Introduction
Oral anticoagulation (OAC) is indicated for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) and at least one risk factor for stroke1. However, managing OAC is a challenge in NVAF patients who also require dual antiplatelet therapy (DAPT) in the setting of a presentation with an acute coronary syndrome (ACS) or where percutaneous coronary intervention (PCI) is undertaken2,3. In 2010, the European Society of Cardiology (ESC) Working Group on Thrombosis, in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the European Heart Rhythm Association (EHRA), published an expert consensus document on antithrombotic management of NVAF patients presenting with ACS or undergoing PCI. Most of the recommendations from the 2010 consensus document were subsequently incorporated into the 2012 focused update of the ESC guidelines for patients with atrial fibrillation1.
Since then, many new studies have been published, and considerable progress has been made in both drug-eluting stent (DES) and transcatheter aortic valve implantation (TAVI) technologies. As a result, a new updated joint consensus document was recently published by the ESC Working Group on Thrombosis, EAPCI, EHRA, and the European Association of Acute Cardiac Care (ACCA), and endorsed by the Heart Rhythm Society (HRS) and the Asia-Pacific Heart Rhythm Society (APHRS)4. This editorial introduces some key recommendations of that document, discusses additional very recently presented trial data, and highlights ongoing and upcoming studies.
New studies driving reappraisal of existing recommendations
Aside from patients in need of concomitant OAC, the optimal duration of DAPT after coronary stenting in general and DES in particular remains unclear5. Prior recommendations for prolongation of DAPT after DES implantation to 12 months were non-evidence-based6, and accumulating evidence suggests that a shorter duration of DAPT may suffice in many patients7-9. Recent joint ESC/European Association of Cardio-Thoracic Surgeons (EACTS) guidelines now recommend six months of DAPT in stable patients after stenting with DES10, though United States guidelines continue to recommend at least 12 months of DAPT11.
In relation to patients receiving OAC who undergo stenting and are in need of additional DAPT with aspirin and clopidogrel, the optimal duration of triple therapy, i.e., OAC plus DAPT, is unknown. On the one hand, the bleeding risks associated with triple therapy are increasingly recognised. A recent registry report from Denmark examined the outcomes of 8,700 hospitalised patients with AF and stable coronary artery disease between 2002 and 201112. An analysis of these patients showed that, relative to OAC monotherapy (approximately 37% of the study population) the risk of bleeding increased when aspirin (hazard ratio [HR] 1.50, 95% confidence interval [CI]: 1.23-1.82), clopidogrel (HR 1.84, 95% CI: 1.11-3.06) or both aspirin and clopidogrel (HR 2.81, 95% CI: 1.82-4.33) were added to OAC. On the other hand, the risk of thromboembolism was comparable in all regimens that included OAC.
One recently examined option to reduce the risk of bleeding in patients receiving OAC who undergo coronary stenting is to omit aspirin therapy and continue OAC and single agent antiplatelet therapy with clopidogrel. This approach was evaluated in the What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial, which randomised 573 patients receiving OAC to either clopidogrel (study treatment) or clopidogrel plus aspirin (control treatment)13. Treatment was continued for one month after bare metal stent (BMS, used in ~31% of patients) and for one year after DES placement (used in ~65%). At one year, the primary endpoint of any Thrombolysis In Myocardial Infarction (TIMI) bleeding was significantly lower in the dual therapy arm (19.4% vs. 44.4%; HR 0.36, 95% CI: 0.26-0.50, p<0.001), though the rates of major bleeding were not different (3.2% vs. 5.6%; HR 0.56, 95% CI: 0.25-1.27, p=0.16). The ischaemic composite of myocardial infarction, stroke, target vessel revascularisation, or stent thrombosis was decreased in the dual therapy arm (11.1% vs. 17.6%; HR 0.60, 95% CI: 0.38-0.94, p=0.025). Interestingly, all-cause mortality was 61% lower in the triple therapy arm versus the dual therapy arm (2.5% vs. 6.3%; HR 0.39, 95% CI: 0.16-0.93, p=0.027), although this observation must be interpreted with caution due to the modest size of the study, the primary endpoint being driven by minor bleeds, the long treatment duration in the triple therapy arm (hence, more bleeding), and the lack of difference in bleeding events likely to have been prognostically important.
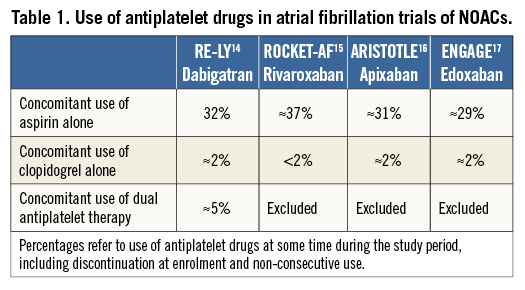
Data sources for understanding the role of non-vitamin K antagonist oral anticoagulants (NOACs) as part of a triple antithrombotic regimen include trials conducted in patients with NVAF and trials conducted in patients with ACS/PCI. In NVAF trials, the use of aspirin on top of OAC represented one third of the study population (with clopidogrel use in 0-5% of patients) (Table 1)14-17. In an ACS trial of low-dose rivaroxaban (2.5 mg BID or 5 mg BID), a triple antithrombotic regimen (i.e., low-dose rivaroxaban plus DAPT) was found to reduce the combined ischaemic endpoint at the price of increased bleeding (HR 3.96, 95% CI: 2.46-6.38, p<0.001)18. Conversely, a phase III ACS trial of apixaban, where the same dose tested in NVAF was used, demonstrated no efficacy benefit of adding apixaban to DAPT, but increased bleeding (HR 2.59, 95% CI: 1.50-4.46, p=0.001)19. Overall, these findings emphasise the need for specifically designed studies to investigate the role of NOACs in patients who also have an indication for DAPT.
Key points from the 2014 consensus document
ACUTE CORONARY SYNDROME OR PERCUTANEOUS CORONARY INTERVENTION
General considerations for periprocedural management of antithrombotic therapies in NVAF patients with ACS or undergoing PCI include the following. First, although specific randomised trial data are lacking for this patient subgroup, radial access might be preferred for coronary angiography/PCI to minimise the risk of access-related bleeding depending on operator expertise and preference. Second, new-generation DES should be preferred over BMS, especially in patients at low risk of bleeding. Third, more potent adenosine diphosphate (ADP) receptor antagonists such as prasugrel and ticagrelor should not be part of a triple therapy regimen in patients with NVAF. Fourth, gastric protection with proton pump inhibitors should be advised in patients on OAC plus antiplatelet therapy. Scenario-specific considerations are summarised below.
– In vitamin K antagonist (VKA)-treated patients undergoing elective PCI, an uninterrupted anticoagulation strategy with no additional heparin boluses in case of therapeutic anticoagulation (INR >2, preferably coupled with radial access) is the preferred strategy in patients at moderate to high risk of thromboembolism, whereas NOACs should be discontinued 48 hours before PCI and periprocedural parenteral anticoagulation used as per standard practice. When the procedure requires OAC interruption for >48 hours, heparin bridging may be considered.
– In patients with NSTE-ACS, an early invasive strategy (i.e., within 24 hours) should be preferred in order to expedite treatment allocation and for delineation of the optimal antithrombotic regimen. Pre-treatment with P2Y12 inhibitors and glycoprotein IIb/IIIa inhibitors is discouraged. In view of the multiple antithrombotic medications used in this setting, it seems prudent to stop OAC and administer unfractionated heparin or bivalirudin only as bail-out or if INR is ≤2 in a patient on VKA, balancing the acute need for additional antithrombotic therapy with the excess bleeding risk and the “thrombus burden”. Bridging with heparin may be considered in low-risk NSTE-ACS patients who are not referred for an early invasive strategy.
– In patients with STEMI, regular or even “routine” use of glycoprotein IIb/IIIa inhibitors is discouraged.
A key part of the document concerns long-term antithrombotic management in patients undergoing elective PCI or presenting with an ACS; recommendations are summarised in Figure 1, where a four-step approach is proposed. The combination and duration of antithrombotic therapies should be tailored according to the individual baseline characteristics based on: i) the risk of stroke (CHA2DS2-VASc score=1 or ≥2), ii) the risk of bleeding (HAS-BLED=0-2 or ≥3), and iii) the clinical presentation (stable coronary artery disease undergoing PCI versus ACS). The ESC Task Force felt that there is no strong evidence to suggest that NOACs behave differently from well-controlled adjusted-dose VKAs in the setting of ACS or PCI, although specific data are limited. Therefore, the principle of continuing an existing OAC and –in case of NOACs– the use of the lower tested dose for stroke prevention in AF is judged to be reasonable at present until new trials are available. Where a VKA is used in combination with antiplatelet therapy, a desired target INR range of 2.0-2.5 should be considered, but particular attention is needed to the quality of INR control, as reflected by a high (>70%) average time in therapeutic range (TTR) given the close relationship of TTR to thromboembolism and bleeding risks20,21. In a patient with NVAF and stable vascular disease (arbitrarily defined as being free from any acute ischaemic event or repeat revascularisation for ≥one year) OAC alone should be preferred, although adding an antiplatelet agent may be considered in patients at very high risk of coronary events (i.e., stenting of the left main, proximal bifurcation, recurrent myocardial infarction, etc.). The Task Force concluded that the evidence was too weak to provide clear guidance on duration of therapy, but emphasised that the duration of a triple antithrombotic regimen should be as short as possible.
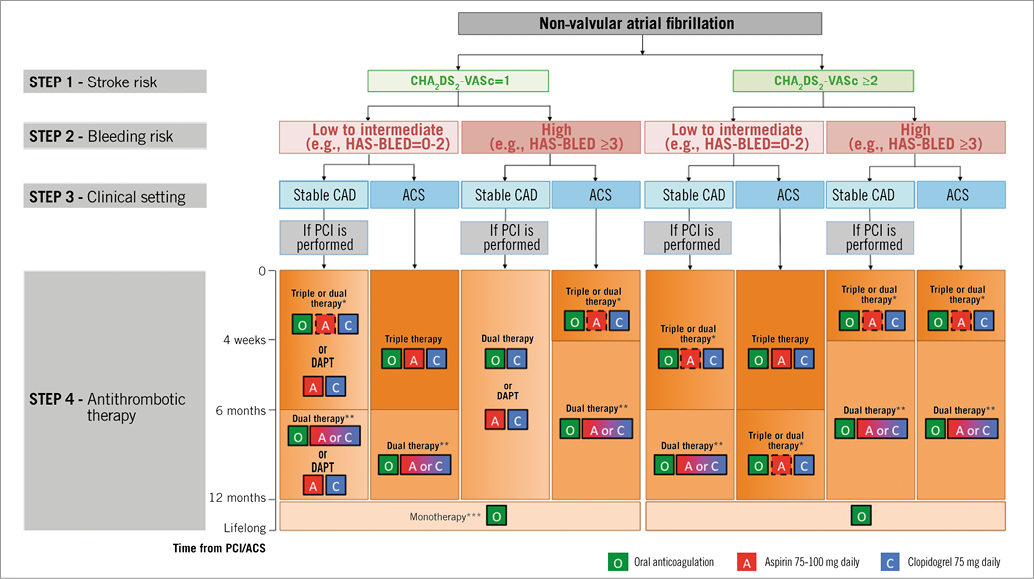
Figure 1. Choice of antithrombotic therapy, including combination strategies of oral anticoagulation (O), aspirin (A) and/or clopidogrel (C). For Step 4, background colour and gradients reflect the intensity of antithrombotic therapy (i.e., dark background colour=high intensity; light background colour=low intensity). Solid boxes represent recommended drugs. Dashed boxes represent optional drugs depending on clinical judgement. New-generation DES are generally preferable over bare metal stents, particularly in patients at low bleeding risk (HAS-BLED 0-2). When vitamin K antagonists are used as part of triple therapy, INR should be targeted at 2.0-2.5 and the time in the therapeutic range should be >70%. *Dual therapy with oral anticoagulation and clopidogrel may be considered in selected patients. **Aspirin as an alternative to clopidogrel may be considered in patients on dual therapy (OAC plus single antiplatelet). ***Dual therapy with oral anticoagulation and an antiplatelet agent (aspirin or clopidogrel) may be considered in patients at high risk of coronary events. DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention. Reproduced with permission from Lip GY et al. Eur Heart J. 2014 4.
TRANSCATHETER AORTIC VALVE IMPLANTATION
Transcatheter aortic valve implantation (TAVI) is an area where information on the efficacy/safety balance of different periprocedural and long-term antithrombotic strategies is limited. Notably, the burden of atrial fibrillation in TAVI is ~40-50%, with ~30% of patients having a history of paroxysmal or chronic atrial fibrillation, and an additional 10-15% of patients with new-onset episodes of atrial fibrillation after the procedure. Routine use of triple antithrombotic therapy is not recommended after TAVI. Although DAPT is otherwise recommended after TAVI in patients without NVAF, if OAC is indicated, then DAPT should not be prescribed unless another indication for DAPT exists, e.g., concomitant PCI. No data exist on the use of NOACs in the TAVI setting. Procedural anticoagulation trials in TAVI are warranted to select drugs that have the potential to limit bleeding complications (e.g., bivalirudin) compared with heparin, and to investigate different strategies of bridging and long-term management for patients with NVAF on OAC.
New studies/evidence since the publication of the 2014 consensus document
Two recent trials that have yet to be published have studied outcomes of OAC-treated patients who underwent stenting with DES. The ISAR-TRIPLE trial is the largest randomised trial comparing different durations of triple therapy22. Overall, 614 patients were randomised to either a six-week clopidogrel therapy (n=307) or a six-month clopidogrel therapy (n=307). The study tested the hypothesis that shortening clopidogrel therapy from six months to six weeks would be associated with superior net clinical outcomes, defined as a composite of death, myocardial infarction, definite stent thrombosis, stroke or TIMI major bleeding at nine months. The main finding was that six weeks of treatment was not superior to six months (9.9% versus 8.9%; HR 1.14, 95% CI: 0.68-1.91, p=0.63) (Sarafoff et al presented at: Transcatheter Cardiovascular Therapeutics; September 15, 2014; Washington, DC, USA). Moreover, cardiac death, definite stent thrombosis or TIMI major bleeding did not differ between treatment groups. Although there was no evidence that a six-week therapy was superior, the comparable outcomes between the groups may be clinically important and tentatively support a role for either treatment duration based on the individual ischaemic and bleeding risks of the patient.
The Zotarolimus-eluting Endeavor Sprint Stent in Uncertain DES Candidates (ZEUS) trial recently demonstrated that the use of a second-generation DES, followed by a DAPT duration regimen dictated by patients’ characteristics and not by stent type, is superior to conventional BMS implantation in patients at increased risk of thrombosis and bleeding (Valgimigli et al presented at: American College of Cardiology/i2 Scientific Session; March 31, 2014; Washington, DC, USA). The study included a subgroup of patients receiving OAC who were recommended for a 30-day course of DAPT23. Although the primary comparison was between two stents (BMS versus early-generation zotarolimus-eluting stents) there was no obvious signal of hazard in the patients who received DES and short duration DAPT.
A recent joint EHRA/EAPCI expert consensus document addresses the use of left atrial appendage (LAA) occlusion in patients with NVAF, prior stroke and potential contraindications to OAC24. The authors acknowledge that OAC remains the standard-of-care in these patients at present, though LAA occlusion therapy should be considered. Moreover, although randomised control trial data exist only for patients who are eligible for both OAC and LAA occlusion25, patients with a high risk of bleeding on OAC or those with contraindications to OAC represent the most accepted clinical indication for LAA occlusion. The authors clarify that patients who require prolonged triple therapy after transcatheter intervention may fall into these categories and become candidates for this treatment though intervention for this indication is not supported by specific trial data.
Upcoming studies in an evolving field
The new ESC joint consensus document provides a framework for clinical decision making in a field where data are sparse. A set of new studies is expected to expand our knowledge on treatment strategies for NVAF patients who need a combination of OAC and antiplatelet therapy in the coming years.
The LEADERS-FREE trial is testing the potential benefit of a newer-generation drug-coated, polymer-free stent (BioFreedom™; Biosensors, Morges, Switzerland) in 2,456 patients considered at high risk of bleeding (including patients on OAC) using a BMS (Gazelle™; Biosensors) as control and recommending short duration (one month) DAPT26. The primary safety endpoint is a composite of cardiac death, myocardial infarction and stent thrombosis according to a non-inferiority design, whereas the primary efficacy endpoint (clinically driven target lesion revascularisation) will be analysed according to a superiority analysis. This trial should help to characterise better a subset of PCI patients at high risk for bleeding, with the potential to support one-month duration of DAPT in DES-treated patients. However, the potential advantage of this strategy in patients with NVAF, compared with current high-efficacy new-generation DES combined with either clopidogrel and OAC (a WOEST approach) or a six-week duration of triple therapy (an ISAR-TRIPLE approach), may be difficult to demonstrate.
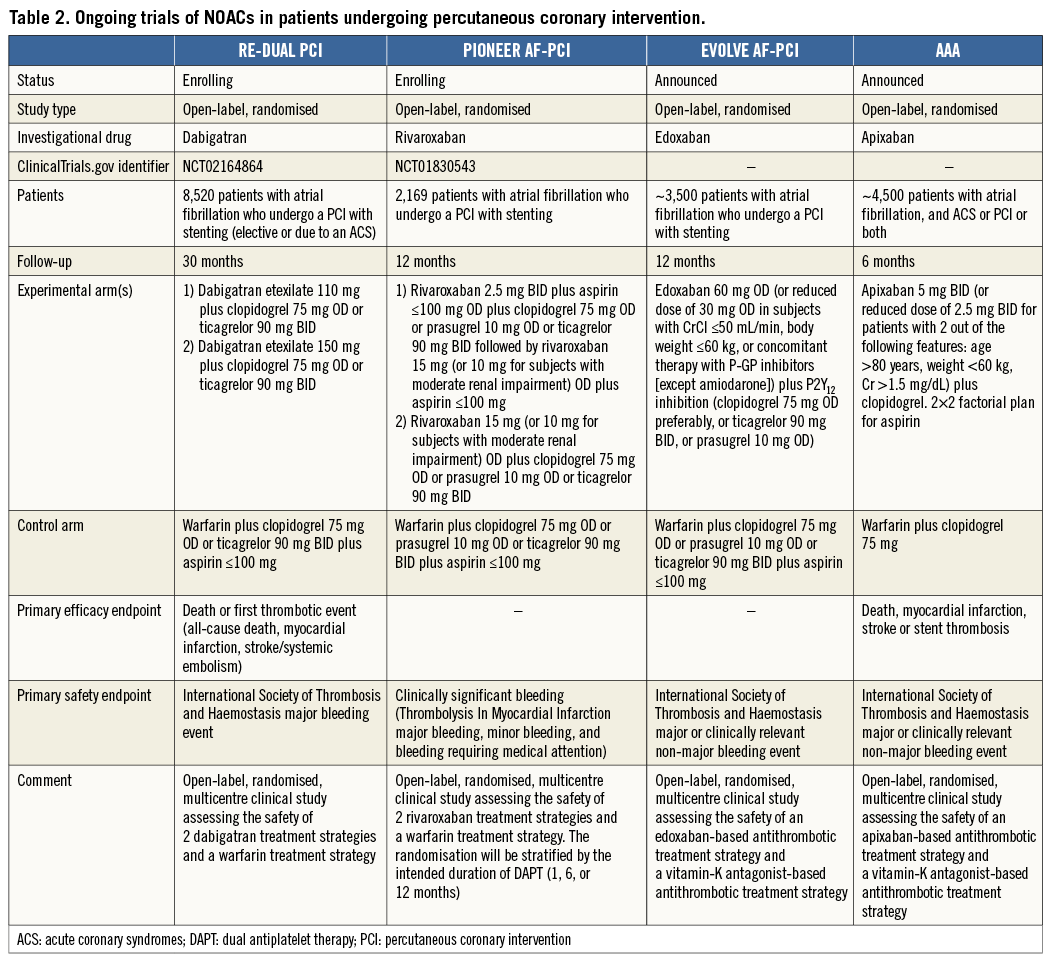
The merits (or otherwise) of NOACs in the landscape of NVAF patients undergoing PCI will be clarified by ongoing studies comparing multiple antithrombotic regimens, including PIONEER AF-PCI for rivaroxaban (NCT01830543) and RE-DUAL PCI for dabigatran (NCT02164864), as well as the recently announced EVOLVE AF-PCI for edoxaban and AAA for apixaban (Table 2). In a landscape which is constantly changing, the impact of data from recently presented large-scale randomised controlled trials concerning the optimal duration of DAPT in patients without an indication for OAC (late-breaking at time of going to press) will also have knock-on effects for the management of patients with concomitant NVAF27,28.
Finally, in the field of TAVI, the POPular-TAVI trial (NCT02247128) is comparing clopidogrel plus VKA versus VKA alone in patients undergoing TAVI with a concurrent indication for OAC, and aspirin versus DAPT in patients without an indication for OAC. Conversely, more insights into the appropriate use of NOACs will come from the ongoing ATLANTIS trial, where apixaban is compared with VKA in TAVI patients with an indication for OAC, and versus DAPT or single antiplatelet therapy in patients without an indication for OAC.
Conflict of interest statement
D. Capodanno reports receiving speaker’s honoraria from Eli-Lilly/Daiichi Sankyo, AstraZeneca, Bayer and serving as an advisory board member for Eli-Lilly, Daiichi Sankyo and AstraZeneca. G. Lip reports serving as a consultant for Bayer, Astellas, Merck, Sanofi, Bristol-Myers Squibb /Pfizer, Daiichi Sankyo, Biotronik, Medtronic, Portola and Boehringer Ingelheim and on a speakers bureau for Bayer, Bristol-Myers Squibb /Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Medtronic. S. Windecker reports receiving lecture fees from Biosensors, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, Eli Lilly and AstraZeneca and research grants to the institution from Biotronik and St. Jude Medical. K. Huber reports receiving lecture fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo and Pfizer, and Bristol-Myers Squibb. P. Kirchhof reports receiving consulting fees and honoraria from Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, MSD, Medtronic, Pfizer, Sanofi, Servier and research grants from Bristol-Myers Squibb, Pfizer Cardiovascular Therapeutics, Daiichi Sankyo, Sanofi, and St. Jude Medical. G. Boriani reports receiving speaker’s fees from Boehringer Ingelheim, Boston Scientific and Medtronic. D. Lane has received investigator-initiated educational grants from Bayer Healthcare and Boehringer Ingelheim and served as a speaker for Boehringer Ingelheim, Bayer Healthcare, and BMS/Pfizer. In addition, Dr Lane is on the Steering Committee of a Phase IV apixaban study (AEGEAN) and has participated in various clinical trials of stroke prevention in atrial fibrillation. M. Gilard reports receiving lecture fees from Bayer, Daiichi Sankyo and AstraZeneca. J. Collet reports receiving research grants from Bristol-Myers Squibb, Sanofi-Aventis, Eli Lilly, Medtronic, Boston Scientific, Cordis, and Stago, consulting fees from Sanofi-Aventis, Eli Lilly, and Bristol-Myers Squibb, and lecture fees from Bristol-Myers Squibb, Sanofi-Aventis, Eli Lilly, and AstraZeneca. R. Byrne reports receiving lecture fees from Biotronik and B. Braun. The other authors have no conflicts of interest to declare.

