Drug Eluting Stents (DES) constitute the current standard of care for the treatment of restenotic lesions1-4. Whereas the stent, the drug and the delivery system are all important components of the DES system, polymer coatings control local delivery of drugs. The first generation DES are based on biostable polymers that remain in the body well after the drug is exhausted, and if the polymers are not biocompatible, they can elicit inflammatory response. It has been proposed in literature that hypersensitivity to polymers with less than satisfactory biocompatibility could be responsible for delayed healing and late stent thrombosis5-8. Bioabsorbable polymers in DES coatings could potentially degrade and be metabolised when all the drug is delivered leaving behind the bare metal stent. However, presently available bioabsorbable polymers either degrade too slowly to be beneficial, or degrade too rapidly and elicit an inflammatory response. Degradation kinetics therefore largely determine polymer biocompatibility and utility. It is believed that well designed biostable polymers can be viable alternatives to bioabsorbable polymers for drug eluting stent coatings.
Polymer hydrophilicity is an important property for any implant application. Hydrophilic polymers would be expected to generate less inter-facial tension in the aqueous body environment potentially, eliciting a minimal foreign body response. Therefore, such polymers are expected to be biocompatible. However, since most of the antiproliferative drugs tend to be hydrophobic, first generation drug eluting stent coatings are based on biostable hydrophobic polymers. This approach has allowed formulators to disperse the drug uniformly in the coating, to obtain diffusion controlled elution. These drug eluting stents have also exhibited a tendency to elicit inflammatory response9-10.
In contrast, the phosphoryl choline (PC) based polymer used in the second generation drug eluting stent Endeavor is hydrophilic. The zwitterionic PC head groups associate with a large number of water molecules to provide the hydrophilicity11,12 and hence tends to be highly biocompatible. This characteristic of PC polymer may contribute to the observed safety, notably zero late stent thrombosis in clinical trials involving 1,300 patients, all of whom have been followed out to at least two years.
The next generation of drug-eluting stents, the Endeavor Resolute from Medtronic, is based on the proprietary BioLinx™ Polymer System. It is a unique blend of three polymers, a hydrophilic C19 polymer, water soluble polyvinyl pyrrolidinone (PVP) and a hydrophobic C10 polymer. Like the PC polymer, it is also designed to mimic the biological chemistry of the body. Analogous to the zwitterionic phosphoryl choline group in PC polymer, the vinyl pyrrolidinone units in the BioLinx™ Polymer System (Figure 1) offer the hydrophilicity. Indeed, the BioLinx™ polymer coating, even with the hydrophobic C10 polymer present, exhibits a disproportionately higher concentration of surface vinyl pyrrolidinone units.
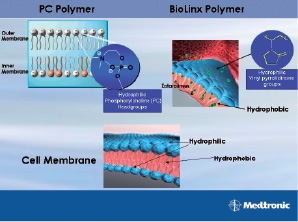
Figure 1. Like the PC polymer, BioLinx™ Polymer System is also designed to mimic the biological chemistry of the body.
The same is confirmed by a number of analytical tools like ATR (Attenuated Transmission Reflectance) spectra, XPS (X-ray Photoelectron Spectroscopy) and contact angle measurements13. Evidence of biocompatibility of BioLinx™ polymer was borne out in in vitro studies of interactions of activated monocytic and vascular smooth muscle cell using real-time based gene profiling and FACS-BD cytokine array13. In addition, the polymer system also promoted viability of endothelial and vascular smooth muscle cells.
The molecular architectures of the component polymers in BioLinxTM have been designed to provide a robust coating (Figure 2).
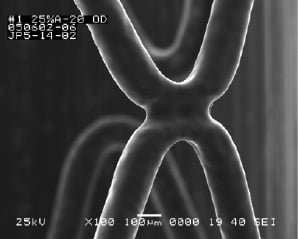
Figure 2. A deployed Endeavor Resolute stent after tracking 3 times in a 5 Fr guide catheter.
It is imperative that the polymer coating is tough and adheres well to the stent surface; should not crack or peel when the stent is tracked through in the vasculature or calcified lesions.
The component polymers in the blend are also designed to offer extended drug release. It is important that in a polymer blend, the components are either miscible, or mutually compatible, so as not to phase separately. Phase separated polymer systems are often unable to provide diffusion controlled kinetics because of preferential migration of drugs into phases of different physico-chemical characteristics. The three component polymers in BioLinx™ are compatible with each other. Differential scanning calorimetry (DSC) of the BioLinx™ polymer system does not show any phase separation14. C10 polymer alone seems to lock in the drug, while the soft C19 polymer elutes quite rapidly. PVP in the blend offers the initial drug burst and enhances the overall drug elution rate. C10 polymer also provides adequate hydrophobicity and rigidity to the blend to hold zotarolimus as well as offering extended diffusion controlled elution kinetics.
Indeed, the unique coating of the Endeavor Resolute stent enables a finer control of the rate of drug elution. The in vitro elution profile of the Endeavor Resolute stent demonstrates approximately 50% elution within the first week, although the remaining drug elutes beyond 31 days (Figure 3).
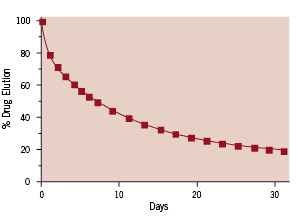
Figure 3. The rate of in vitro elution of the Endeavor Resolute zotarolimus-eluting stent systems. Approximately half of the drug contained within the coating of an 18 mm stent is eluted within the first week, but the remaining drug is eluted beyond 4 weeks.
A relative comparison of drug in arterial tissue between Endeavor and Endeavor Resolute contrasts how two different elution strategies both result in effective drug levels in the porcine model (Figure 4).
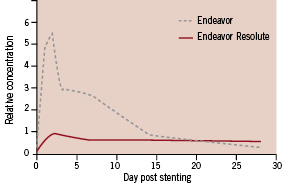
Figure 4. Relative arterial tissue concentrations of zotarolimus in the porcine coronary artery model at various times post-stent implantation. Endeavor maintains effective drug levels through initial loading of arterial tissue with drug during the first two weeks of elution while Endeavor Resolute sustains an effective drug level in tissue through continued, long-term elution.
Although the zotarolimus dose is identical for both the original Endeavor stent and the Endeavor Resolute stent that being 1.6 µg per mm2 of stent surface, the Endeavor stent delivers its load of drug to the arterial tissue early where it is retained, owing to its relatively high lipophilicity, providing drug in tissue for 28 days. In contrast, the Endeavor Resolute stent releases the drug more gradually, producing a more tightly constrained drug level in the tissue, but sustaining it for a longer duration. The Endeavor Resolute stent elutes 85% of its zotarolimus content during the first 60 days post-procedure, and the remainder of the drug is completely eluted by 180 days15.
Conclusions
BioLinx™ polymer, designed on sound principles of polymer chemistry, offers Endeavor Resolute a biocompatible surface mimicking the biological chemistry of the body coupled with coating durability and extended drug elution.

