
Abstract
Aims:The Xeltis bioabsorbable pulmonary valved conduit (XPV), designed to guide functional restoration of patients’ own tissue, is potentially more durable than current pulmonary bioprosthetic valves/valved conduits. The aim of this study was to assess the haemodynamic performance of the novel XPV implanted in an ovine model.
Methods and results:The XPV was surgically implanted in adult sheep under general anaesthesia and cardiopulmonary bypass (XPV group, n=20). Sheep that received a Hancock bioprosthetic pulmonary valved conduit served as a control group (HPV group, n=3). Transthoracic echocardiograms from VARC-2 recommended time points at 3, 6, 9, 12, 18 and 24 months (XPV group) and at 3 and 6 months (HPV group) after the procedure were analysed in an independent core laboratory. The primary endpoint was favourable valved conduit performance, defined as peak systolic pressure gradient <40 mmHg, no severe pulmonary regurgitation (PR), and a maximum conduit patency index of -20%. In the latter, negative values denote luminal narrowing and vice versa. The valvular peak systolic pressure gradient (mmHg) was 25.6±9.7 (3 months), 19.6±7.1 (6 months), 10.0±9.2 (24 months) in the XPV group and 18.4±6.6 (3 months), 17.7±4.6 (6 months) in the HPV group. The patency index (%) of the conduit at the valvular level was +30.3±13.6 (6 months) and +64.1±1.4 (24 months) in the XPV group and +2.0±15.9 (6 months) in the HPV group. PR was trace or mild at all visits, except in one animal with persistent moderate PR in the XPV group, up to 24 months.
Conclusions: The XPV showed a favourable and durable haemodynamic performance (up to two years after implantation), without conduit narrowing/obstruction or severe regurgitation.
Abbreviations
ETR: Endogenous Tissue Restoration
HPV: Hancock pulmonary valved bioprosthesis
PA: pulmonary artery
PR: pulmonary regurgitation
RV: right ventricle
RVOT: right ventricular outflow tract
XPV: Xeltis pulmonary valved conduit
Introduction
Congenital defects involving the right ventricular outflow tract (RVOT) and the pulmonary artery (PA), such as tetralogy of Fallot, pulmonary atresia, or transposition of the great arteries with pulmonary stenosis, represent about 20-40% of patients with congenital heart disease who survive until adulthood1,2. Reconstruction of the RVOT is part of the surgical repair of these conditions, and inevitably portends recurrent RVOT dysfunction requiring the implantation of a right ventricle (RV)-to-PA conduit or a prosthetic pulmonary valve. Unfortunately, the lifespan of these conduits is much shorter than the lifespan of the recipient patients because they degenerate, resulting in pulmonary regurgitation (PR), with resultant progressive RV dilation and failure3-6. In addition, these conduits do not allow for the natural growth of the child. In order to overcome these limitations, the development of more biocompatible conduits that have the potential to grow has been a challenging quest in recent years. The approaches to engineering a biocompatible pulmonary valve with growth potential typically involve the seeding of cells (endothelial cells, stem cells, amniotic fluid-derived cells, or autologous progenitor cells) to populate various scaffolds composed of biodegradable polymers, autologous tissue, or allograft or xenograft matrixes7-13. More recently, a new technology was developed based on a biodegradable polymer matrix designed to enable Endogenous Tissue Restoration (ETR) without the use of stem cells or animal-derived products14. The novel polymeric valved conduit allows the patient’s own cells to infiltrate and trigger a cascade of physiological events leading to gradual replacement of prosthetic material by native tissue. In this study, we sought to investigate the midterm performance of a novel biodegradable polymeric pulmonary valved conduit (XPV) in a sheep model.
Methods
STUDY DESCRIPTION
The study included 23 adult Swifter sheep (age: two to four years, weight: 60-90 kg). The pulmonary valved conduit (Xeltis BV, Eindhoven, the Netherlands) was implanted in 20 sheep (XPV group) while the 22 mm Hancock® bioprosthetic valved conduit (Medtronic, Minneapolis, MN, USA), which consists of a porcine aortic valve sutured into the centre of a woven fabric conduit was implanted in three control sheep (HPV group). Two animals died early after implantation of the XPV because of arrhythmia and extensive clamping during the procedure. A third animal was sacrificed after five months due to infective endocarditis. Nine animals have no data available. Nine animals eventually constituted the XPV group available for follow-up. The study flow chart is shown in Figure 1.
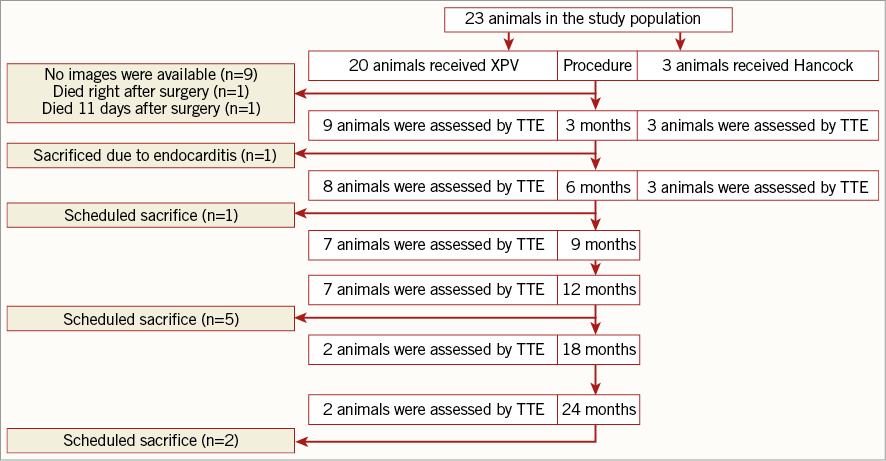
Figure 1. Flow chart of the study and the key echocardiographic assessment time points. TTE: transthoracic echocardiography
The study protocol adhered to the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes and the Guide for the Care and Use of Laboratory Animals and was reviewed and approved by the Test Facility’s Ethical Committee for compliance with regulations prior to study initiation (EC approval no. EC MxCl 2014 - 027).
THE BIODEGRADABLE PULMONARY VALVED CONDUIT DESCRIPTION
The XPV is a flexible, highly porous conduit with three leaflets, fabricated by electrospinning from a novel supramolecular elastomer. The XPV has a length of 5 cm and an inner diameter of 21 mm. The concept of XPV is based on ETR, in which gradual restoration of the absorbable leaflet and conduit wall by the patient’s own cells takes place. The leaflet and conduit are infiltrated by inflammatory cells, which release growth factors and promote smooth muscle cell infiltration and matrix (proteoglycans and collagen with focal elastic tissue) production, while the same inflammatory cells also ensure the absorption of the polymeric implants.
SURGICAL TECHNIQUE AND POSTOPERATIVE MANAGEMENT
The conduits (XPV 21 mm or HPV 22 mm) were surgically implanted under general anaesthesia and normothermic cardiopulmonary bypass on the beating heart. The heart was exposed by a left anterolateral thoracotomy through the third intercostal space. After transection of the native pulmonary artery, the graft was implanted as an interposition pulmonary artery vascular graft 0.5-1.0 cm above the native pulmonary valve, the leaflets of which had been surgically removed. The antithrombotic regimen consisted of enoxaparin (Clexane®; Sanofi Aventis, Diegem, Belgium) 20 mg twice daily and aspirin (Aspegic®; Sanofi Aventis) subcutaneous injection 250 mg for five days.
ECHOCARDIOGRAPHIC ACQUISITION
Echocardiographic acquisition was performed using a Vivid-I (GE Healthcare, Chicago, IL, USA) ultrasound machine in accordance with a pre-specified protocol in the Animal Test Facility (Medanex, Diest, Belgium). Transthoracic echocardiography (TTE) was performed on conscious animals at the following time points: preoperative, one week after the procedure, every four weeks thereafter up to two years, and one week before animal sacrifice. Standard echocardiographic views were used to obtain two-dimensional (2D), spectral Doppler, and colour Doppler interrogation of the pulmonary valve, RVOT, and the main PA. For the assessment of the pulmonary valved conduit performance, echocardiographic acquisition included the following views: 2D images of the entire conduit as well as pulsed wave (PW) Doppler, continuous wave (CW) Doppler, and colour Doppler at the subvalvular, valvular, and distal conduit regions.
CORE LABORATORY ECHOCARDIOGRAPHIC ANALYSIS
As recommended by the Valve Academic Research Consortium (VARC)-2 guidelines, we chose mandated timelines for echocardiographic analysis15. Transthoracic echocardiograms at 3, 6, 9, 12, 18 and 24 months (XPV group) after implantation were analysed for XPV primary and secondary performance endpoints. Comparison between the XPV and HPV groups was performed at 3 and 6 months after the procedure.
All transthoracic echocardiograms were analysed in an independent core laboratory (Cardialysis, Rotterdam, the Netherlands) using the Image Arena version 4.6.3 workstation (TOMTEC, Munich, Germany). Measurements of Doppler velocities and 2D dimensions were performed in accordance with published guidelines16-19. Figure 2 displays the locations at which the echocardiographic measurements of the conduit diameters and performance indices were performed.
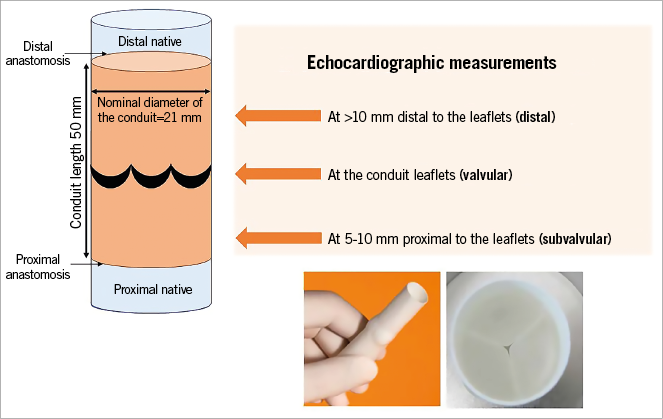
Figure 2. Diagram displaying the locations of echocardiographic measurements of the pulmonary valved conduit morphology and function.
CONDUIT FUNCTION
Haemodynamic assessments included peak velocity (m/sec) and mean and peak pressure gradients (mmHg) at the subvalvular and valvular levels of the conduit. Mean and peak pressure gradients were derived from PW or CW Doppler by manually tracing the spectral systolic velocity curves.
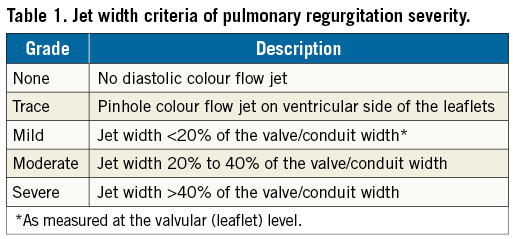
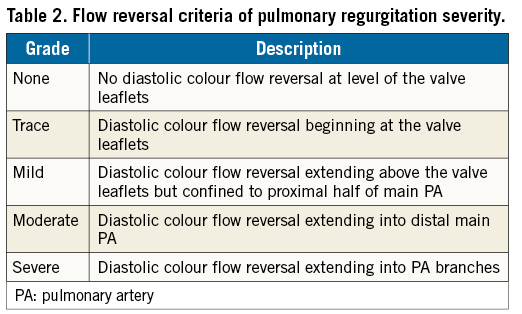
Severity of pulmonary valve regurgitation (PR) was graded on a five-grade scale as none, trace, mild, moderate, or severe based on jet width criteria (Table 1) and/or colour Doppler flow reversal criteria (Table 2)20. Clinically relevant PR was defined as at least mild PR.
CONDUIT MORPHOLOGY
Measurements of the conduit diameter at early to mid-systole were intended at the level of the conduit leaflets (valvular), 5-10 mm proximal to the leaflets (subvalvular), and >10 mm distal to the leaflets (distal).
The conduit patency index (CPI) was calculated to assess narrowing or expansion of the conduit at the subvalvular and valvular levels from three months post implantation to a given follow-up time point (=[conduit diameter at follow-up – conduit diameter at 3 months]/conduit diameter at 3 months%). A negative CPI value suggests conduit narrowing due to, e.g., neointimal hyperplasia, and a positive CPI suggests conduit expansion.
The primary endpoint of the study was the XPV performance at postoperative 3, 6, 9, 12, 18 and 24 months. A favourable performance of the XPV was defined as freedom from all of the following: 1) a transvalvular peak systolic pressure gradient >40 mmHg, 2) conduit narrowing as demonstrated by a reduction of the CPI of 20% or more, and 3) severe PR.
STATISTICS
Continuous variables with normal distribution were expressed as mean±SD, while continuous variables with non-normal distribution were expressed as median and interquartile range (IQR). No formal statistics were performed in this study due to the small sample size and the exploratory nature of the study.
Results
As shown in Table 1, all echocardiographic parameters were measurable in the majority of cases (88-100%), except for the distal conduit diameter, which could be reliably measured in only one third of cases. Therefore, measurements at the subvalvular and valvular conduit levels are reported in the results.
STUDY PRIMARY ENDPOINT (Table 3)
A favourable valve haemodynamic performance was achieved in all study animals. The average peak systolic pressure gradient (PG) at the valvular level was 25.6±9.7 mmHg (3 months), 10.0±9.2 mmHg (24 months) in the XPV group and was 18.4±6.6 mmHg (3 months), 17.7±4.6 mmHg (6 months) in the HPV group. Except for a single case that showed a temporary rise of peak PG >40 mmHg at the three-month evaluation, all other cases did not show a significant rise (>40 mmHg) at any time point and tended to decline over time (Figure 3). Therefore, although they tended to be higher early post procedure, peak PG values were eventually lower in the XPV group at the end of follow-up than in the HPV group at 3 and 6 months (Table 3).
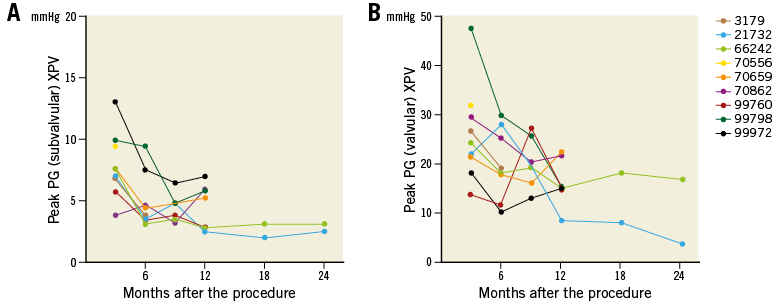
Figure 3. Serial changes of peak pressure gradient of individual animals at the subvalvular (A) and valvular (B) levels of the biodegradable pulmonary valved conduit.
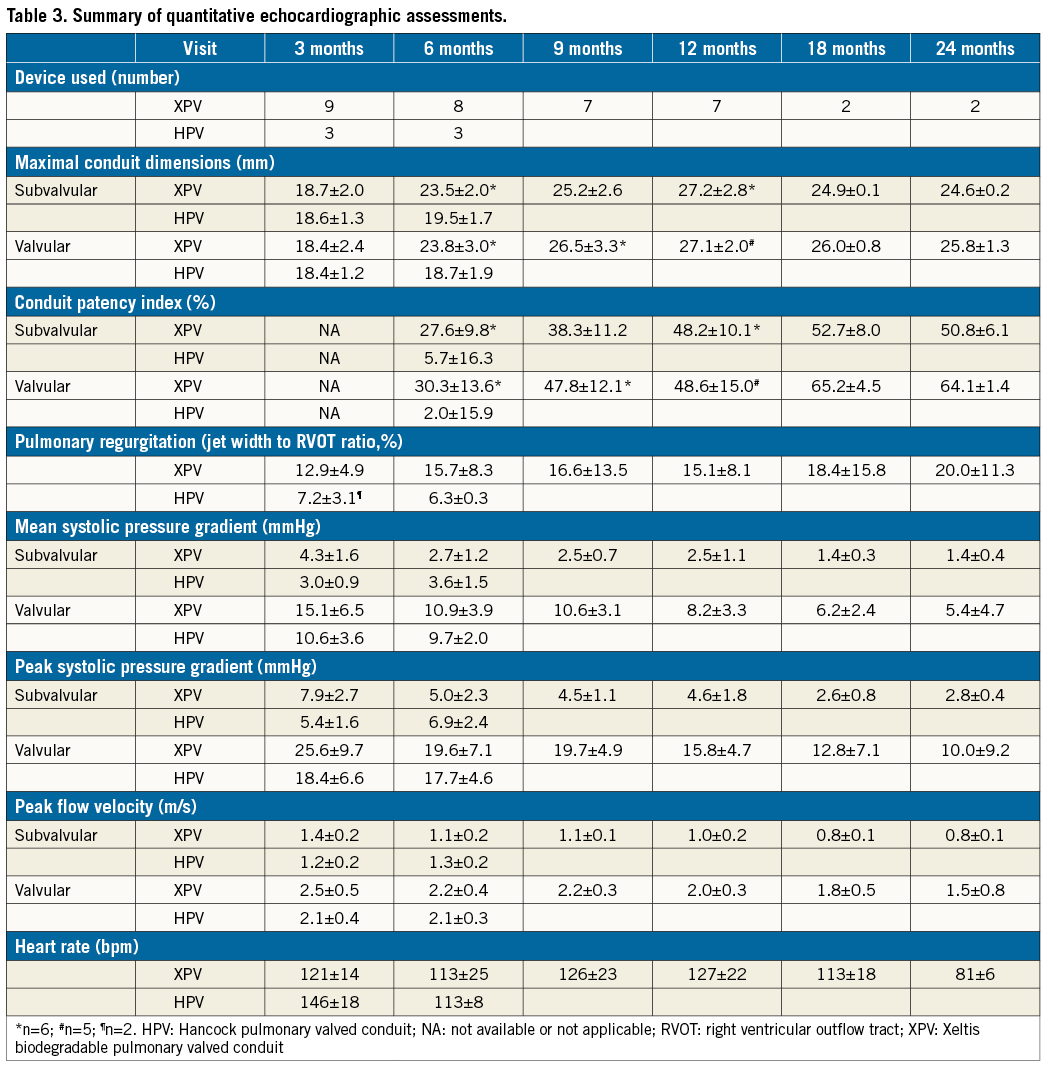
The CPI (%) in the XPV group was +27.6±9.8 at six months and +50.8±6.1 at 24 months post procedure at the subvalvular level, while it was +30.3±13.6 at six months and +64.1±1.4 at 24 months at the valvular level. At six months, the CPI in the HPV group was +2.0±15.9 at valvular level and +5.7±16.3 at subvalvular level (Table 3). None of the animals in the XPV group had a narrowing >20% across the conduit at any time point (Figure 4).
Figure 4. Serial changes of the conduit patency index of individual animals at the subvalvular (A) and valvular (B) levels of the Xeltis biodegradable pulmonary valved conduit.
PR severity was trace or mild at all follow-up time points in eight animals and consistently moderate in one animal in the XPV group, while PR was trace in the HPV group animals up to six months (Table 4, Figure 5). In the XPV group, the PR jet width to RVOT ratio was <20% in five animals and >20% in two animals 12 months after implantation. According to the flow reversal criteria, PR severity was trace or mild in six animals and moderate in one animal 12 months after procedure in the XPV group. There was a discrepancy of the PR severity between jet width criteria and flow reversal criteria in a single animal, in which PR was eventually adjudicated as mild.
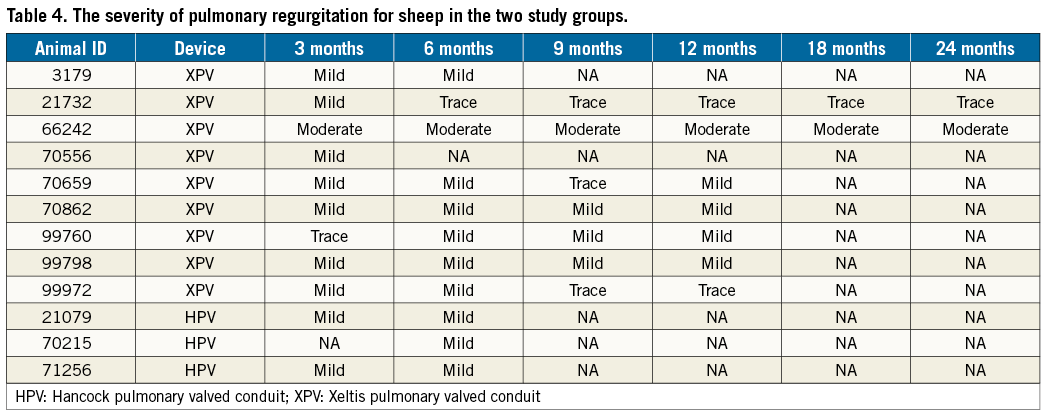
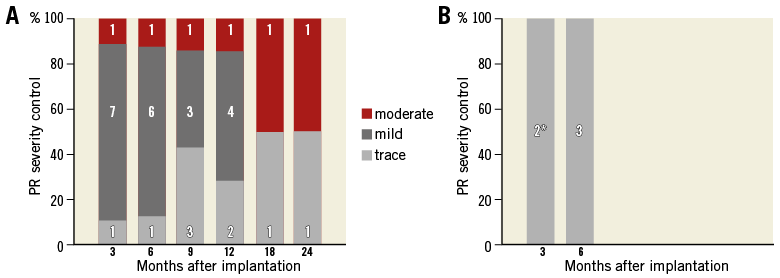
Figure 5. The severity of pulmonary regurgitation at different time points after implantation of the biodegradable pulmonary valved conduit (A) and the Hancock valved conduit (B). The number of subjects remaining at each time point, for each severity, is shown in the bars. *Animal #70215 images were non-analysable.
OTHER STUDY ENDPOINTS
Other parameters of valve performance were examined in the XPV group at 12 and at 24 months after the procedure and compared to the respective measurements performed at 3 and 6 months in the HPV group. Mean systolic PG at the valvular level was 15.1±6.5 mmHg (3 months), 10.9±3.9 (6 months) and 5.4±4.7 mmHg (24 months) in the XPV group and was 10.6±3.6 mmHg (3 months) and 9.7±2.0 mmHg (6 months) in the HPV group. Mean systolic PG was <20 mmHg in all XPV group animals at both the subvalvular and the valvular levels of the conduit 12 months (n=7) and 24 months (n=2) after the procedure. Mean systolic PG decreased over time in the XPV group while it did not change in the HPV group between 3 and 6 months after implantation (Figure 6).
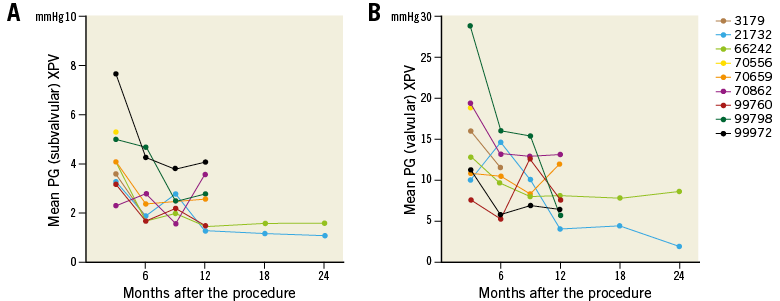
Figure 6. Serial changes of mean pressure gradient of individual animals at the subvalvular (A) and valvular (B) levels of the biodegradable pulmonary valved conduit.
None of the animals in the XPV group had a peak velocity >3 m/s at subvalvular or valvular levels of the conduit 12 and 24 months after the procedure (n=7). Although one animal had a peak velocity >3 m/s at the valvular level 3 months after the procedure, the peak velocity at 6 months decreased to <3 m/s. Peak velocity decreased over time in the XPV group (Figure 7, Table 3), while peak velocity in the HPV group did not change between 3 and 6 months (Table 3).
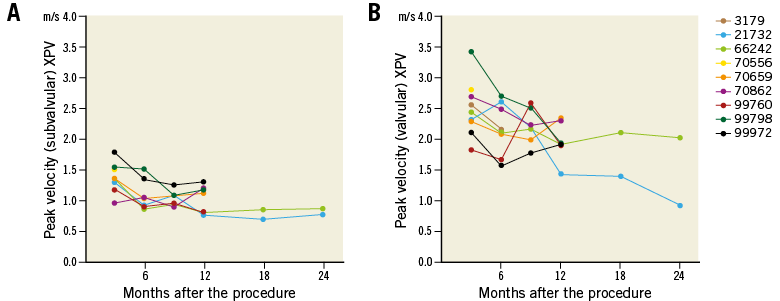
Figure 7. Serial changes of peak velocity of individual animals at the subvalvular (A) and valvular (B) levels of the biodegradable pulmonary valved conduit.
The subvalvular conduit diameter of XPV increased from 18.7±2.0 at 3 months to 27.2±2.8 and 24.6±0.2 at 12 and 24 months, respectively. In contrast, the subvalvular conduit diameter in the HPV group was 18.6±1.3 and 19.5±1.7 at 3 and 6 months, respectively. Similarly, the valvular conduit diameter of XPV increased from 18.4±2.4 at 3 months to 27.1±2.0 and 25.8±1.3 at 12 and 24 months, respectively, while in the HPV group it was 18.4±1.2 vs. 18.7±1.9 at 3 and 6 months, respectively (Figure 8, Table 3).
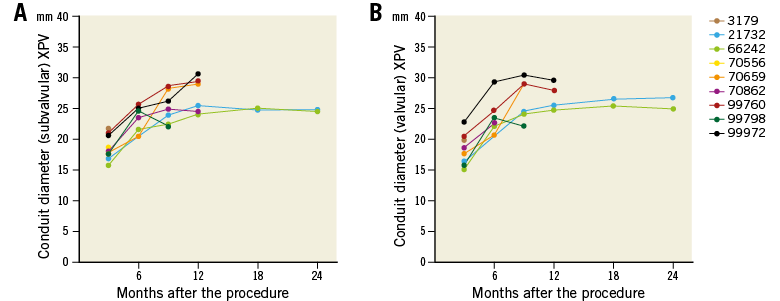
Figure 8. Serial changes of conduit diameter of individual animals at the subvalvular (A) and valvular (B) levels of the biodegradable pulmonary valved conduit.
Discussion
In 20-40% of adults with congenital heart disease, there is an RVOT abnormality requiring implantation of an artificial conduit between the RV and the PA1,2. Regardless of the technique used to reconstruct the RVOT, conduit dysfunction is common, and freedom from degeneration at 10 years is 51% in the case of homografts implanted before the age of 10 years5. Accordingly, many patients have their third RVOT intervention by the age of 20 years21. Reintervention is associated with discomfort, disfigurement, cost, morbidity, and mortality.
The XPV is a next-generation conduit fabricated from a novel supramolecular elastomer that enables endogenous cells to populate the scaffold and to produce matrix. In the present study, echocardiographic analysis by an independent core laboratory showed a favourable haemodynamic performance as well as an acceptable regurgitation severity of the XPV, sustained up to two years after implantation. Morphologically, the XPV showed no narrowing or abnormal growth during the follow-up period. The definition of a favourable valve haemodynamic performance in this report was based on the best clinical judgement, published data on established pulmonary prosthetic valves/conduits, and practice guidelines19,22,23.
In two animals, haemodynamic findings warrant more discussion. Animal #99798 initially had a high peak systolic PG at the valvular level at three months, which was consistently improving on later echocardiograms, eventually approaching acceptable values. The peak transvalvular gradient decreased to <30 mmHg at six months and to <20 mmHg on the 12-month echocardiogram. There are no clear echocardiographic findings, or other clinical ones, to explain this temporarily high PG. However, a transient thrombus is a likely explanation.
Moderate PR was consistently seen in animal #66242 up to 24 months after the procedure. This animal showed a CPI of up to +70%, denoting a considerable increase in the conduit diameter during follow-up, ranking highest among all the animals in terms of luminal expansion. On the other hand, the actual diameter of the XPV in that animal (#66242) was 25 mm, which is the same diameter as another animal (#21732) at the latest follow-up at 24 months. These values are, in fact, only 4 mm larger than the nominal implantation diameter of 21 mm. Likewise, another animal (#99972) with even more increased diameter at 12-month follow-up had only trace PR.
There are limited data on precise echo Doppler cut-off values to define prosthetic pulmonary valve dysfunction. The American Society of Echocardiography guidelines recommend using RV-to-PA peak velocity as well as peak and mean PG for the evaluation of prosthetic pulmonary valve function. The normal performance of a pulmonary prosthetic valve is defined by a peak transprosthetic velocity of <3.2 m/s and a mean transprosthetic pressure gradient <20 mmHg18. In the Melody Transcatheter Pulmonary Valve Post-Approval Study (NCT01186692), a mean pressure gradient of ≤30 mmHg and PR
In line with the haemodynamic data of established transcatheter and surgical pulmonary valve prostheses as well as the Hancock 22 mm conduit used in this study, the XPV has favourable haemodynamic data. These data are to be confirmed in a human clinical study, which is currently underway.
Limitations
Interpretation of this study is limited by the relatively small number of echocardiographic evaluations available. Echocardiograms at the immediate postoperative time point (t=0) were not available. Absence of a clear electrocardiographic signal on echocardiographic images was another limitation, particularly for PR assessment. Ideally, the conduit dimensions should be quantified on a three-dimensional imaging modality.
Conclusions
The XPV shows encouraging results in an adult sheep model up to 24 months after implantation without luminal narrowing or haemodynamic deterioration.
| Impact on daily practice The use of biodegradable valves and conduits, such as the Xeltis pulmonary valved conduit endogenous tissue restorative technology would minimise or even eliminate the complications related to the use of prosthetic materials. Patients would have fewer thrombotic or degenerative prosthetic valves, thus less need for antithrombotic use as well as less need for reintervention. Another important aspect of this novel endogenous restorative technology is that it allows growth of the implanted material. The latter is essential in treating children with congenital heart disease. This study suggests that haemodynamic performance of a novel pulmonary valved conduit is favourable up to two years after implantation in a preclinical setting. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat, Paris, and University Paris VII, Paris, France.
Conflict of interest statement
M. Brugmans and M. Cox are employed by Xeltis. P.W.Serruys is part of the Scientific Advisory Board of Xeltis. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

