Abstract
Aims: Antiplatelet treatment can cause a change in plasma levels of platelet microRNAs (miRNAs). However, it is not clear whether the plasma level of platelet miRNAs can predict clinical outcomes of antiplatelet treatment. The present study aimed to evaluate the association of plasma miR-16, miR-21, miR-126, miR-26b, and miR-223 with the risk of clinical outcomes in dual antiplatelet-treated patients after percutaneous coronary intervention (PCI).
Methods and results: A total of 491 Han Chinese patients who had received PCI and dual antiplatelet therapy were sequentially recruited to the study and followed for up to one year. Plasma concentrations of five candidate miRNAs early the next morning after PCI were determined by quantitative reverse transcription PCR. The effect of the plasma miRNA level on major adverse cardiovascular events (MACE) within one year and bleeding within six months were assessed. We found that a higher plasma miR-126 level was significantly associated with a higher risk in terms of time-to-MACE. When compared with the plasma miR-126 level in the first three quartiles, the hazard ratio (HR) for the plasma miR-126 level in the fourth quartile was 2.61 (95% CI: 1.32-5.18, p=0.006). Multivariable Cox regression analysis showed that diabetes mellitus, ejection fraction, hypertension and a higher plasma miR-126 level were independent risk factors for MACE. Plasma miR-223 level was not an independent predictive marker for MACE. There was no significant association between the level of five plasma miRNAs and bleeding events during six-month follow-up.
Conclusions: Based on these results, we suggest that plasma miR-126 could be a potential marker for predicting major adverse cardiac events in patients after PCI.
Introduction
One of the significant advances in the treatment of coronary artery disease (CAD) has been percutaneous coronary intervention (PCI). However, PCI can cause mechanical endovascular injury, which may result in activation and aggregation of platelets and thrombus formation, and later neointimal hyperplasia, and in-stent restenosis (ISR)1. Although standard medications, including dual antiplatelet treatment of clopidogrel and aspirin, are used, some patients are still at high risk of adverse cardiovascular events, in part as sequelae of the pathophysiologic changes mentioned above. Therefore, intensive biomarker research and clinical validation are critically important for an effective monitoring of therapeutic efforts.
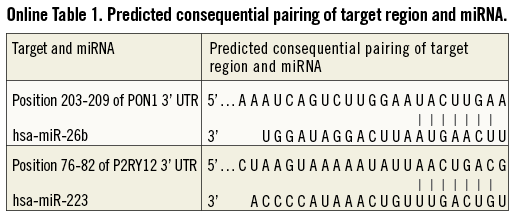
MicroRNAs (miRNAs) are a class of small non-coding cellular RNAs that regulate gene expression at the post-transcriptional level2. Some miRNAs are involved in the biological regulation of endothelium and smooth muscle cells. For example, miR-16 reduces blood vessel formation in vivo3. MiR-21 is found to play an important role in vascular smooth muscle cell proliferation and apoptosis, in cardiac cell growth and death, and in cardiac fibroblast functions4. MiR-126 is highly enriched in platelets5,6 and endothelial cells, and plays a pivotal role in maintaining endothelial homeostasis and vascular integrity7. Abnormal expression of miRNA regulating the biology of endothelium and smooth muscle cells may affect re-endothelialisation of diseased vessels. MiRNAs may also play an important role in the regulation of the pharmacokinetic and pharmacodynamic processes of the antiplatelet drug clopidogrel. For example, paraoxonase 1 (PON1), identified by Bouman et al8 as a key enzyme for clopidogrel metabolic activation, is predicted to have a conserved target of miR-26b (Online Table 1). MiR-223 is abundant in platelets5,6 and can target the mRNA of the P2Y12 receptor (Online Table 1), a direct target of the active metabolite of clopidogrel9. Abnormal expression of miRNA regulating key genes in the pharmacokinetic and pharmacodynamic pathway of clopidogrel may modify the clinical outcome of dual antiplatelet-treated patients after PCI.
MiRNAs were recently identified in several types of body fluid, from both healthy individuals and patients with various types of diseases, and may have potential to serve as novel non-invasive biomarkers10,11. Studies have shown an abundance of miRNAs exists in platelets and could transfer to the plasma compartment6,12. More recently, both Willeit et al6 and de Boer et al12 reported that antiplatelet treatment could cause a change in plasma levels of platelet microRNAs. For example, the administration of aspirin could lead to reduced levels of circulating miR-126 12. However, it is not clear whether the plasma level of these platelet miRNAs can predict clinical outcomes of dual antiplatelet treatment.
The present study evaluated whether the above five candidate miRNAs in plasma were associated with the risk of time to major adverse cardiac events (MACE) and occurrence of bleeding events in dual antiplatelet-treated Han Chinese patients after PCI.
Methods
ETHICS STATEMENT
This study was approved by the Medical Ethical Review Committee of Guangdong General Hospital. All patients who participated in the study provided written informed consent for the genetic and blood biomarker analysis.
PATIENTS AND TREATMENT
A total of 550 CAD patients undergoing PCI were sequentially enrolled in Guangdong General Hospital between January 2010 and October 2010. All patients were between 26 and 80 years old and were unrelated Han Chinese with a clinical presentation of acute coronary syndrome (ACS) who were undergoing drug-eluting stent placement. ACS was diagnosed as myocardial infarction (MI)13 or unstable angina14. Myocardial infarction was defined by an elevated troponin value in the setting of symptoms or electrocardiographic changes (both ST-segment elevation and non-ST-segment elevation changes) consistent with an MI13. Unstable angina was diagnosed if the patient had a negative cardiac biomarker and any one of the following: new-onset angina (<2 months) of at least class III according to the Canadian Cardiovascular Society, prolonged (>20 minutes) rest angina, recent (<2 months) worsening of angina, or angina that occurred within two weeks of an MI14.
All patients were pretreated with a loading dose of 300 mg clopidogrel before the procedure. Clopidogrel and aspirin maintenance doses were 75 mg and 80-100 mg daily, respectively. Demographic data, medical history, and medication were collected from the hospital information database. All patients used clopidogrel and aspirin during the entire follow-up period or at least until the bleeding event took place. Exclusion criteria included concomitant use of oral coumarin derivates or other anticoagulant treatment and, with INR larger than 1.5, any contraindication to aspirin or clopidogrel therapy, pre-existing bleeding disorders, being pregnant or lactating or planning to become pregnant, advanced cancer, and haemodialysis.
STUDY ENDPOINTS AND FOLLOW-UP
Follow-up information was collected based on inpatient and outpatient hospital visits, and telephone contacts with the patients or their family six months after PCI and on the date of last contact by June 2011. We adopted the primary efficacy and safety outcomes as study endpoints in the study. The primary clinical efficacy endpoint was the cumulative incidence of major adverse cardiac events (MACE), including cardiac death, non-fatal MI, stent thrombosis, and ischaemic stroke, during a one-year follow-up period.
The primary clinical safety endpoint of the study was the six-month incidence of combined alarming, internal, and nuisance bleeding events defined according to Serebruany et al15. Alarming bleeding included bleeding requiring a transfusion, intracranial bleeding, and life-threatening bleeding. Internal bleeding included haematoma, epistaxis, blood loss from the mouth, vagina, melaena, eye bleed, haematuria, and haematemesis. Nuisance bleeding included easy bruising, bleeding from small cuts, petechiae, and ecchymosis.
COLLECTION OF PLASMA
All blood samples were collected and anticoagulated with ethylenediaminetetraacetic acid (EDTA) dipotassium salt early the next morning before administering a maintenance dose of aspirin and clopidogrel, and after 20±2.1 hrs of PCI. The plasma was isolated within an hour by centrifugation at 3,000 g for 15 min at 4°C to retrieve plasma. The plasma was then stored at –80°C until assayed.
EXTRACTION OF TOTAL RNA
Total RNA containing small RNA was extracted plasma using a TRIzol® LS kit (Invitrogen, Shanghai, China) according to the manufacturer’s protocol with the following modifications. Into 250 μl plasma, 10 μl proteinase K was added and mixed and incubated for one hour at 56°C. Then the TRIzol® LS reagent was mixed in a 3:1 ratio with plasma and incubated for five minutes. After the addition of chloroform, tubes were shaken vigorously and centrifuged to separate the upper aqueous phase which was carefully transferred to a fresh tube. Isopropanol (0.5 ml) was then added to the aqueous phase for overnight storage at 4°C followed by centrifugation at 12,000×g for 10 minutes at 4°C. The RNA precipitate was washed with 75% ethanol and centrifuged at 12,000×g for five minutes. RNA was precipitated and collected by further centrifugation and storage in RNase-free water at –80°C.
MIRNA QUANTITATIVE REVERSE TRANSCRIPTION (QRT) PCR
Plasma concentrations of miR-16, miR-21, miR-126, miR-26b, miR-223 and reference snRNA U6 were determined by qRT-PCR. Two μg of total RNA were reverse-transcribed using M-MLV Reverse Transcriptase (Invitrogen, Shanghai, China) in accordance with the manufacturer’s protocol. In short, the 20 uL reactions were incubated for 30 min at 42°C, 5 min at 85°C, and then stored at –20°C.
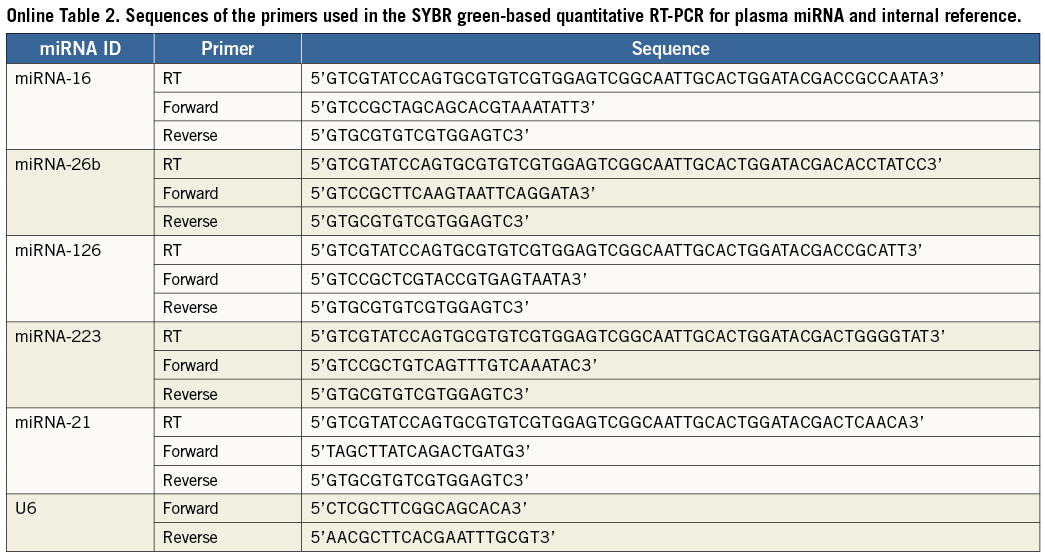
qRT-PCR were analysed using the Bulge-Loop™ miRNA qRT-PCR Detection Kit (Ribobio Co., Guangzhou, China) and TransStart™ Green qPCR SuperMix (TransGen Biotech, Beijing, China) in accordance with the manufacturer’s protocol with the MJ Opticon II Quantitative PCR System (MJ Research, Inc., Waltham, MA, USA). Briefly, the reactions were incubated at 95°C for five min, followed by 40 cycles of 95°C for 12 sec, 65°C for 50 sec. Triplicate samples, validated endogenous controls and interassay controls were used throughout. The relative expression level of each miRNA was calculated from the equation 2−ΔCt, where ΔCt=mean CtmiRNA−mean CtU6. The sequences of the primers used in the qRT-PCR reaction are listed in Online Table 2.
STATISTICAL ANALYSIS
Demographic and clinical characteristics were summarised using counts (percentages) for categorical variables and mean±standard deviation if data were normally distributed, or the median (interquartile range, Q1-Q3) for continuous variables if data were skewed for continuous variables. The normality of the distribution of continuous variables was checked by the Shapiro-Wilk test.
The primary endpoints of the time-to-MACE among three groups were categorised according to plasma miRNA levels using log-rank tests. All plasma miRNA levels were treated as three-category variables (levels below the first quartile coded as 0, levels between the first and the third quartile coded as 1, levels above the third quartile coded as 2). The crude hazard ratios (HRs) of plasma miRNA level and baseline characteristics of demographics, medical history, and medication data for time-to-MACE were determined using univariate Cox regression analyses. The adjusted HRs of variables for time-to-MACE were further estimated using multivariate Cox regression analyses with stepwise selection. A significance level of 0.10 is required to allow a variable into the multivariable Cox regression model, and a significance level of 0.10 is required for a variable to stay in the model.
A univariate logistic regression model was used to compare characteristics of baseline demographics, medical history, medication and plasma miRNA level during hospitalisation between the group with and the group without bleeding within six months. The adjusted odds ratios (ORs) of variables for bleeding were further estimated using multivariate logistic regression analyses with stepwise selection. A significance level of 0.10 is required to allow a variable into the multivariable logistic regression model, and a significance level of 0.10 is required for a variable to stay in the model.
Variables included age, gender (coded as 0/1), smoking status (as 0/1), diabetes mellitus, hypertension, previous infarction, previous stroke, left main coronary artery (coded as 0/1) and use of medications (coded as 0/1). The significance level for all statistical tests was set at p<0.05. Data analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
SAMPLE SIZE CALCULATION
In the present study, we performed sample size calculation using Stata 10 (StataCorp, College Station, TX, USA). According to previous data, patients with ACS in the fourth quartile of plasma miR-133a levels had a 2.5-fold higher risk of death compared with patients in the first three quartiles. Therefore, if the true hazard ratio of candidate miRNAs in plasma is 2.5 in patients undergoing PCI, the probability of MACE within one year is 0.08 and the proportion of withdrawals of patients is 0.1, so we would need to recruit 520 patients to be able to reject the null hypothesis that the survival curves among different plasma miRNA groups are equal with a power of 0.80. The Type I error probability was set to 0.05.
Results
BASELINE CHARACTERISTICS AND THEIR EFFECT ON MACE
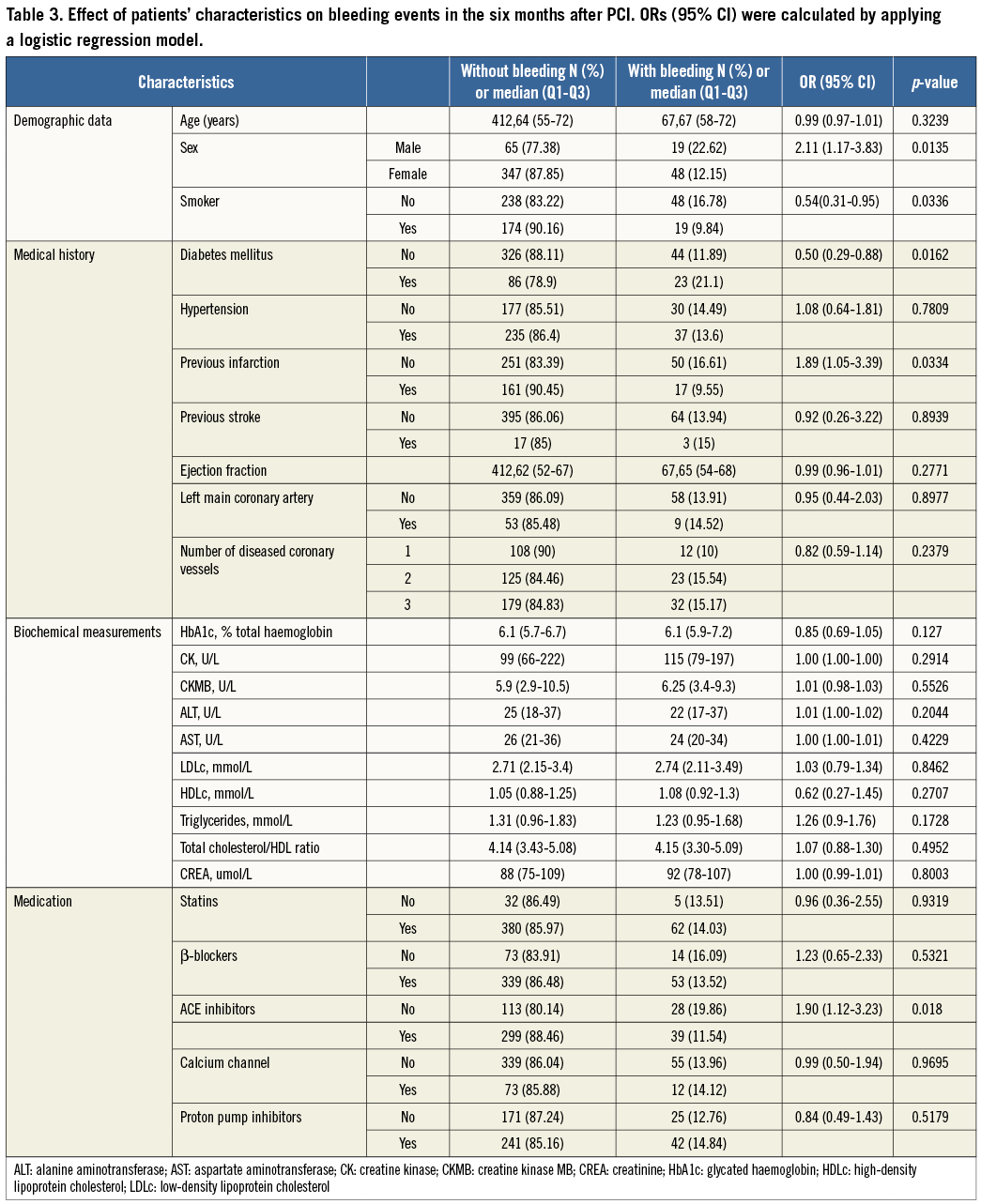
An overview of the enrolment and follow-up of the participants is presented in Figure 1. A total of 491 Han Chinese patients undergoing PCI and who had received clopidogrel therapy were available for analysis in the study. The patients’ characteristics and their association with MACE are given in Table 1. Their median age was 64 (Q1-Q3 56-73) years, approximately 83% were males, and 40% were active smokers. Higher age was associated with a higher risk for time-to-MACE (HR 1.04; 95% CI: 1.00-1.07). Conventional risk factors for coronary artery disease were frequent, 23% for diabetes and 59% for hypertension, and were associated with a higher risk for time-to-MACE after PCI (HR 2.54; 95% CI: 1.28-5.07 for diabetes, and HR 3.09; 95% CI: 1.5-6.4 for hypertension). Higher ejection fraction was associated with a lower risk for time-to-MACE (HR 0.97; 95% CI: 0.94-0.99). The use of comedications was frequent, and predominately statins (92.46%), β-blockers (81.87%), angiotensin-converting enzyme inhibitors (ACEIs) (70.47%), calcium channel blockers (26.68%), and proton-pump inhibitors (59.06%) were prescribed.
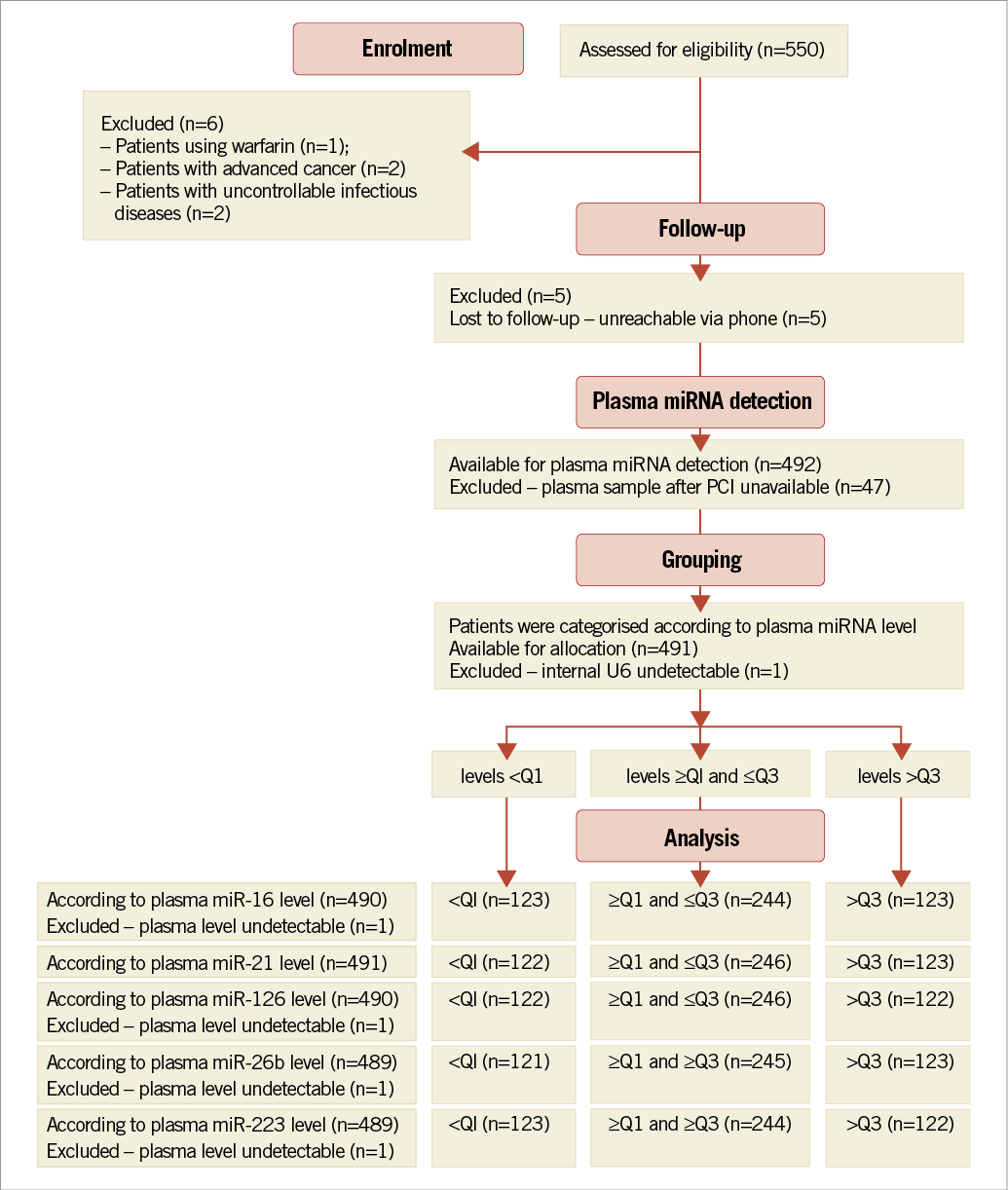
Figure 1. Flow chart of the enrolment and follow-up of the participants.
EFFECT OF PLASMA MIRNA LEVEL ON TIME-TO-MACE
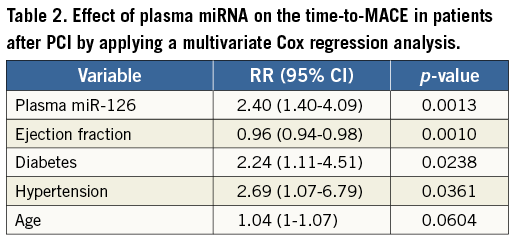
Among five plasma miRNAs, a higher plasma miR-126 level and a higher miR-223 level were associated with a higher risk for time-to-MACE (both p>0.05) (Figure 2). When compared with plasma miR-126 level in the first three quartiles, HR for plasma miR-126 level in the fourth quartile was 2.61 (95% CI: 1.32-5.18). When compared with plasma miR-223 level in the first three quartiles, HR for plasma miR-223 level in the fourth quartile was 2.98 (95% CI: 1.50-5.90). Multivariable Cox regression analysis revealed that a higher plasma miR-126 level, diabetes mellitus, and hypertension were independent risk factors for time-to-MACE with HR (95% CI) of 2.40 (1.40-4.09), 2.24 (1.11-4.51), and 2.69 (1.07-6.79), respectively, while a higher ejection fraction was a protective factor for time-to-MACE with HR (95% CI) of 0.96 (0.94-0.98) (Table 2). Plasma miR-223 level was out of the final model indicating plasma miR-223 level was not an independent risk factor for MACE.

Figure 2. Kaplan-Meier analysis of event-free survival from MACE in patients stratified by relative expression level of plasma miR-16 (A), miR-21 (B), miR-126 (C), miR-26b (D), or miR-223 (E).
EFFECT OF BASELINE CHARACTERISTICS AND PLASMA MIRNAS ON BLEEDING WITHIN SIX MONTHS
The primary safety endpoint of bleedings within six months occurred in 72 patients (14.1%) of the study population, including 45 patients with petechia or ecchymosis events, 26 with unusual bleeding events from the mouth or gums, nine with melaena or gastrointestinal bleeding events, two with epistaxis events, two with intracranial bleeding events, one with a haematuria event, and one with an eye bleeding event.
Baseline characteristics of the study population according to the status of bleeding within six months are shown in Table 3. In the univariate analysis, female sex (OR 2.11; 95% CI: 1.17-3.83) and diabetes mellitus (OR 1.941; 95% CI: 1.14-3.32) were associated with a higher risk of bleeding events, while previous infarction (OR 0.54; 95% CI: 0.31-0.95, p=0.0421), being a current smoker (OR 0.54; 95% CI: 0.31-0.95), and use of ACEIs (OR 0.51; 95% CI: 0.30-0.84) were associated with a lower risk of bleeding events. There was no significant difference in expression level of plasma miR-16, miR-21, miR-126, miR-26b, or miR-223 between patients with bleeding and without bleeding during six-month follow-up after PCI with or without adjustment for confounding factors (Figure 3).
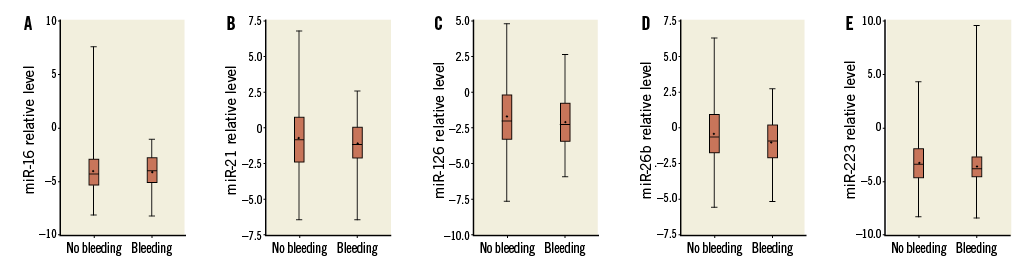
Figure 3. Comparison of expression level of plasma miR-16 (A), miR-21 (B), miR-126 (C), miR-26b (D), or miR-223 (E) between patients with bleeding and without bleeding during six months of follow-up after PCI.
Discussion
The results of our study show, for the first time, that a higher plasma miR-126 level was associated with a significantly higher risk for time-to-MACE in dual antiplatelet-treated patients after PCI, which was consistent with published data6,12. However, we did not observe a significant association between the levels of five plasma miRNAs with bleeding during six-month follow-up.
Recently, it has been reported that antiplatelet treatment could change circulating levels of platelet-derived microRNAs. Willeit et al6 reported that the dose escalation of aspirin in combination with prasugrel resulted in increasing platelet inhibition, and more potent platelet inhibition resulted in a reduction of plasma miR-126 and miR-223. de Boer et al12 also reported that the administration of aspirin led to reduced levels of circulating miR-126. The above results indicate that platelet miRNAs could serve as a surrogate marker of the efficacy of antiplatelet therapy. A lower level of plasma miR-126 and miR-223 may be related to more potent platelet inhibition, while a higher level may be associated with deficient platelet inhibition, which could result in the occurrence of MACE.
MiR-223 is the most abundant miRNA in platelets, followed by miR-1265, and could target the mRNA of P2RY12. Univariate Cox regression analysis showed a higher plasma miR-223 level was associated with a higher risk for time-to-MACE. However, in the multiple Cox regression analysis, miR-223 was removed from the model, when miR-126 was added into the model. The dependent effect of plasma miR-223 to plasma miR-126 may be because they were packaged in the same microparticles and released together into the circulating blood16.
The predictivity of plasma miR-126 is based not only on its response to antiplatelet therapy, but also on its functionality on target cells. PCI causes endothelial denudation and activation and aggregation of platelets and thrombus formation, which may lead to restenosis and symptoms of myocardial ischaemia. Therefore, re-endothelialisation is essential for restoration of normal vascular homeostasis and regulation of neointimal hyperplasia1. The endothelial-specific miR-126 governs vascular integrity7,17, and may be essential for restoration of normal vascular homeostasis and regulation of neointimal hyperplasia. Previous studies indicated miR-126 played an important role in the endothelium through vascular endothelial growth factor (VEGF) signalling pathways17. Our in vitro study showed overexpression of miR-126 enhanced, whereas downregulation of miR-126 diminished, VEGF mRNA expression and protein expression in human EA.hy 926 endothelial cells (data not shown). Published data also showed that miR-126 could target VEGF-A and decrease the expression of VEGF in human lung carcinoma cell lines A549, Y-90 and SPC-A1, and can inhibit the growth of these cells18,19. We speculated that the increased plasma miR-126 level could decrease the expression of VEGF in endothelial cells, delay re-endothelialisation of the injured vessel, and therefore increase the risk of MACE after PCI.
Based on the potential function of circulating miRNA, we hypothesised that abnormal expression of plasma miRNA targeting PON1 and P2RY12 may modify its antiplatelet effects. However, we did not observe an independent effect of plasma miR-26b and miR-223 on the risk of time-to-MACE and occurrence of bleeding events. This null effect of plasma miR-26b may be explained by the limited effect of PON1 on the metabolic activation of clopidogrel20, which was also reflected in the controversial results of the association between genetic variation within PON1 and cardiovascular outcomes among patients undergoing PCI who were treated with clopidogrel8,20-22.
Limitations
Our study has limitations that merit mention. First, it was a single-centre study and the sample size was not large enough to ensure adequate power. Further studies with a larger sample size, including the study of an external reference population, are needed to validate the results. Second, we initially included some cardiac-specific miRNAs (including miR-1 and miR-133a) in the candidate miRNA list. However, our primary experiment data showed the levels of these two miRNAs were undetectable or very low. We therefore excluded these miRNAs from the analysis. Third, in this candidate plasma miRNA association study, the number of plasma miRNAs was small. Some important plasma miRNAs may have been missed. Whole plasma miRNA profiling should be performed to overcome this limitation in the future.
Conclusion
In conclusion, we have identified plasma miR-126 as a potential marker for predicting MACE in antiplatelet-treated Han Chinese patients after PCI. The results provide a basis for future efforts to develop plasma miRNA-based assays to predict adverse cardiovascular response to PCI in antiplatelet-treated patients.
Funding
This work was supported by the National Key Technology R&D Program of China (2011BAI11B07), National Science Foundation of China (81072701), Natural Science Foundation of Guangdong Province, China (S2011010005830).
Conflict of interest statement
The authors have no conflicts of interest to declare.