
Abstract
Aims: We sought to evaluate the impact of stent size on angiographic and clinical outcomes after implantation of everolimus-eluting bioresorbable stents (BRS) in routine clinical practice.
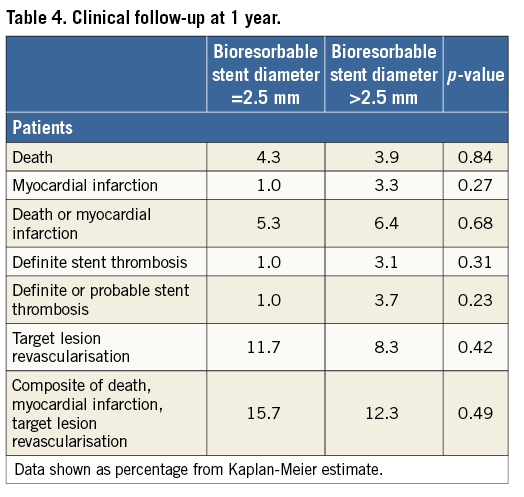
Methods and results: All consecutive patients undergoing BRS implantation at two centres in Munich, Germany, were included prospectively. The patient population was divided according to the diameter of the implanted BRS. Angiographic surveillance was scheduled at six to eight months after stent implantation and films were analysed in a core laboratory. A BRS with 2.5 mm diameter was implanted in 101 patients and BRS >2.5 mm diameter in 318. Baseline patient characteristics were similar in both groups. Reference vessel diameter was 2.36±0.22 mm in patients with an implanted 2.5 mm BRS and 3.03±0.40 mm in the other group (p<0.001). At angiographic follow-up, in-stent late luminal loss (0.28±0.47 mm vs. 0.25±0.52 mm, p=0.74) was similar in both groups, though binary angiographic restenosis was numerically higher in patients treated with a 2.5 mm BRS (12.5% vs. 6.1%, p=0.05). After 12 months, the rate of the composite of death, myocardial infarction or target lesion revascularisation was 15.7% vs. 12.3% (p=0.49). Definite stent thrombosis was detected in 1.0% vs. 3.1% (p=0.31).
Conclusions: In patients treated with BRS in routine clinical practice, angiographic and clinical outcomes were comparable in patients treated with a 2.5 mm stent as compared with those treated with a larger stent size.
Abbreviations
BRS: bioresorbable stent
DES: drug-eluting stent
QCA: quantitative coronary angiography
RVD: reference vessel diameter
TLR: target lesion revascularisation
Introduction
Bioresorbable stents (BRS) are an emerging technology for the treatment of coronary artery stenosis, designed to improve late outcomes, in comparison to standard metallic drug-eluting stents (DES), by reducing the risk of late stent thrombosis caused, at least in part, by a chronic inflammatory reaction to permanent stent implants1,2. Additional potential benefits of BRS include late luminal enlargement after stent resorption, restoration of vasomotor function and opportunities for non-invasive imaging surveillance2,3.
Initial randomised controlled trials demonstrated comparable results between BRS and conventional metallic DES4,5. However, further trials and data from large-scale clinical registries suggested higher rates of stent thrombosis with BRS as compared with standard DES6-8. The higher rate of events, particularly in the first 30 days after implantation, suggests that mechanical issues such as stent strut thickness and radial strength, as well as procedure-related factors and inappropriate lesion selection, may have increased the event rates. In a subgroup analysis of the ABSORB III trial, concerns regarding the implantation of BRS in small coronary vessels were raised, since patients with a median reference vessel diameter (RVD) of ≤2.63 mm showed a higher rate of target lesion failure (9.8% vs. 5.7%) and patients with an RVD of <2.25 mm had a higher risk for target lesion revascularisation (TLR) (6.6% vs. 2.2%) than those with a larger RVD9,10.
The aim of the present analysis was to determine the impact of BRS size on angiographic and clinical outcomes in patients undergoing stent implantation in daily practice.
Methods
PATIENT POPULATION
Between September 2012 and June 2014, all consecutive patients treated with at least one everolimus-eluting BRS (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) for single-vessel or multivessel percutaneous coronary intervention (PCI) of de novo lesions at two high-volume centres in Munich, Germany, were included. Further details have been published previously8. To analyse the impact of BRS diameter on the outcome, the total patient cohort was divided into two groups according to the diameter of the implanted BRS: one group comprised patients with at least one BRS with a diameter of 2.5 mm and the other group included patients who underwent implantation of a 3.0 or 3.5 mm BRS.
STUDY DEVICE AND IMPLANTATION
The investigated BRS consists of a poly-L-lactic acid backbone of circumferential hoops, which are linked by bridges. The 150 µm struts are coated with a mixture of poly-D-L-lactic acid and anti-inflammatory everolimus in a 1:1 ratio. The ultimate products of its dissolution are water and carbon dioxide, and the complete degradation process takes approximately 24-36 months11.
After thorough predilatation, BRS implantation was performed respecting the rated burst pressure of the delivery balloon. Post-dilatation and use of intravascular imaging guidance by optical coherence tomography or intravascular ultrasound were left to the operator’s discretion. Periprocedural unfractionated heparin or bivalirudin was administered for anticoagulation. All patients received 500 mg of aspirin loading followed by aspirin 100 mg indefinitely and an additional ADP receptor antagonist (clopidogrel, prasugrel or ticagrelor), including an initial loading dose for at least 12 months according to the clinical presentation and existing guidelines12.
ANGIOGRAPHIC ASSESSMENT AND CLINICAL ENDPOINTS
Assessment of the baseline, post-implantation and follow-up coronary angiography was performed off-line with an automated edge-detection software (CMS version 7.1; Medis medical imaging systems bv, Leiden, The Netherlands) at the quantitative angiographic (QCA) core laboratory (ISAResearch Center, Munich, Germany). Nitroglycerine was administered intracoronarily before recording cineangiograms. All measurements were performed as an in-stent as well as an in-segment (in-stent area and 5 mm proximal and distal to the stent) analysis in the same single worst view projection, and the contrast-filled non-tapered catheter tip was used for calibration. Standard criteria were used for morphological lesion characterisation13.
Angiographic success was defined as final TIMI 3 flow and a residual diameter stenosis of less than 30% as assessed by QCA. Angiographic follow-up was scheduled six to eight months after the index procedure and results were evaluated off-line at the QCA core laboratory. Parameters of interest comprised percentage diameter stenosis, in-segment binary restenosis, and in-stent late luminal loss, which was defined as the difference between the minimal lumen diameter after implantation and the minimal lumen diameter at the follow-up angiography.
Clinical follow-up was conducted via telephone or office visits. Clinical endpoints of interest were a composite of death, myocardial infarction and ischaemia-driven percutaneous or surgical TLR, components of the composite endpoint and the occurrence of stent thrombosis according to the Academic Research Consortium criteria14. All clinical endpoints were assessed after 12 months.
STATISTICAL ANALYSIS
Categorical variables are shown as counts and percentages and compared by chi-square or Fisher’s exact test, whereas continuous variables are shown as mean with standard deviation or median with interquartile range, which were compared by t-test. Event rates are presented as Kaplan-Meier estimates, and for comparison of both groups the Cox proportional hazards model was applied. A p-value of <0.05 was considered as statistically significant. Statistical analysis was performed with S-PLUS 4.5 (S-PLUS; Insightful Corp, Seattle, WA, USA).
Results
BASELINE CHARACTERISTICS AND PROCEDURAL FINDINGS
A total of 419 patients were treated with an everolimus-eluting BRS, 101 patients with a 2.5 mm BRS and 318 patients with a BRS larger than 2.5 mm.
Baseline patient characteristics were similar in both groups and are presented in Table 1. In both groups, most of the patients had multivessel coronary artery disease (80.2% vs. 74.8%; p=0.27). The rates of non-ST-elevation myocardial infarction (18.8% vs. 19.2%) and ST-elevation myocardial infarction (5.0% vs. 9.4%) were comparable in both groups (p=0.62).
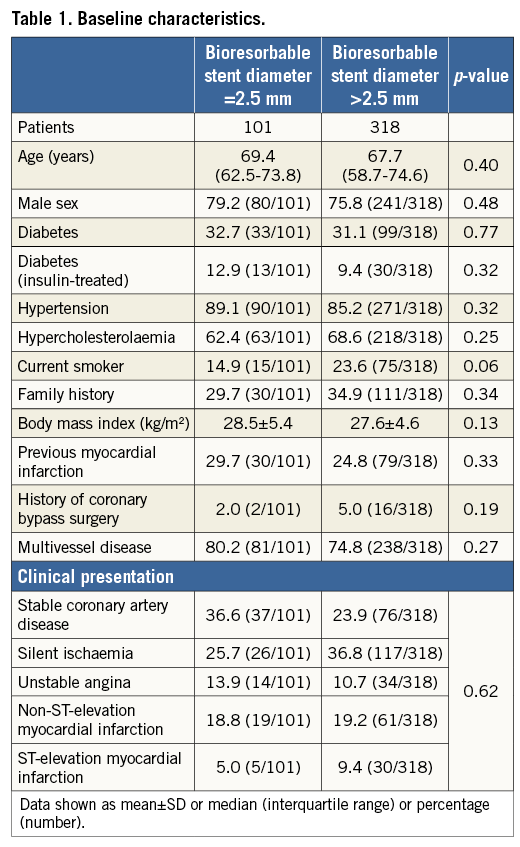
An overview of lesion and procedural characteristics is presented in Table 2. Of 133 lesions treated with a 2.5 mm BRS, 48.7% were classified as complex lesion morphology, whereas 49.1% of a total of 440 lesions treated with larger BRS sizes were considered to be complex (type B2/C lesions according to the ACC/AHA classification) (p=0.94). Bifurcation lesions (15.9% vs. 13.2%; p=0.45) as well as chronic total occlusions (0.9% vs. 1.6%; p=1.0) were treated in a comparable proportion in both groups. The mean reference vessel diameter by QCA was 2.36±0.22 mm and 3.03±0.40 mm, respectively (p<0.01). The mean lesion length was shorter in the patient group treated with a 2.5 mm BRS (13.4±5.8 mm vs. 16.5±10.0 mm; p<0.001).
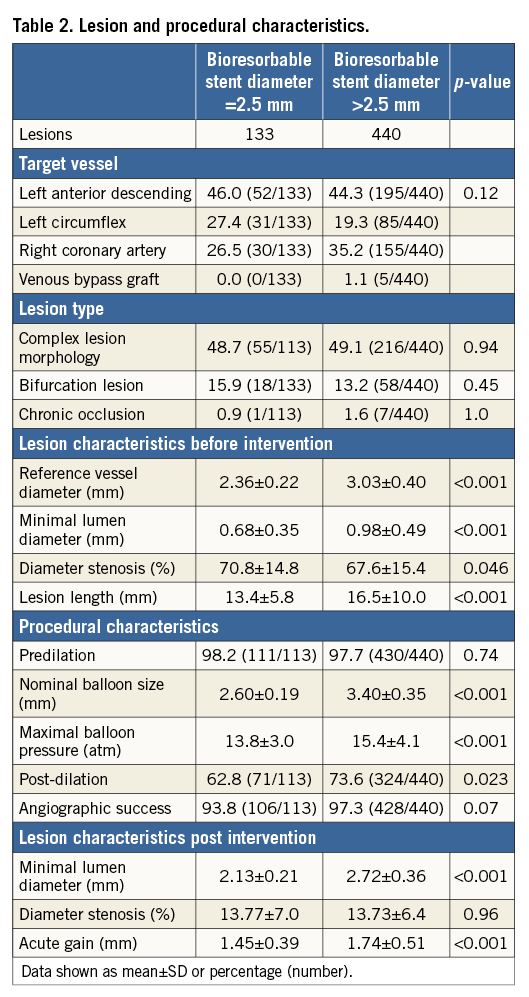
Almost all lesions in both groups were predilated (98.2% vs. 97.7%; p=0.74). Post-dilatation was more often applied in lesions treated with BRS >2.5 mm (62.8% vs. 73.6%; p=0.023). The maximum balloon size measured by QCA was 2.60±0.19 mm versus 3.40±0.35 mm (p<0.001), and the maximum balloon pressure was lower in patients treated with a 2.5 mm stent (13.8±3.0 atm vs. 15.4±4.1 atm; p<0.001). Acute gain was lower in patients treated with a 2.5 mm stent (1.45±0.39 mm vs. 1.74±0.51 mm, p<0.001), though relative acute gain (as a percentage of baseline reference vessel diameter) was higher (61.5% vs. 57.4%, p=0.020). Angiographic success at the end of the procedure was high in both groups (93.8% vs. 97.3%; p=0.07). The mean number of stents per lesion was 1.02±0.13 versus 1.18±0.47, respectively (p<0.001).
ANGIOGRAPHIC FOLLOW-UP DATA
Angiographic surveillance was performed in 73.3% (74/101) of the patients treated with a 2.5 mm BRS and in 66.7% (212/318) of the patients with a larger BRS size (p=0.21). In terms of mean in-stent late lumen loss (0.28±0.47 mm vs. 0.25±0.52 mm; p=0.74) and in-segment diameter stenosis (28.8±17.1% vs. 27.1±15.8%; p=0.43), there was no statistically significant difference between both groups. In addition, a trend towards a higher rate of binary restenosis was observed in patients treated with a 2.5 mm BRS, which was of borderline statistical significance (12.5% vs. 6.1%; p=0.05).
A total of 33 (32.7%) patients treated with a 2.5 mm BRS also had lesions treated with a larger stent. Sensitivity analysis with exclusion of these patients did not substantially alter the angiographic results (mean in-stent late lumen loss 0.29±0.43 mm vs. 0.25±0.53 mm, p=0.57; binary restenosis 11.8% vs. 6.0%, p=0.14). Details of QCA analysis at follow-up are presented in Table 3 and Figure 1.
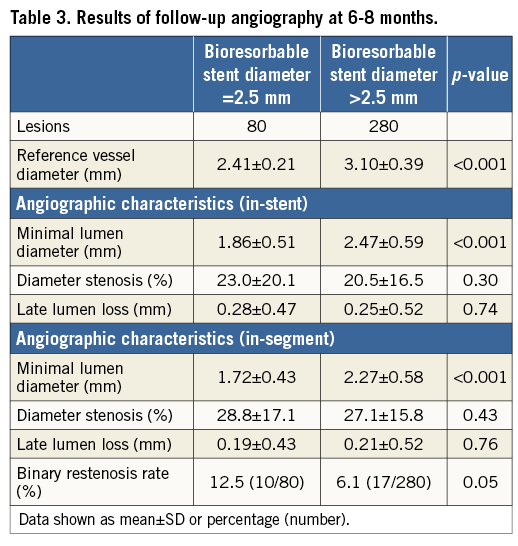
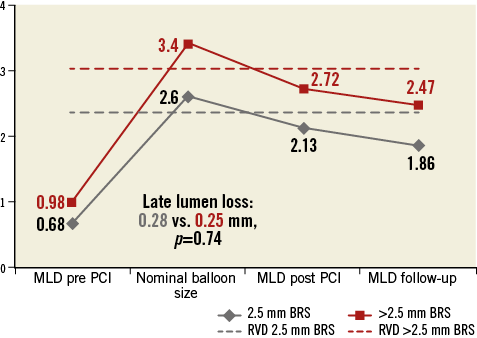
Figure 1. Comparison of angiographic findings at time of implantation and follow-up according to implanted stent size.
CLINICAL OUTCOME DATA
The median follow-up duration was 360 (268-360) days. The incidence of the composite of death, myocardial infarction and TLR was 15.7% in the group treated with 2.5 mm BRS and 12.3% for patients with a larger size BRS implanted (p=0.49) (Figure 2). The rate of TLR was also comparable in both groups (11.7% vs. 8.3%; p=0.42). Definite stent thrombosis was observed in 1.0% of patients treated with a 2.5 mm BRS versus 3.1% of patients treated with a larger stent (p=0.27) (Figure 3). The composite safety endpoint of death and myocardial infarction did not differ between both groups (5.3% vs. 6.4%; p=0.68). Details of clinical outcome can be found in Table 4.
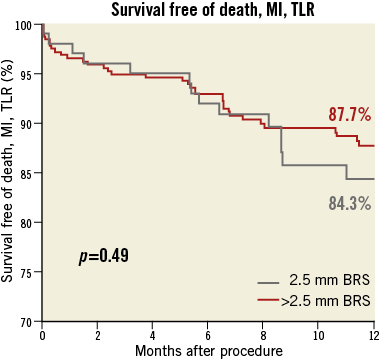
Figure 2. Freedom from the composite of death, myocardial infarction or target lesion revascularisation at 12 months.
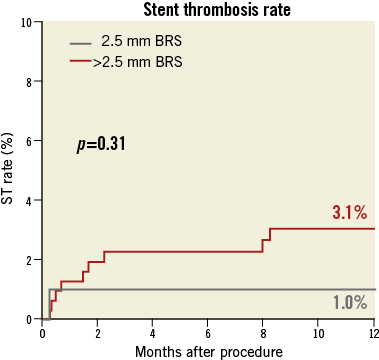
Figure 3. Definite stent thrombosis at 12 months.
SUBGROUP ANALYSIS OF PATIENTS WITH VERY SMALL VESSEL SIZE
We further divided patients treated with a 2.5 mm stent into tertiles based on measured RVD. The lowest tertile comprised patients with a measured RVD of 2.27 mm or less. Of these patients, during follow-up one patient underwent TLR and no patient suffered stent thrombosis.
Discussion
In our study of consecutive patients treated with everolimus-eluting BRS in routine practice, we evaluated the impact of implanted stent size on clinical and angiographic outcomes. The major findings of the present analysis are:
1. The baseline characteristics of patients treated with a 2.5 mm versus a larger stent were similar.
2. The angiographic success rates were broadly comparable between both groups, implying that BRS implantation in small vessels appears technically feasible.
3. In selected patients undergoing angiographic surveillance after six to eight months, the angiographic antirestenotic efficacy was high in patients treated with a 2.5 mm BRS, as well as those treated with a larger BRS.
4. No significant differences were detected regarding overall clinical outcome and rates of stent thrombosis after one year, in relation to the size of the implanted stent.
The experience with BRS implantation in patients with stenosis in small vessels is limited. The best evidence comes from subgroup analyses of the ABSORB III randomised trial9. In that trial, although there was no statistically significant interaction between vessel size and treatment effect in pre-specified subgroub analysis, a trend towards a higher rate of adverse events was seen in BRS-treated patients with QCA-measured vessel size <2.63 mm - target lesion failure (9.8% vs. 5.7%), stent thrombosis (2.3% vs. 0.8%) - though this trend was also seen in patients treated with conventional metallic DES9. However, despite the fact that vessel size <2.5 mm diameter was an exclusion criterion, approximately 19% of the patients treated with BRS had a measured RVD of <2.25 mm. Indeed, in a post hoc analysis, these patients had a significantly higher risk for TLR (6.6% vs. 2.2%) and stent thrombosis (4.6% vs. 0.9%) when compared with patients with a larger vessel diameter10. Against this, in a subgroup analysis of the ABSORB Cohort B trial, all patients treated with a 3.0 mm BRS were evaluated according to the RVD of <2.5 mm or ≥2.5 mm19. This study demonstrated no relevant differences between groups regarding the clinical outcome and angiographic results after two years. Moreover, a significant increase in lumen area from 5.71±0.98 mm2 to 6.20±1.27 mm2 (p=0.0155) over follow-up was observed in the small-vessel group19. Indeed, in terms of angiographic outcomes, in that study the 12-month in-stent late luminal loss was remarkably similar to that seen in our small-vessel group (0.27±0.32 mm in ABSORB Cohort B vs. 0.28±0.47 mm in the present report) supporting an overall high antirestenotic efficacy of BRS in small vessels. However, some substantial differences were found regarding the clinical outcome after 12 months, since the incidence of TLR was 2.4% in ABSORB Cohort B and 11.7% in our study. This may be due to the treatment of a more complex patient population in our investigation: though the reference vessel sizes in both small-vessel groups were comparable (2.29±0.14 mm vs. 2.36±0.22 mm), B2/C lesions, bifurcation lesions and patients with acute coronary syndrome were excluded from ABSORB Cohort B. Nevertheless, the rate of stent thrombosis in small vessels treated with BRS was low in both reports (0.0% vs. 1.0%).
Overall, a numerically higher rate of TLR (11.7% vs. 8.3%; p=0.42) and the composite of death, myocardial infarction and TLR (15.7% vs. 12.3%; p=0.49) was observed in patients treated with a 2.5 mm BRS. A possible explanation may be that post-dilatation was less often performed (62.8% vs. 73.6%; p=0.023) and the maximum balloon pressure was significantly lower (13.8 vs. 15.4 atm; p<0.001) in patients treated with a 2.5 mm BRS. Indeed, recent studies suggest that more liberal use of post-dilatation in patients treated with BRS may be associated with improvement in clinical outcomes20,21.
Limitations
Some limitations of the data have to be acknowledged. Firstly, the definition of small vessels is not consistent across the available studies. In our analysis, we categorised patients based on the nominal diameter of the implanted BRS in order to reflect better the perspective of the physician in routine practice. However, QCA-measured reference vessel data from a core laboratory were available for all patients. Secondly, only two centres participated in this study, and the number of patients is relatively small compared to other large-scale randomised trials or registries. Moreover, the study was not powered to detect differences between the two treatment groups, especially with regard to infrequently occurring endpoints such as stent thrombosis. Thirdly, not all patients underwent angiographic follow-up; this must be considered when interpreting the angiographic results. Fourthly, patients treated very early in the experience with BRS were also included and the impact of changes in implantation strategy must be considered20,21. Fifthly, analysis based on stent footprint following deployment was not carried out. However, the clinical importance of this parameter remains to be established. Finally, follow-up was available only up to one year and further long-term follow-up at a time point when the BRS is expected to be fully absorbed would provide a more comprehensive assessment of the clinical performance of the device.
Conclusion
In patients treated with BRS in routine clinical practice, angiographic and clinical outcomes were comparable in patients treated with a 2.5 mm stent as compared with those treated with a larger stent size. Further data from long-term follow-up of large-scale randomised clinical trials will define better the comparative efficacy of these devices versus conventional metallic DES.
| Impact on daily practice Concerns have been raised regarding the safety of bioresorbable stent implantation in patients with lesions in small vessels. We compared angiographic and clinical results of patients with an implanted 2.5 mm bioresorbable stent and patients treated with a larger stent size. Overall, we found comparable results in patients treated with a 2.5 mm stent as compared with those treated with a larger stent size, suggesting that vessel size may not be a key determinant of outcomes in patients treated with these stents. |
Conflict of interest statement
A. Kastrati reports submission of patent applications in relation to drug-eluting stent technology. R. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific, and institutional research grants from Boston Scientific and HeartFlow. The other authors have no conflicts of interest to declare.

