CASE SUMMARY
Background: A 73-year-old man was admitted to our Institute for repeated episodes of congestive heart failure. The patient was affected by arterial hypertension, dyslipidaemia, severe chronic obstructive pulmonary disease, and recurrent atrial fibrillation. Two months earlier he had undergone aortic valve replacement with a biological prosthesis for severe stenosis. Echocardiography showed a left-to-right shunt localised in the left ventricular outflow tract, normal morphology and function of the aortic valve prosthesis, and a hyperkinetic left ventricle.
Investigation: Physical examination, electrocardiography, transthoracic and transoesophageal echocardiography, right heart catheterisation, left ventricular angiography.
Diagnosis: Post-surgical perimembranous interventricular defect with a clinically significant left-to-right shunt.
Management: Percutaneous closure with a ventricular septal defect occluder.
Keywords: aortic valve surgery, percutaneous closure, perimembranous interventricular defect
PRESENTATION OF THE CASE
A 73-year-old man was admitted to our Institute for repeated episodes of congestive heart failure. His past medical history included arterial hypertension, dyslipidaemia, severe chronic obstructive pulmonary disease, prior episodes of lower intestinal bleeding, and recurrent atrial fibrillation. At physical examination, the patient presented a grade 3/6 holosystolic murmur which could be heard on the entire praecordium. Routine laboratory analyses showed mild renal impairment and anaemia. Atrial fibrillation with a normal ventricular response rate and complete right bundle branch block were observed on baseline 12-lead electrocardiography. Two months earlier, the patient, affected by severe aortic stenosis, had been implanted with a 25 mm Perceval S biological prosthesis (Sorin Biomedica Cardio S.r.l., Salugia, Italy). Postoperative course had been complicated by atrial fibrillation (treated successfully with an electrical cardioversion, but subsequently the patient presented an early relapse), anaemia (corrected through red blood cell transfusions), and congestive heart failure (which required therapy with intravenous diuretics). The patient had also presented fever with an Enterococcus faecalis-positive blood culture for which targeted antibiotic therapy was instituted.
During the hospital stay in our Institute, the patient presented two episodes of acute pulmonary oedema, which were triggered by a combination of pulmonary disease exacerbation and hypertensive crisis. Transthoracic echocardiography showed a normally functioning aortic valve prosthesis and a hyperkinetic left ventricle. Moreover, in the left ventricular outflow tract, a left-to-right shunt was detected, which was associated with normal dimensions of the right ventricle and atrium, normal systolic pulmonary arterial pressure, and moderate tricuspid regurgitation. Subsequently, transoesophageal echocardiography revealed the presence of a perimembranous interventricular defect (Moving image 1) in the absence, however, of any vegetation on the aortic valve prosthesis (Moving image 2). Magnetic resonance was performed, but was inconclusive due to motion artefacts caused by the aortic valve prosthesis. Therefore, the patient underwent left ventricular angiography (Figure 1 and Moving image 3), performed in a left anterior oblique view with 40 ml of Iomeron 400 (Bracco Imaging S.p.A., Milan, Italy) injected at 10 ml/s and the left-to-right shunt was clearly evident. Right heart catheterisation revealed a mild increase in intracardiac pressures (mean pulmonary arterial pressure 23 mmHg) with a Qp:Qs ratio of 1.3.
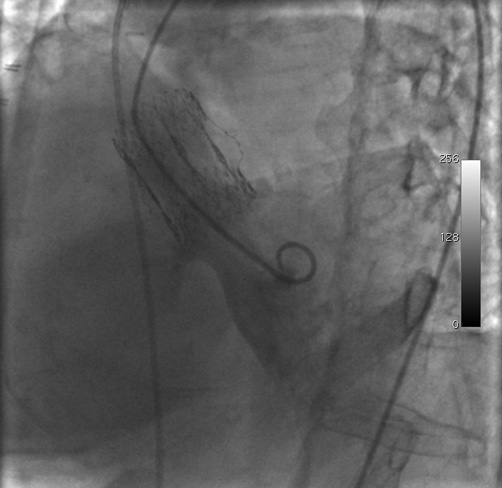
Figure 1. Left ventricular angiography performed in a left anterior oblique view. The left-to-right shunt originated below the aortic valve prosthesis. Before image acquisition, a Swan-Ganz catheter had been placed in the pulmonary artery for right heart catheterisation.
The sum of the clinical and instrumental data pointed to the existence of a left-to-right shunt which did not, however, reach the generally accepted cut-off for haemodynamic significance (i.e., 1.5). Nevertheless, after discussion of the case by the multidisciplinary heart team, it was felt that the left-to-right shunt could not be neglected and, in fact, played a central role in the recurrent episodes of heart failure experienced by the patient. Surgical correction of the defect was considered high-risk due to the recent aortic valve replacement. Therefore, the decision was taken to attempt a percutaneous closure of the perimembranous interventricular defect. The procedure was particularly challenging because of the general condition of the patient. Indeed, he presented poor mobility, a high anaesthesiological risk (due to an unfavourable neck anatomy and coexisting severe pulmonary disease), and a history of lower intestinal bleeding. The resultant American Society of Anesthesiologists score was 4, which is assigned to patients with a severe systemic disease that is a constant threat to life.
How would I treat?
THE INVITED EXPERT’S OPINION
Michele Coceani et al describe a case of a symptomatic iatrogenic perimembranous ventricular septal defect (pmVSD) after surgical aortic valve replacement. In general, this complication is rare but not completely uncommon. In the present case, the occurrence of a pmVSD after implantation of a sutureless Perceval S (Sorin) bioprosthesis, which is thought to be a less traumatic device compared to suture-based aortic valve replacements, might have been related to septal injury during excision of the native valve, a prosthesis/annulus mismatch, or a trauma during the standard balloon post-dilation. The fact that the patient started to suffer from repeated episodes of congestive heart failure immediately after valve replacement might indicate that the pmVSD was already present but overlooked in the acute postoperative phase.
Despite the apparently insignificant shunt appearance with a Qp:Qs ratio of 1.3 under resting conditions, and the lack of right chamber enlargements or significant pulmonary hypertension, the pmVSD is very likely to have a significant clinical impact which unfolds particularly during hypertensive states. Therefore, my recommendation would be the closure of the defect. For that, we have two options: surgical or percutaneous defect closure. The surgical approach with either direct or patch closure is a standard technique associated with a lower risk for conduction abnormalities, but carries a significant procedure-related risk for mortality and morbidity due to its invasiveness, particularly in the given redo situation. The percutaneous approach has progressed significantly over the past 10 years, and today offers a very safe and effective solution. In particular, the introduction of dedicated devices such as the Amplatzer membranous VSD occluder (St. Jude Medical, St. Paul, MN, USA) has improved technical success rates and therefore I would propose this approach for our patient.
Technically, there are different ways to perform the procedure. The presence of a bioprosthesis instead of a mechanical prosthesis in the aortic position certainly facilitates the situation, although even mechanical valves can be safely crossed as previously described1 and do not preclude a percutaneous approach. Crossing of the defect with a standard or hydrophilic wire over a right coronary artery catheter or an Amplatz catheter retrogradely placed in the left ventricular outflow tract is usually easily achieved, while defect crossing from right to left is significantly complicated by poor defect accessibility. The device itself can be delivered in either direction when using a symmetric double-disc device, but must be delivered from a venous access when using the Amplatzer membranous VSD occluder (i.e., deployment of left disc first). Device delivery from right to left usually requires the creation of an arteriovenous loop by snaring and exteriorising the wire through a venous access, and has the advantage of providing a very stable and rigid rail for the delivery sheath and device introduction which is mandatory in the majority of anatomies. In addition, the high profile access is on the venous instead of the arterial site which significantly reduces access complications. Device delivery from left to right requires the exchange of the initial crossing wire to a stiffer guidewire followed by delivery sheath and device introduction in a retrograde fashion. Either way, device deployment occurs in a standard manner: developing the distal disc first, pulling the partially deployed device into the defect followed by the deployment of the proximal disc. Once a correct and stable device position has been achieved and confirmed, either angiographically or by echocardiography, the device is released and pusher cable and sheath are retracted. Potential procedure-related complications include device embolisation, atrioventricular conduction disturbances, (aortic) valvular regurgitation, air embolism, haemolysis, and access issues, but serious complications are very rare, particularly in experienced hands.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
The standard therapy for perimembranous ventricular septal defects (pmVSD) is surgical correction. Percutaneous closure via transcatheter techniques is well described and, given this patient’s high surgical risk and recent surgical aortic valve replacement, is a reasonable alternative. With the close proximity of perimembranous ventricular septum to the tricuspid and aortic valves, the atrioventricular node and conduction bundles, percutaneous closure is associated with valvular dysfunction and complete AV block (cAVB). Especially in the setting of a right bundle branch block, cAVB remains a significant concern.
Computed tomographic angiography with 3-D reconstruction and 3-D echocardiography is helpful in understanding better the pmVSD dimensions and the course of the tract within the membranous septum. Appropriately sized devices can help reduce the incidence of cAVB. Furthermore, tortuous and misaligned direction of the tract in relation to the access approach can make wire crossing and device delivery a challenge. Many cases have reported successful crossing of pmVSDs via a retrograde transaortic approach. Once the wire is across the pmVSD, it is snared through either the femoral or internal jugular vein, creating an arteriovenous (AV) rail that provides support for delivery. Either retrograde transaortic or antegrade venous advancement can be employed depending on the pmVSD proximity to the valves and the angle of delivery. Furthermore, retrograde transseptal with venovenous rail and retrograde transapical (TA) advancement, either through surgical or percutaneous approaches, provide additional alternative methods.
Nonetheless, with the recent implantation of a Perceval S (Sorin) sutureless aortic bioprosthesis, concern remains regarding manipulation through the valve and the subsequent risk of valve injury or embolisation. For this case, we propose that an entirely percutaneous TA approach using CTA-fluoroscopic fusion imaging is perhaps the most appealing option. Given our experience with percutaneous TA for the treatment of paravalvular leaks, left ventricular (LV) pseudoaneurysms and post-surgical VSDs, this would be our first choice1. Pre-procedural, helical CTA fused with fluoroscopic images using proprietary software such as the HeartNavigator system (Philips Healthcare, Best, The Netherlands) can be utilised to identify important cardiac structures and the route of TA access such that entry into the LV is aligned with the pmVSD and away from lung parenchyma and coronary arteries (Moving image 4). Wire crossing/equipment delivery across the pmVSD can be aided by creating an AV rail if necessary. Transapical access closure is performed with the off-label use of an Amplatzer device (St. Jude Medical).
The retrograde TA approach provides direct access to the pmVSD with favourable angles for crossing and delivery, especially in patients with mechanical aortic valves or recent surgical implantation, such as in this case. Furthermore, CTA-fluoroscopic fusion imaging aids in procedural guidance. Typically, TA access is performed under general anaesthesia, which needs to be taken into consideration given this patient’s anaesthesiological risk. Finally, with the potential for cAVB after percutaneous closure, we would recommend a transvenous pacing wire in this high-risk patient for 24 hours with close telemetry observation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 4. Percutaneous transapical closure of a post-surgical perimembranous ventricular septal defect.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
General anaesthesia was not performed due to the risks linked to sedation, and the patient remained awake during the entire procedure. As a result, transoesophageal echocardiography was not employed and the procedure was conducted exclusively under fluoroscopic guidance. Sheaths, size 6 Fr (Cordis Corporation, Miami Lakes, FL, USA), were inserted in the right femoral artery and vein and in the left femoral artery. Anticoagulation was obtained with 5,000 UI of unfractionated heparin administered intravenously. The biological aortic valve was crossed with an Emerald 150 cm 0.038” J-tip wire (Cordis Corporation) introduced from the right femoral artery on which a pigtail catheter (Cordis Corporation) was advanced into the left ventricle. Left ventricular angiography confirmed the interventricular defect, which was localised just below the biological prosthesis. Subsequently, from the left femoral artery, the aortic valve was crossed with a 260 cm 0.035” hydrophilic wire (ZIPwire; Boston Scientific, Natick, MA, USA) on which a Judkins right diagnostic catheter (Cordis Corporation) was advanced. During this phase, the patient presented asystole which was managed initially through external pacing and, shortly after, a temporary pacemaker was placed through the left femoral vein. At this point, the hydrophilic wire was directed towards the interventricular defect with the aid of the right diagnostic catheter, pushed across the defect, and captured by an 18 mm Hooker snare (Med-Italia Biomedica S.r.l., Medolla, Italy). Its distal end was exteriorised from the right femoral vein to complete an arteriovenous loop (Figure 2) and the two ends of the wire were secured to the sterile drape in the proximity of the vascular sheaths with surgical clamps. Next, the right venous sheath was exchanged for an 8 Fr Amplatzer Torq Vu delivery sheath (AGA Medical, Golden Valley, MN, USA) which was advanced over the wire into the apex of the left ventricle, after which the wire was removed from the left femoral artery (Figure 3). A 7 mm membranous Amplatzer ventricular septal defect occluder (AGA Medical) was thereafter delivered through the sheath which, in turn, was gently retracted towards the right ventricle (Figure 4 and Figure 5 and Moving image 5). After angiographic confirmation (Moving image 6), the device was released and remained in a stable position (Moving image 7). Final left ventricular angiography showed minimal intraprosthetic shunting (Figure 6 and Moving image 8). Haemostasis was achieved by manual compression of the veins, whereas two FemoSeal™ closure systems (St. Jude Medical) were used to seal the arterial access sites. Following the procedure, the patient underwent implantation of a permanent dual-chamber pacemaker and did not present any heart failure recurrence.
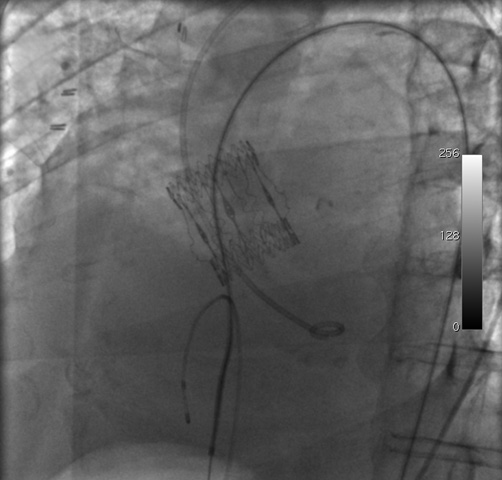
Figure 2. Left anterior oblique view of the arteriovenous loop formed by the hydrophilic wire, inserted through the left femoral artery and removed from the right femoral vein. A Judkins right diagnostic catheter was advanced over the wire. During this phase, because the patient presented asystole, a temporary pacemaker was placed in the right ventricle via the left femoral vein.
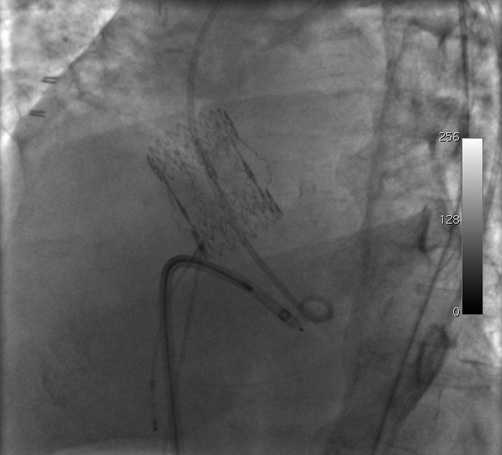
Figure 3. Positioning of the delivery sheath in the left ventricle During advancement, the hydrophilic wire was pushed from the left femoral artery so as to direct the sheath towards the apex and avoid any contact with the aortic valve prosthesis.
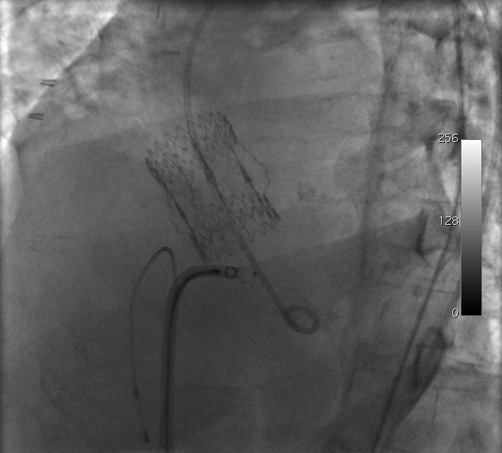
Figure 4. Retraction of the delivery sheath and contemporary advancement of the septal defect occluder.
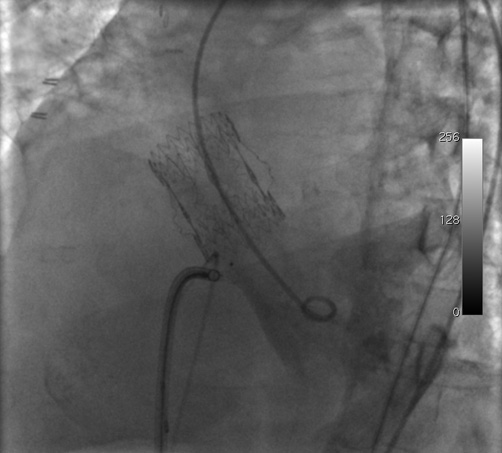
Figure 5. Retraction of the delivery sheath and contemporary advancement of the septal defect occluder.
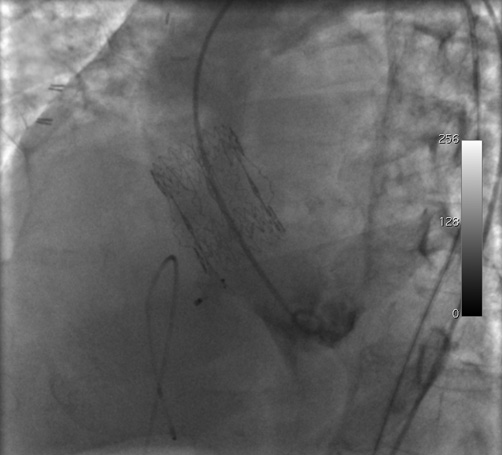
Figure 6. Final left ventricular angiography which showed adequate positioning of the septal defect occluder and absence of significant residual shunting.
Discussion
Iatrogenic interventricular septal defect is a rare complication of aortic valve replacement surgery. Typically, these defects involve the membranous part of the interventricular septum and correction is indicated only in the rare cases in which the resulting shunt is haemodynamically significant. Surgical repair is associated with a non-negligible complication rate and may be technically challenging due to the position of the defect. For this reason, percutaneous closure represents an appealing alternative1,2.
Our patient did not have a Qp:Qs ratio above the threshold value of 1.5, which characterises interventricular defects with significant shunting, nor did he present any echocardiographic sign of right ventricular overload or dysfunction. However, the interventricular defect was identified as an important contributing factor to the repeated episodes of heart failure and, as a result, percutaneous closure was programmed. Even though the patient had presented fever and a positive blood culture following the initial valve replacement, we do not believe that the defect was caused by endocarditis, also because transoesophageal echocardiography performed prior to closure of the defect did not reveal any type of vegetation on the aortic valve prosthesis.
The percutaneous closure was technically challenging for two reasons: the patient had significant comorbidities and we could not depend on transoesophageal echocardiographic guidance. The latter aspect did not permit an accurate sizing of the occluder and we were forced to choose the diameter of the device using an “eyeball technique”. The procedure involved retrograde crossing of an aortic prosthesis, which may lead to catheter and wire entrapment. However, this particular complication has been reported exclusively in patients with mechanical heart valves, especially older generation ones such as the Bjork-Shiley valve3. To avoid retrograde crossing of an aortic prosthesis, we could have used a venovenous loop via a transseptal approach4, but this option is less attractive in the case of perimembranous defects due to the steep angulation that the delivery sheath needs to form and because damage of the mitral valve apparatus may ensue. Of note, during advancement of the delivery sheath the hydrophilic wire was pushed from the left femoral artery so as to form a loop in the apex of the ventricle and prevent any contact between the sheath and the aortic valve prosthesis.
Our patient developed complete heart block during the procedure, a complication whose incidence varies considerably, averaging 5% in most series but with reported rates as high as 22%5. The asystole observed in our patient was probably favoured by the position of the defect (which lay in close proximity to the His bundle) and by the pre-existing complete right bundle branch block. In perimembranous defects associated with baseline conduction defects, perhaps it may be prudent to place a temporary pacemaker at the beginning of the procedure. With regard to device selection, the Amplatzer membranous ventricular septal defect occluder has already been extensively employed in the past and its use is supported by several studies6, although it should be noted that, compared to our patient, the individuals enrolled in these studies were much younger and had fewer comorbidities. Interestingly, a second-generation Amplatzer occluder has been recently introduced and tested with success7. The new device has been modified in several ways –elliptical and concave shape of the left-sided disc, thinner nitinol wire which decreases the rigidity and radial pressure of the device, and increased waist length which reduces the force against the ventricular septum– so as to minimise the risk of atrioventricular block.
Conflict of interest statement
The authors have no conflicts of interest to declare.

