to assess normal cardiac growth and function. One week after MI, a baseline MRI was performed on all swine to assess LV function and infarct size. The next day, six of the MI swine received an intracoronary injection of ~100x106 umbilical cord blood derived cultured unrestricted human somatic stem cells (USSC) in 10 mL unconditioned culture medium. The other six MI swine received 10 mL medium intracoronary. Cells or medium were injected slowly (1 mL/min) in the infarct related artery. Four weeks later all animals underwent a second MRI to assess the effect of USSC treatment. The pigs were sacrificed afterwards and the hearts were examined by histology. All swine received 8 mg/Kg/day cyclosporine A, starting one week after MI.
Four weeks after USSC injection in this porcine model of reperfused MI, we could not detect a beneficial effect of USSC treatment on global or regional LV function. Furthermore, there was no reduction observed in infarct size as measured with delayed enhancement MRI compared to medium only treated animals. Infarct size actually increased slightly after USSC injection. Histology showed a transmural infarct in all MI-swine, containing abundant collagen and fibrous tissue. In USSC treated swine an increased number of calcifications and inflammatory cells (CD3+ and CD45+) were detected. Immunohistochemistry showed that four weeks after injection USSC had not transdifferentiated into a cardiac or vascular phenotype. However, some USSC had become CD45 positive.
The different results between the preclinical studies predominantly performed in small rodents and in our own laboratory, can be explained by the differences between the experimental models (Table 1). Differences in heart size, heart rate and haemodynamics between large mammals, such as the pig and rodents, could account for the differences found in cell therapy. We were the only study which created an infarct model with reperfusion, which closely matches clinical settings, but creates a very different environment for the injected cells. Furthermore, we injected cells intracoronary instead of intramyocardial or intravenously. The different infarct model and method of cell delivery we used could account for the differences observed after UCB cell therapy.
There are also large differences between the different UCB derived cell types which were injected in the studies; freshly isolated mononuclear cells13,16,20, CD34+ cells14 and CD133+ cells17,20 were used but also cultured mononuclear cells12, mesenchymal stem cells18, CD34+19 cells and USSC15. Furthermore the number of injected cells, the timing of cell injection and the follow-up time differs in all studies. Taken together all these differences in experimental set-up could be the reason why our results contradict earlier reports of the effect of UCB on myocardial infarction.
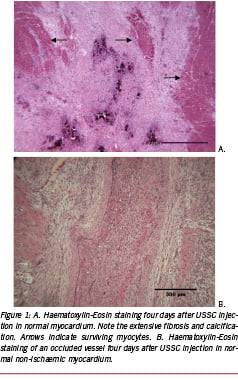
The study by Kim et al15, which was also performed in swine showed a beneficial effect on LV function of the same USSC cell line as used in our studies. However in that study, cells were injected intramyocardially, and furthermore, the MI was created by permanent LAD ligation. To get more insight in the contradictory findings between the study of Kim et al15 and our study we performed some additional experiments. We chose to inject cells into the healthy myocardium in five swine and sacrificed the animals four days later. Extensive micro infarctions (Figure 1A) could be observed four days after USSC injection in non-ischaemic myocardium caused by occluded vessels (Figure 1B). In situ hybridisation showed that the cells in the occluded vessel were predominantly of human origin. Micro infarctions were also observed by Vulliet et al22 after intracoronary injection of cultured mesenchymal stem cells in dogs. These findings suggest that intracoronary injection of these cultured USSC is not suitable, but intramyocardial injection might be preferable. Further studies are required to investigate whether USSC occlude vessels due to their large cell size (~20 µm). Bone marrow derived mononuclear cells, however, are only ~5-7 µm in size.
In some studies immune suppression was administrated to the animals14,15,18. Min et al18 showed that seven days after injection, human cells could not be detected any more in rats which did not receive immune suppression. With immune suppression human UCB derived cells could still be detected eight days after injection. Three other studies16,17,20 showed that after injection of human cells in rodents, these cells could be detected in the hearts of some, but not all, of the injected animals. Overall, it is still unclear whether UCB cells are really hypo immunogenic. Kim et al15 even observed a higher number of CD3 positive cells and macrophages in cell treated swine. But they could also detect human cells which were positive for cardiomyocyte specific markers such as troponin I and myosin heavy chain. All other preclinical studies showed that the human UCB derived cells were detected near vessels and myocytes or myofibroblast, but the injected cells predominantly kept their haematopoetic phenotype.
Conclusions
Treatment of infarcted myocardium with UCB derived cells is promising but there should be caution when using these cells in patients. The results of our own experiment suggest that intracoronary injection of cultured UCB derived stem cells is not suitable. More studies in large animal models with reperfused MI are needed to investigate the optimal mode of administration for these cells. Furthermore, since the immunogenicity of these cells is not yet determined, this should be tested as well in appropriate models.
Since an infarct area is a very hostile environment for injected cells due to ischaemia and inflammation, it is not surprising that very few cells seems to survive, and that thus far limited evidence exists that injected cells are able to differentiate towards a cardiomyocyte-like phenotype. Potential approaches to overcome these limitations might be the overexpression of survival genes or to direct the differentiation of the UCB cells towards a cardiac lineage before injection.

