In recent years, increasing attention has been given to capturing, evaluating, and improving the patient experience in the cardiac catheterisation laboratory. To this end, the European PATient Experience in the CATHeterisation Laboratory (PATCATH) questionnaire (Supplementary Figure 1) was developed1. This is a novel tool, produced by the Patient Initiatives Committee of the European Association of Percutaneous Cardiovascular Interventions (EAPCI), aiming to assess the patient’s experience of cardiac catheterisation. We report the initial experiences of using the tool in a high-volume tertiary referral centre.
The questionnaire was developed by the EAPCI in association with the European Society of Cardiology Patient Forum and divided into 3 domains, assessing experience before, during and after coronary angiography or intervention. Responses were recorded on a scale of strongly agree, agree, disagree, or strongly disagree. The questionnaires were distributed to patients attending the catheterisation lab for angiography or percutaneous coronary intervention (PCI) during the time period of the pilot study. A research nurse distributed the questionnaire and explained the rationale for the study but did not assist the patient with completion of the questionnaire. Participation was anonymous and voluntary. Information on patients who declined to fill out the questionnaire was not available. All questionnaires that were completed were analysed, even if they were only partially filled.
A total of 100 responses from patients in the catheterisation laboratory undergoing either elective or inpatient coronary angiography or PCI were received. A total of 52% of patients were grouped in the higher age category (≥66 years), most were male, and most underwent a diagnostic coronary angiogram (Supplementary Table 1). Of note, 21% of respondents included were >75 years old. The response rate for individual questions was on average 94.7±0.1%. Patient response indicated a high level of satisfaction with the experience before the procedure: 98.6% of patients strongly agreed or agreed with statements confirming that they were aware why the procedure was recommended and felt supported whilst awaiting it (Figure 1A). Furthermore, 98.5% strongly agreed or agreed with statements affirming a positive sense of comfort and safety during the procedure, with clear communication from the operator (Figure 1B). After the procedure, 59.8% reported a positive experience, with issues identified in comprehending necessary lifestyle changes (60%), the benefits of a rehabilitation program (46%), the rationale behind medication recommended (58%) and treatment duration (53%) (Figure 1C).
Patient-reported experience measures are a key element of the transition from volume-based to value-based cardiac care. Our initial pilot experience with the PATCATH tool was positive. Although response rates varied by specific question, the non-response rate was not greater than 30% for any individual question.
Some limitations of our study should be considered. Due to the pilot nature of the study, the survey was distributed by a research nurse. We cannot discount that this may have introduced bias in responses and may have resulted in a higher than usual participation rate. The fact that the questionnaire was administered shortly after the procedure might be a limitation. Administration at a later point may be preferable, although the value of an immediate evaluation for the experience would be missed, and the potential impact on the response rate should be considered. Our surveys were administered on paper, but an electronic form might further improve response rates and would allow capture of the completion time. In general, patient satisfaction was very high, which, although reassuring, suggests that further calibration of the tool might prove useful. Responses in relation to postprocedural care showed a higher degree of uncertainty. This may be due to information overload, where the patient is provided with so much information all at once that it is difficult to absorb and retain, or due to incorrect assumptions about the patient’s pre-existing level of knowledge. It may also be that some questions in the post-procedure domain are not relevant to all patients, e.g., those without findings of obstructive coronary artery disease. The impact of the local practice of routine administration of low-dose benzodiazepine (intravenous midazolam) at the time of the procedure should also be considered, although most questionnaires were administered just prior to discharge, when the residual effects of periprocedural sedation were likely minimal.
The results of this analysis suggest that a novel tool to assess patient satisfaction in patients undergoing coronary angiography and intervention was easy to administer and generally well understood. Higher uncertainty in the post-procedure domain suggests that further calibration of the tool may be helpful.
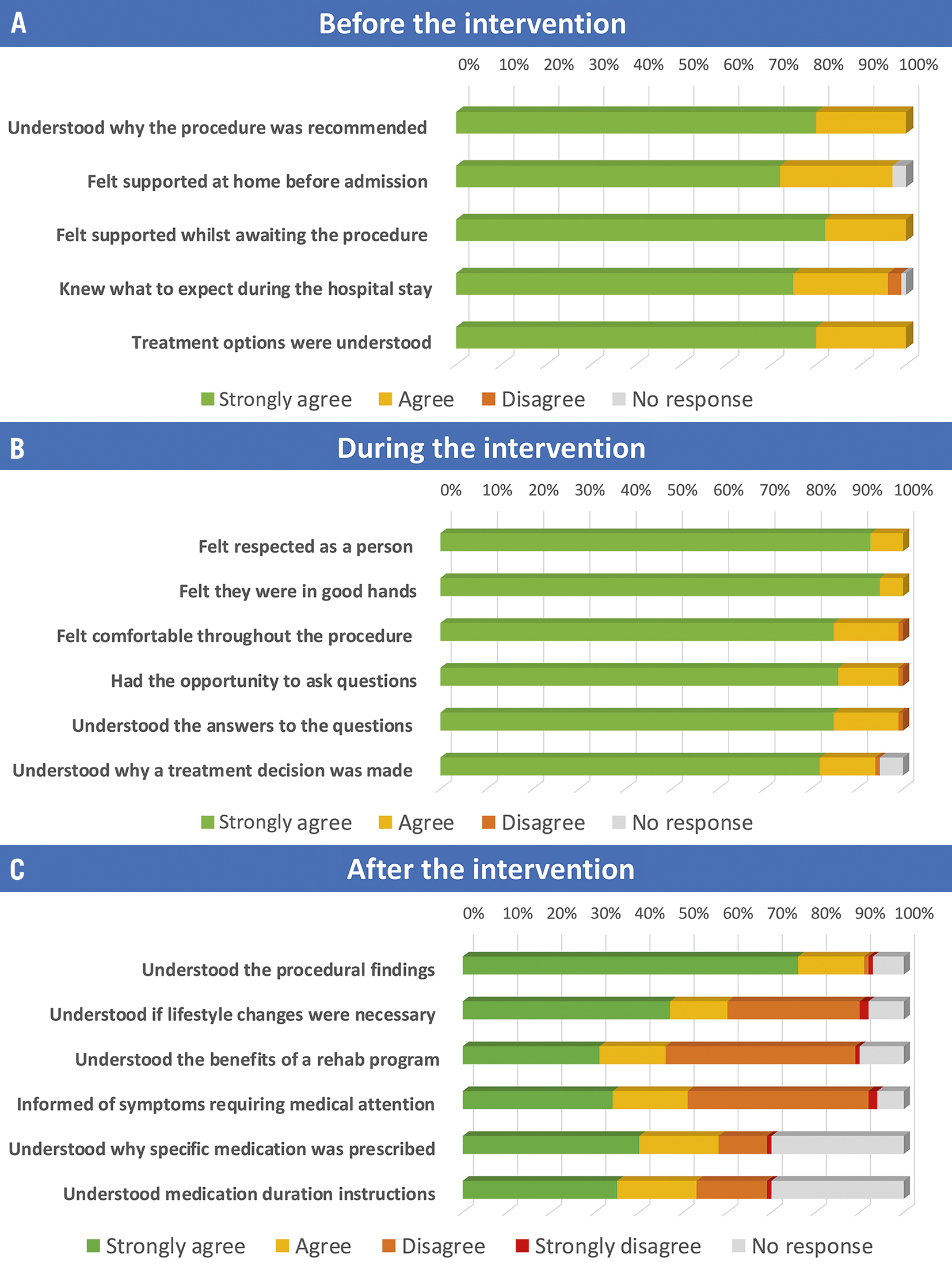
Figure 1. Patients’ responses to the PATCATH questionnaire. A) Before the procedure. Assessment of the patient’s understanding of the procedural indication, support whilst awaiting the procedure, and expectations of the hospital stay. B) During the procedure. Assessment of the patient’s experience of the procedure, in particular, their sense of safety, comfort and ability to understand the findings communicated during the procedure. C) After the procedure. Assessment of the aftercare instructions, in particular, the diagnosis, advice on lifestyle findings, benefits of a rehabilitation programme, and choice and duration of pharmacological treatment.
Funding
No extramural funding was used for this study.
Conflict of interest statement
R.A. Byrne reports research or educational funding to the institutions of employment (Mater Private Network and RCSI University) from Abbott Vascular, Biosensors, Boston Scientific, and Translumina, none of which impact in any way on his personal remuneration. R.A. Byrne does not receive personal fees from any medical device or pharmaceutical company. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

