The no-reflow phenomenon
The no-reflow phenomenon has been defined in 2001 by Eeckhout and Kern as inadequate myocardial perfusion through a given segment of the coronary circulation without angiographic evidence of mechanical vessel obstruction1. Rates of cardiac death and non-fatal cardiac events are increased in patients with compared to those without no-reflow2,3. The term “no reflow” encompasses the slow-flow, slow-reflow, no-flow and low-flow phenomenon. Its incidence depends on the clinical setting, ranging from as low as 2% in elective native coronary percutaneous coronary interventions (PCI) to 20% in saphenous venous graft (SVG) PCI and up to 26% in acute myocardial infarction (AMI) mechanical reperfusion4-6. Depending on the clinical setting, the mechanism of the no-reflow phenomenon differs. Distal embolisation and ischaemic-reperfusion cell injury prevail in patients with AMI, microvascular spasm and embolisation of aggregated platelets occur in native coronary PCI, whereas embolisation of degenerated plaque elements, including thrombotic and atherosclerotic debris are encountered during SVG PCI7. The no-reflow phenomenon is classified according to its pathophysiology with potential implications for its treatment in the categories provided in Table 1.

Diagnosis of no-reflow
The no-reflow phenomenon should be suspected in any situation of impaired TIMI flow. The adequate assessment of no-reflow necessitates to rule out several potential complications that can mimic this phenomenon. First, spasm of the epicardial coronary arteries should be excluded by the administration of i.c. nitroglycerin boluses preferably followed by a sub-selective contrast injection through a microcatheter applying a pull back. This manoeuvre enables to unmask an epicardial coronary dissection and/or other macrovascular obstruction. Although improved epicardial blood flow has been shown to reduce mortality in AMI, TIMI flow 3 does not exclude a no-reflow phenomenon. Continuous chest pain, haemodynamic compromise, new ST-segment changes during elective PCI or lack of ST-segment resolution in the setting of acute myocardial infarction should remind the operator to assess the quality of myocardial perfusion. The myocardial perfusion grade (blush) is the most convenient method to quantify the no-reflow phenomenon in the catheterisation laboratory and represents an independent predictor of mortality8. Myocardial contrast echocardiography, contrast enhanced MRI or CT are excellent additional diagnostic tools to investigate myocardial perfusion, although not readily available in the catheterisation laboratory.
Prevention of no-reflow
Due to the adverse impact of the no-reflow phenomenon on prognosis, effort should be performed in order to avoid its appearance. The first step to prevent the no-reflow phenomenon is to identify patients at risk prior to the procedure. Several predictors have been identified and are summarised in Table 27.
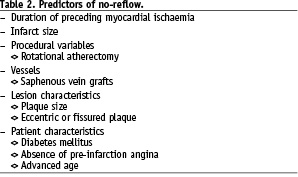
Moreover, the clinical setting of PCI is of primordial importance since preventive measures may vary. In the setting of a ST-segment elevation AMI, GP IIb/IIIa inhibitors and mechanical thrombus aspiration should be considered in order to prevent no-reflow. Both therapeutic modalities have been shown beneficial in terms of improving TIMI flow as well as clinical outcome6,9. Preventive measure of no-reflow are less well established in patients with non-ST-segment elevation MI. Thus, GP IIb/IIIa inhibitors have shown only a modest impact on surrogate endpoints such as cardiac biomarker elevations10. Conversely, distal embolisation protection devices have been shown to significantly reduced the rate of no-reflow and improve clinical outcome in the setting of SVG PCI in the SAFER trial11. In the setting of elective native coronary PCI, no universal preventive measure of no-reflow has been identified except for pre-loading with clopidogrel, which may be associated with a reduction in peri-interventional release of cardiac biomarkers. Furthermore, several recipes have been proposed to minimise no-reflow during rotational atherectomy12,13 (Figure 1).
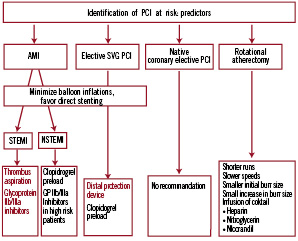
Figure 1. Algorithm for the prevention of the no-reflow phenomenon.
Treatment of no-reflow
Once the diagnosis of no-reflow has been established, initial treatment aims to restore a stable haemodynamic status by the use of fluid administration, vasopressor agents, oxygen delivery, and the use of an intra-aortic balloon pump in case of refractory hypotension. In parallel, the status of anticoagulation is assessed by measuring the ACT aiming at a target of >200-250 s. Despite lack of objective evidence to improve clinical endpoints, vasodilators are widely used owing to their beneficial impact on myocardial perfusion in several studies14-16.
An algorithm for the treatment of no-reflow is depicted in Figure 2.
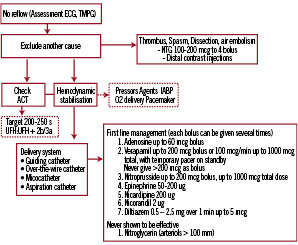
Figure 2. Algorithm for the treatment of no-reflow. The use of a microcatheter should be strongly considered for diagnosis and treatment.
Per consensus adenosine is considered as a first line drug owing to its strong vasoactive properties, ease of application and short half-life. Notwithstanding, the haemodynamic condition may favour the use of one drug over another. Thus, epinephrine may be preferred in cases of severe hypotension or haemodynamic compromise17.
Introduction of coronary perforations
Coronary perforation is a very rare but feared complication due to the associated morbidity and mortality. The incidence ranges from 0.1% to 3%. Clinical predictors of coronary perforations encompass the elderly, female gender, tortuous, calcified and small arteries18. Procedural predictors are rotational atherectomy, the use of particular guide wires (stiff & hydrophilic wires), oversized balloons (ratio artery to balloon of >1.2-1.3) and balloon rupture (pin hole effect)19. Ellis and coworkers20 introduced an angiographic classification of perforation distinguishing four types, a concept largely adopted in clinical practice. Distal perforations related to use of hydrophilic and/or rigid guide wires have not been accommodated in this classification, and we therefore propose to add a fifth type of perforation (type V) (Figure 3).
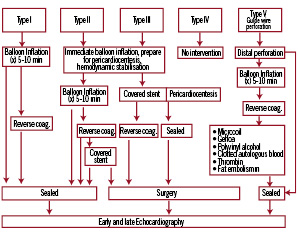
Figure 3. Algorithm for the management of coronary perforation. The traditional Ellis classification has been extended by a type V perforation, encompassing distal coronary perforations.
Treatment of coronary perforations
Management strategies depend on the severity of the perforation (i.e class I to V). The first aim is to prevent cardiac tamponade, which can be achieved by immediate balloon inflation proximal to the perforation site. Type I perforations are generally without clinical sequelae and seal spontaneously. Type II perforations can usually be managed by balloon inflation and require only rarely additional pericardiocentesis. Repeated balloon inflations lasting up to five to 10 minutes will seal the perforation (using low pressure to promote haemostasis) in the majority of cases. In case of failure, reversal of anticoagulation should be considered and can be achieved by administration of intravenous protamine sulphate, whereas patients treated with abciximab may require platelet transfusions. Type III perforations usually require emergency pericardiocentesis to prevent haemodynamic compromise. Moreover, the implantation of a covered stent should be immediately attempted whenever technically feasible21. Type V coronary perforations are inflicted by distal guidewire manipulation and should be treated first by balloon inflation. Alternatively and particularly in case of persistent leakage, embolisation of the artery may be considered to seal type V perforations. The use of microcoils22, gelfoam23, clotted autologous blood24, subcutaneous tissue25 as well as polyvinyl alcohol26 and thrombin27 have been described to manage this complication.
Once the perforation has been sealed, early echocardiography is advised to establish a baseline followed by late (24 hours) echocardiography in order to capture persistent leaks, which may complicate distal perforations and become clinically relevant only after several hours.

