Abstract
The current standard of treatment of valvular diseases with severe functional and/or clinical consequences is the repair or replacement of the valve, which is usually surgical or, in specific scenarios, percutaneous. The available prosthetic valves, however, are not a magic bullet in the physicians’ arsenal for the management of valvular diseases, since the age-dependent structural valve deterioration (SVD) and the need for prolonged systemic anticoagulation in the case of metallic prosthetic valves are not inconsequential during the lifespan of a patient with an implanted prosthetic valve. Based on decades of research combining the scientific disciplines of supramolecular chemistry, electrospinning and regenerative medicine, endogenous tissue restoration has emerged as a very promising domain to provide this magic bullet, in the form of valves, which enables functional restoration by the body itself. The concept of a restorative material that will set the framework for the creation of a new, endogenous valve is very appealing and, recently, proof of concept studies have been completed at both preclinical and clinical levels. These studies have shown favourable pathologic, anatomic and haemodynamic characteristics compared to currently available prosthetic valves, in sheep and in young children undergoing right ventricular outflow tract reconstruction, and may represent an alternative to the bioprosthesis made of xenopericardial tissue. The present manuscript reviews the rationale, background knowledge and historic development of endogenous tissue restoration and presents preliminary data about the Xeltis valve, which appears to have the potential to make restorative valve therapy a reality in clinical practice.
Introduction
Surgical and percutaneous bioprostheses use biological tissue from animals, such as bovine, porcine, equine leaflets; however, one of the major limitations of this technology is its durability. Tissue of animal origin tends to degenerate and becomes calcified with time, so that, in the span of a lifetime, reintervention is frequent after one or two decades in patients who received bioprostheses implanted surgically or percutaneously. Long-term data on percutaneously implanted mechanical or bioprosthetic aortic valves are limited by several factors, including the relatively small number of valves with long-term follow-up available and the limited life expectancy of the typical patients in whom most of these valves have been implanted. Most of the few follow-up studies (at best moderate in size) performed so far have not shown significant deterioration-related problems, but longer-term data in large cohorts are needed to conclude whether durability is also an issue with percutaneously implanted valves1.
The age-dependent structural valve deterioration (SVD) in the Carpentier-Edwards PERIMOUNT pericardial bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) clearly indicates that the risk in patients who have been operated below the age of 50-65 years is greater compared to more elderly patients (70-80 years)2. Age-stratified freedom from reoperation due to SVD at 15 and 20 years post surgery was 70.8±4.1% and 38.1±5.6%, respectively, for the group aged 60 years or less, 82.7±2.9% and 59.6±7.6% for those 60 to 70 years, and 98.1±0.8% for the oldest group. Expected valve durability is 19.7 years for the entire cohort (Figure 1).
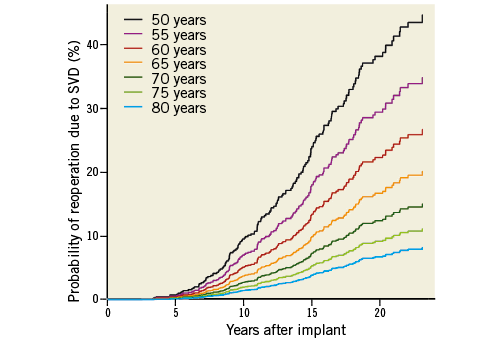
Figure 1. Very long-term outcomes of the Carpentier-Edwards PERIMOUNT valve in the aortic position as a function of the age at which the patient was implanted. (Adapted with permission Bourguignon et al2).
Current bioprosthetic valves are based on animal-derived glutaraldehyde-fixed pericardial tissue (more rarely the native valve of the animal), which creates several issues, such as material access considerations, consistency of material performance, manufacturing difficulties and chronic inflammatory responses. Importantly, the chronic inflammatory responses result in biocompatibility concerns manifesting as leaflet thickening and thrombosis as well as accelerated structural valve degeneration. As a clinical consequence, there is a need for adjunctive pharmacotherapy (long-term aspirin therapy and short-term systemic anticoagulation)3-5 and repeat hospitalisation(s) with or without reintervention(s) (repeat surgery or valve in valve). In addition, any material unable to grow with the patient constitutes a major obstacle for treatment in non-fully grown-up patients. Endogenous tissue restoration – described in the following paragraph – has the following advantages: dry storage, reduced profile of the valve, scalable and predictable manufacturing, improved biocompatibility resulting in less leaflet thickening/thrombosis, reduced need for anticoagulation and improved durability.
Endogenous tissue restoration
The philosophy of endogenous tissue restoration (ETR) relies on the fact that bioabsorbable material (a polymer as scaffold) could become replaced by the body’s own tissue and therefore offers an alternative to the foreign body material that has been used for decades. ETR is based on decades of research combining three scientific disciplines: the field of supramolecular chemistry, the domain of electrospinning and knowledge about regenerative medicine.
In 1997, Jean-Marie Lehn received the Nobel Prize for his work on supramolecular chemistry6,7, the development of which in the following decades has led to the current polymer material platform. Today, there is a large library of tunable supramolecular polymers that are characterised by different degrees of mechanical strength and rates of bioabsorption.
The second component of this new technology is electrospinning, which is defined as a fibre production method which uses electric force to draw charged threads of polymer solutions or polymer melts up to fibre diameters in the order of some hundred nanometers. Using the technique of electrospinning, unique bioabsorbable matrices can be created in which the supramolecular polymers have been assembled in a random fashion, creating a matrix that can be easily penetrated by endogenous cells, such as red cells, platelets, macrophages, fibroblasts, myofibroblasts, and so on (Figure 2).
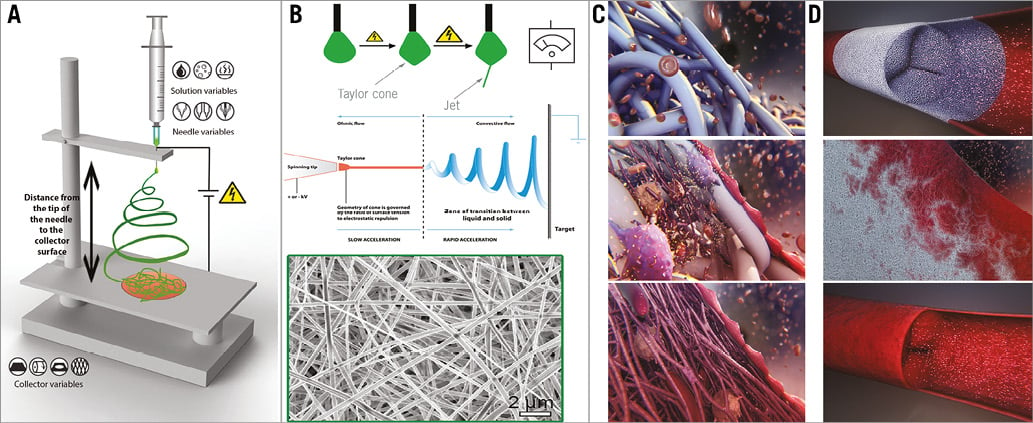
Figure 2. A synopsis of the use of ETR in the treatment of valvular diseases. A) General electrospinning set-up including a syringe with a polymer solution, a voltage power source and a collector: the end product can be adjusted by modifying a variety of parameters related to the solution, the syringe, the distance between the tip of the needle and the collector surface and finally the collector itself. B) Upper panel. From solution to nano-fibres: the droplet at the needle tip is transformed under the effect of the increasing power of the electrical field from a cone (Taylor cone), to a jet and finally to a thin fibre (transition from a liquid to a solid state). B) Lower panel. Microscopic electron image of an electrospun scaffold created by the accumulation of the microfibres in the collector. Subsequent processing of a similar scaffold results in the creation of the bioresorbable valve conduit. C) Serial changes of the bioresorbable conduit at the microscopic level: gradual infiltration of the microfibre conduit skeleton by blood elements (red cells, platelets, macrophages), myoblasts, fibroblasts with subsequent enzymatic bioabsorption of the fibres. D) Serial changes of the bioresorbable conduit at the macroscopic level: gradual replacement of the synthetic valve by a new endogenous valve. Modified with permission from: Mol et al11, https://commons.wikimedia.org/w/index.php?curid=5521755 (Joanna Gatford - The New Zealand Institute for Plant and Food Research Ltd) and http://www.xeltis.com/#videoLightbox (Xeltis, Eindhoven).
The first cell and organ cultures were performed at the beginning of the 20th century8, but the term tissue engineering was conceived at the end of the 20th century9 and, as far as restorative valve therapy is concerned, the development of this interdisciplinary field was subsequently boosted by the work of Professor Frank P.T. Baaijens and his group in 200810,11.
ETR guides the body to restoring itself. There are basically three important phases: phase 1, implantation of the body prosthesis; phase 2, neo-tissue formation; and phase 3, functional restoration. The critical balance between the tissue formation and the implant absorption is the key to the success of this technology (Moving image 1).
From a physiopathological point of view, ETR is defined as replacement of the absorbable leaflet and conduit by the patient’s own native cells that infiltrate the polymeric matrix and trigger a cascade of physiologic events with gradual replacement by native tissue. As absorption begins, the leaflets and conduit are infiltrated by inflammatory cells, releasing growth factors, promoting smooth muscle cell infiltration and matrix production (proteoglycans, collagen with focal elastic tissue). Renu Virmani and the team at the CVPath Institute have investigated the Xeltis pulmonary valved conduit (Xeltis BV, Eindhoven, the Netherlands) with a length of 21 mm and compared it to the physiopathological outcome of the 22 mm Hancock® bioprosthetic valved conduit (Medtronic, Dublin, Ireland). The animal for this preclinical investigation was the sheep and the pulmonary graft was interposed after excision of the native cusp in the adult sheep (Bennink G et al – manuscript under review, personal communication).
Figure 3 shows the ETR in a pulmonary valved conduit at three different levels of the leaflet – at the free margin, at the coaptation site and at the hinge point. Over time (24 months), there is a leaflet collagen replacement with leaflet matrix absorption. From preliminary results presented at EuroPCR 2017, Renu Virmani concluded that degradation of the leaflet material progressed with time but was incomplete at 24 months (Virmani R. Bioresorbable valve therapy – Tomorrow’s world: Endogenous tissue restoration – pathologic insights. Presented at EuroPCR 2017, Paris, France). The intimal thickening of the conduit and leaflet peaked at 12 months, and decreased thereafter with minimal calcification; this contrasted with the outcome of the Hancock prosthesis, where the calcification was much more prominent. The conduit degraded faster than the leaflet, with replacement by proteoglycans and collagen (Figure 4). In brief, the Xeltis pulmonary valved conduit showed encouraging results in a sheep model up to 24 months, and may represent a significant improvement over the current conduits available on the market for children with congenital heart diseases who need reconstruction of their right ventricular outflow tract (RVOT).
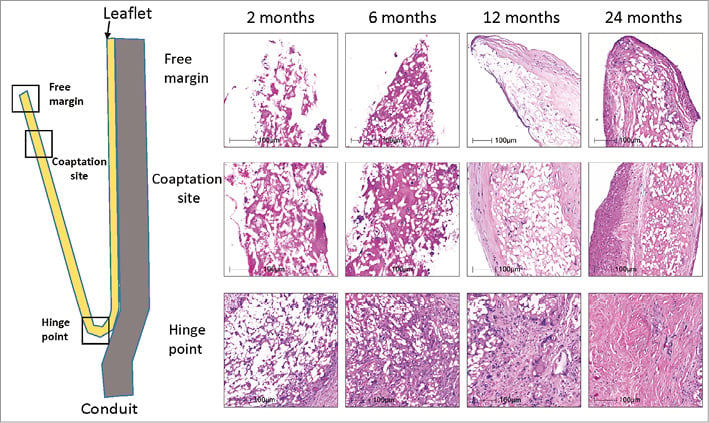
Figure 3. Serial changes in leaflet histology of novel bioabsorbable pulmonary valved conduit over time. (Adapted with permission from Virmani R. Bioresorbable valve therapy - Tomorrow’s world: Endogenous tissue restoration - pathologic insights. Presented at EuroPCR 2017, Paris, France).
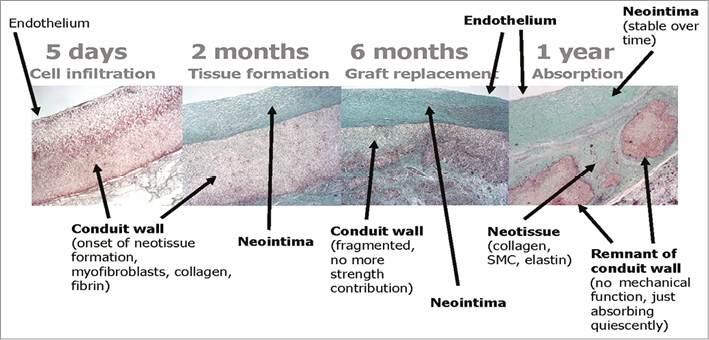
Figure 4. Serial changes of a bioresorbable pulmonary valved conduit implanted in a sheep (preclinical Xeltis valve study). (Modified with permission from Serruys PW. XELTIS - The first bioresorbable valve enabling cardiovascular restoration. Presented at CRT 2017, Washington, DC, USA).
Haemodynamic performance in the preclinical model of pulmonary valved conduit
The core lab of Cardialysis has reported the serial changes in haemodynamic parameters and the severity of pulmonary regurgitation after implantation of the Xeltis pulmonary valved conduit in sheep. Nine animals had follow-up at three months and seven animals had serial assessment at 12 months. Initially, the velocity at the level of the leaflet was 2.5±0.5 m/s at three months, decreasing to 2.0±0.3 m/s at 12 months. Two animals had measurements up to 24 months, showing either a decrease or a stabilisation of the velocity. There was one case of moderate regurgitation at three months, unchanged at 6, 9 and 12 months. Seven cases of mild regurgitation were documented at three months but this regressed in four cases at 12 months; a single case of trace regurgitation was observed at 3 and 6 months, whereas three and two cases with trace regurgitation were documented at 9 and 12 months, respectively (Figure 5).
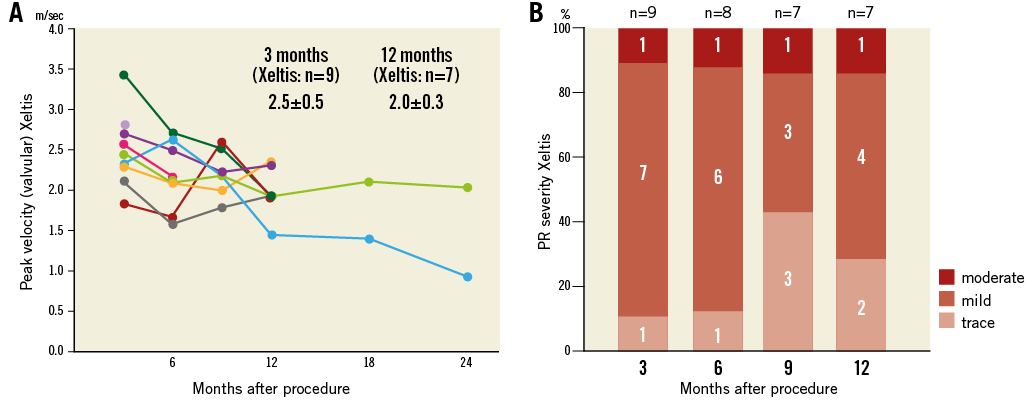
Figure 5. Serial changes of haemodynamics and pulmonary regurgitation severity after implantation of the Xeltis pulmonary valved conduit in sheep27.
The serial changes in the conduit dimension of the Xeltis graft vs. the Hancock prosthesis were also investigated. The conduit diameter in the subvalvular part of the Xeltis pulmonary valved conduit increased with time from 18.7±2.0 to 27.2±2.8 mm; in two cases with a follow-up of 24 months, the dimensions remained stable. It is of note that three Hancock prostheses that were analysed as comparator did not have any significant changes in diameter from three to six months.
First clinical proof of concept: the Xeltis Fontan study and pulmonary valved conduit
In 2016, at the European Association of Cardiothoracic Surgeons (EACTS) meeting, the two-year results were reported of five children who received a 20 mm extracardiac conduit between the inferior vena cava and the pulmonary artery as part of the Fontan procedure12 (Carrel T. Xeltis feasibility clinical trial. Presented at the 30th European Association for Cardio-Thoracic Surgery [EACTS] annual scientific meeting 2016). These children’s ages ranged from 4 to 12 years. No device-related adverse events were reported up to 24 months after surgery12. Imaging study using MRI demonstrated good haemodynamics, and anatomical and functional stability of the graft (Figure 6, Moving image 2).
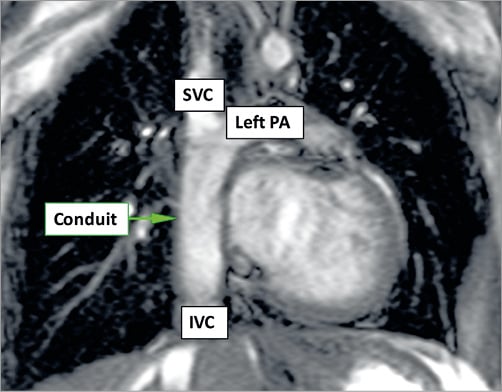
Figure 6. MRI image from the first-in-man study of the Xeltis Fontan conduit. (Adapted with permission from Bockeria et al12)
An EU clinical feasibility study on the Xeltis pulmonary valved conduit was also initiated, with Professor Thierry Carrel from the Inselspital, Bern, Switzerland, as principal investigator (Carrel T. Bioresorbable valve therapy – Tomorrow’s world: Early human experience – right ventricular outflow tract reconstruction. Presented at EuroPCR 2017, Paris, France). Twelve patients, with an age ranging from 2 to 12 years, were enrolled between July and December 2016 in a prospective, non-randomised, open-label study to assess the safety of the Xeltis pulmonary valved conduit, in subjects undergoing RVOT reconstruction; conduits of 16 or 18 mm were implanted and 100% technical success was achieved (Figure 7). All twelve patients have successfully recovered from the procedure. Longer-term data from the study are still being collected and will be reported when all data are available.
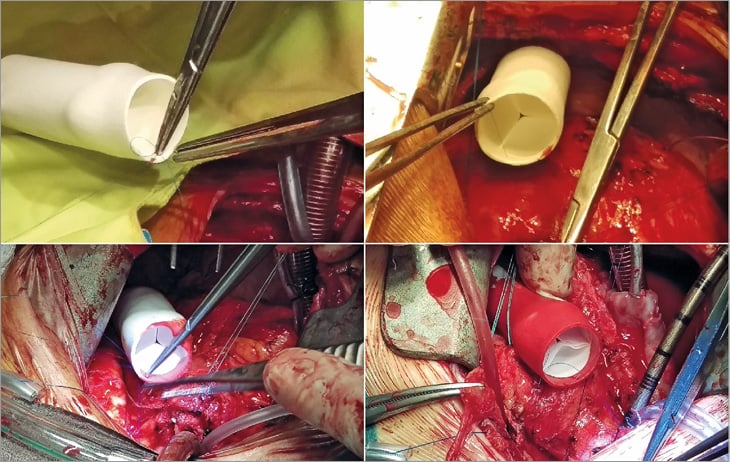
Figure 7. Xeltis pulmonary valved conduit implantation in the RVOT of a patient. (Adapted with permission from Carrel T. Bioresorbable valve therapy - Tomorrow’s world: Early human experience - right ventricular outflow tract reconstruction. Presented at EuroPCR 2017, Paris, France).
The regulatory continuation of this first-in-man study in Europe will be a US early feasibility study (EFS), approved in January 2017 by the FDA. In that study, 10 subjects will undergo an RVOT reconstruction.
Extension of the technology to the aortic valve and to the systemic circulation: preclinical acute result studies
Bioabsorbable material has been integrated into two self-expanding valves (Figure 8). So far, 75 sheep have received one of these valves in the aortic position (Figure 9). The device success rate was 98%. Preliminary results with the first six months of follow-up look promising. Overall, the acute haemodynamics were favourable with a peak pressure gradient of 7.8±3.8 mmHg, a mean gradient of 4.2±1.8 mmHg and an effective orifice area (EOA) of 2.4±0.4 cm2 (Serruys PW. Bioresorbable valve therapy– Tomorrow’s world: Preclinical results. Presented at EuroPCR 2017, Paris, France). To put these results into clinical perspective, in Table 1 we present haemodynamic data of the CoreValve® (Medtronic), the Lotus™ (Boston Scientific, Marlborough, MA, USA) and the SAPIEN 3 (Edwards Lifesciences) valves in clinical series. Although the results between clinical and preclinical studies are not directly comparable, one can get an idea of what the potential of the Xeltis valve in human trials could be, by using the performance of the clinically available valves presented in this Table as a reference.
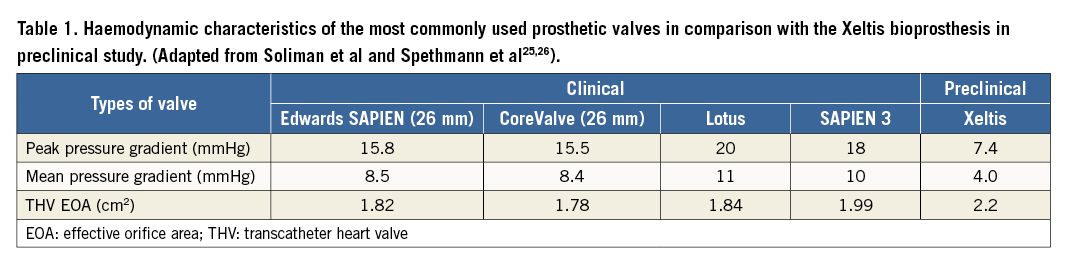
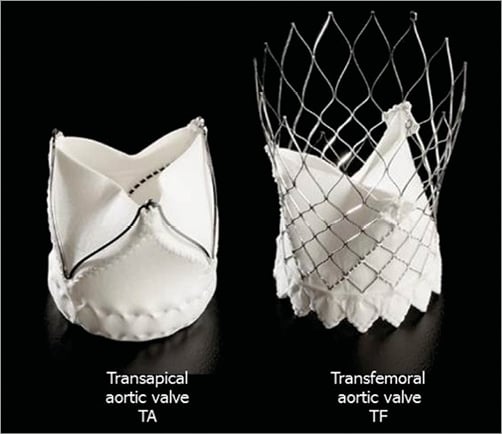
Figure 8. Self-expanding prosthetic aortic valves with integrated bioabsorbable material for transapical and transfemoral implantation.
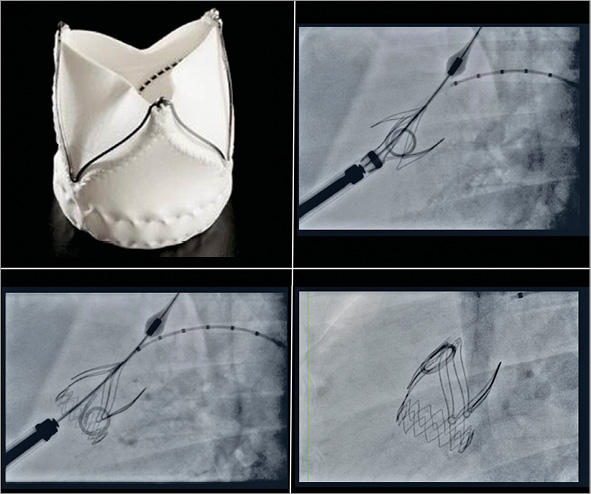
Figure 9. Transapical implantation of the self-expanding Xeltis aortic valve in sheep.
In these animals, aortic regurgitation (AR) was quantified with video-densitometric assessment as described previously13,14 (Figure 10), using a time-density curve in the reference region (ascending aorta) and compared to the region of interest in the left ventricle outflow tract (LVOT). The area under the curve of the time density in the aorta was compared to the time-density curve in the LVOT. The theoretical range of the regurgitation calculated by the two areas under the curve ranges from 0 to 1 (0% to 100%).
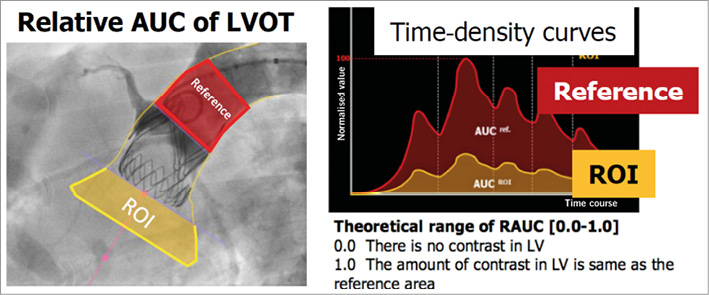
Figure 10. Schematic representation of the video-densitometric method for assessment of aortic regurgitation (Adapted with permission from Tateishi et al14)
Figure 11 shows the cumulative curve of quantitative assessment of AR (video-densitometric AR). The median and interquartile range (IQR) values of AR measurements by quantitative video-densitometry were 6% and 1-12%, respectively. Three cases showed a regurgitation superior to 17% (0.17), a threshold value that corresponds to a more than mild regurgitation on echocardiography with a vital long-term prognostic significance demonstrated in a patient cohort followed up to four years after percutaneous aortic valve implantation13,14. However, in the present preclinical study in healthy animals, these three regurgitations with a quantitative aortic regurgitation of more than 17% have to be attributed not to the initial porosity of the leaflet matrix but to the imperfect clipping of the leaflets by the clipping mechanism of the self-expanding valve at the time of implantation.
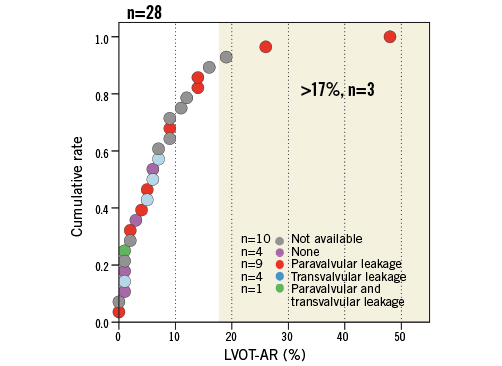
Figure 11. Quantitative assessment of aortic regurgitation (video-densitometric AR) after implantation of the Xeltis aortic valve using the cumulative curve.
Echocardiography was available in 20 of these animals. Among them, there were four cases without a leak, while the predominant reason for regurgitation was paravalvular leak in nine cases and transvalvular leak in four cases. All these echocardiographic assessments were qualitative. Two of the cases with video-densitometric assessment superior to 17% also showed paravalvular leak on echocardiography.
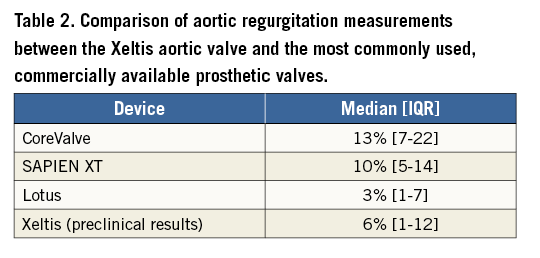
Table 2 presents the results of the quantitative video-densitometric assessment of the SAPIEN, CoreValve and Lotus valves observed in the Brazilian TAVI registry, so that the reader can have a picture of the reference values, acknowledging the fact that there can be no direct comparisons. Pathology studies of the Xeltis valve in the aortic position are awaited and will help to understand the response better.
Overview of other tissue-engineered heart valves
In addition to the promising technology described throughout this report, other attempts have been made to produce tissue-engineered valves derived from polymers, decellularised scaffolds, or non-valve tissue biological scaffolds (e.g., collagen)15. Examples of decellularised scaffolds include decellularised ovine16,17, canine18, and allogenic human19 pulmonary valves. Examples of non-valve tissue biological scaffolds include fibrin and bovine type-1 collagen scaffolds, both tested in an in vitro setting20,21. More data are available on polymeric scaffolds which are bioabsorbable and have been tested at different in vitro and animal levels. Examples include polyglycolic acid (PGA)-based scaffolds (which were extensively tested in vitro as well as in animal models) and poly ε-caprolactone (PCL)-based scaffolds. The latter are characterised by different advantages over the former, including their suitability for electrospinning and the lesser vulnerability of leaflets to compaction and retraction22. More recently, Schmitt et al reported the in vivo functionality and histologic results of a stented decellularised heart valve up to six months after percutaneous implantation in the pulmonary position in a series of 15 sheep23. The valve was produced from ovine vascular cells seeded on a PGA scaffold coated with poly-4-hydroxybutyrate and integrated into a nitinol stent. In vivo performance was assessed serially by echocardiography until explantation (after 8, 16 or 24 weeks). All implantation attempts were successful and all valves showed normal pressure gradients, but stent ovality led to progressive regurgitation.
All the tissue-engineered valves discussed above have been tested at a preclinical level and in a limited number of animals, while no data are available regarding plans for further testing in the context of formal trials. Interestingly, however, Cebotari et al reported, in 2006, the implantation of a pulmonary heart valve engineered with autologous endothelial progenitor cells in two paediatric patients with tetralogy of Fallot19. During the 3.5 years of follow-up, these valves showed satisfactory haemodynamics and there were no signs of valve degeneration or progression of valve regurgitation. To the best of our knowledge, this valve is not undergoing further evaluation in clinical trials.
Conclusions
In summary, the potential benefits of the Xeltis technology may be described as being on three levels: 1) acute benefit, since the restorative material allows profile reduction of the valve and allows dry storage without glutaraldehyde, 2) manufacturability, as scalable good manufacturing practice in polymer production is possible and there is no need for sourcing of animal tissue with a substantial reduction of the sutures to fix the leaflets in the metallic frame, since the polymeric material is attached to the frame by surrounding and encapsulating the metallic struts (Figure 8), and 3) clinical outcome, where a less chronic inflammatory process is expected, since there will be no response to foreign body such as fixate tissue.
In preclinical study, the restorative Xeltis pulmonary valved conduit showed a favourable and consistent haemodynamic performance as well as an acceptable regurgitation severity rate 12 months after surgery. These positive results persist up to two years after surgery. The restorative Xeltis aortic valve demonstrated good haemodynamic parameters with no cases having a more than mild transvalvular AR immediately after implantation of the valve.
In the first-in-man trial, the restorative pulmonary valved conduit has demonstrated the ability to be implanted successfully in various anatomical conditions, using standard surgical techniques. The early clinical outcomes look promising for this technology, with the potential to improve results of RVOT reconstruction, but long-term follow-up studies to confirm these observations are warranted.
In conclusion, the Xeltis platform is a synthetic, bioabsorbable cardiovascular device which, after implantation, enables ETR of a heart valve or blood vessel. It is the world’s first such product to enter clinical trials, even in a limited number of patients with highly selected cardiac substrate. Clinical proofs of concept with the Fontan procedure and reconstruction of the RVOT are promising at short- and medium-term follow-up. Xeltis restorative technology has the potential to redefine heart valve replacement therapy using ETR to regenerate valves with long-lasting durability. Of course, there is still a long way to go until the full picture of the clinical potential of this valve is known, and the publication of long-term results of the initial clinical and preclinical trials is the first step in this exciting trip. These results will probably dictate the next steps that need to be taken. The durability of the initial result, the interplay between the structural changes and the functional performance during the course of the bioresorption process of the valve, the presumed limited intrinsic potential of elderly people to support the restoration process with endogenous cells and the behaviour of the valve in the more demanding environment of the arterial human circulation are only some of the potential limitations of this technology. It is conceivable that, as we sail in uncharted waters, both the translational capacity and the potential limitations of the Xeltis valve will be fully explored in the years to come. In 2002, an editorial on percutaneous valve implantation in the European Heart Journal24 raised a question (Back to the future?) that has been largely answered in the meantime by the extraordinary development of the percutaneous treatment of the four valves of the heart. Today, in 2017, we raise a new question: is restorative valve therapy by ETR tomorrow’s world?
Conflict of interest statement
P.W. Serruys and M.B. Leon are members of the Advisory Board of Xeltis. T. Carrel is Principal Investigator of the Pulmonary Conduit Trial and a member of the Advisory Board of Xeltis. R. Virmani has received institutional research support from 480 Biomedical, Abbott Vascular, ART, BioSensors International, Biotronik, Boston Scientific, Celonova, Claret Medical, Cook Medical, Cordis, Edwards Lifesciences, Medtronic, MicroPort, MicroVention, OrbusNeich, ReCore, SINO Medical Technology, Spectranetics, Surmodics, Terumo Corporation, W.L. Gore and Xeltis; and honoraria from 480 Biomedical, Abbott Vascular, Boston Scientific, Cook Medical, Lutonix, Medtronic, Terumo Corporation and W.L. Gore; and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. M. Cox is employed by Xeltis and has shares of Xeltis. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Xeltis technology - endogenous tissue restoration for bioresorbable valves.
Moving image 2. MRI frontal image and 4D flow MRI study of a patient from the first-in-man study of the Xeltis Fontan conduit at 26 months post implantation.
Supplementary data
To read the full content of this article, please download the PDF.
Xeltis technology - endogenous tissue restoration for bioresorbable valves.
MRI frontal image and 4D flow MRI study of a patient from the first-in-man study of the Xeltis Fontan conduit at 26 months post implantation.

