Abstract
Aims: This study aimed to evaluate the safety and efficacy of using the second-generation DIOR drug-coated balloons (DCB) as an adjunct to plain old balloon angioplasty (POBA) for the treatment of de novo coronary lesions.
Methods and results: Valentines II was designed as a prospective, multicentre, multinational, web-based registry. Eligible patients with stable or unstable angina, and/or documented ischaemia on stress testing with de novo lesions of >50% stenosis were prospectively enrolled. Patients underwent POBA followed by DCB treatment. In cases of suboptimal angiographic success (Thrombolysis In Myocardial Infarction [TIMI] flow <3 and/or residual stenosis of >30%), additional bail-out bare metal stenting (BMS) was left at the operator’s discretion. The primary endpoint was major adverse cardiac events (MACE; all-cause death, myocardial infarction [MI], target vessel revascularisation [TVR] and vessel thrombosis) at six to nine months. A subset of patients underwent angiographic follow-up. One hundred and nine lesions in 103 patients were treated. Mean age was 62.6±10.2 years; 79.6% were men. Lesion stenosis at baseline and post treatment was 83.3±9.5% and 10.4±10.6%, respectively. Procedural success was 99%. Coronary dissections occurred in 14.7%, and bail-out BMS implantation was required in 13 patients (11.9%). Mean follow-up was 7.5 months; follow-up rate was 99%. Cumulative MACE at follow-up was 8.7%, with 1% all-cause death, 1% MI, 6.9% overall TVR, of which 2.9% were target lesion revascularisations, and no vessel thrombosis. Angiographic follow-up on a subset of patients (n=35) demonstrated late luminal loss of 0.38±0.39 mm for both the in-balloon and in-segment analyses.
Conclusions: The Valentines II trial demonstrates the feasibility of using a second-generation DIOR DCB as adjunct to POBA in de novo coronary lesions. This approach achieved high procedural success with acceptable rates of bail-out stenting and low MACE rates at mid-term follow-up, and offers an attractive alternative for revascularisation of patients who are unsuitable candidates for drug-eluting stents.
Introduction
Drug-coated balloon (DCB) technology is an exciting area of innovation in percutaneous coronary revascularisation. DCBs are semi-compliant angioplasty balloons covered with an antirestenotic drug (paclitaxel), which is rapidly released into the vessel wall during balloon contact1,2. Its rationale for development derives mainly from the limitations of delivering a stent, or in complex coronary lesions where the rate of stent failure is high even with drug-eluting stents (DES)3,4. Moreover, the potential advantage of DCB use as an alternative to DESs lies in its antiproliferative properties. DCBs do not need a stent or durable polymer, thereby potentially reducing the risk of accrued late and very late stent thrombosis and the need for prolonged dual antiplatelet therapy (DAPT)5. For the treatment of in-stent restenosis (ISR), DCBs have demonstrated superior results when compared to regular balloon angioplasty and paclitaxel-eluting stents (PESs)6-9. The potential application of DCBs in de novo coronary lesions has been tested in limited studies.
DCBs show promise in treating de novo lesions in small vessels, although their efficacy appears attenuated when used in conjunction with a bare metal stent (BMS)10,11. DCB use also appears feasible in treating bifurcation lesion side branches despite the heterogeneity of the techniques employed12-14. In small studies involving de novo lesions, DCBs were used in conjunction with stents, showing mixed results15-17. The present study aimed to evaluate the safety and efficacy of the DCB technology as an adjunct to plain old balloon angioplasty (POBA) without the need for stenting in the treatment of de novo coronary lesions using a second-generation DIOR® DCB (Eurocor GmbH, Bonn, Germany).
Methods
The Valentines II trial was designed as a prospective, multicentre, multinational, web-based registry to assess the safety and efficacy of the second-generation DIOR DCB technology used as an adjunct to POBA in patients with de novo coronary lesions. The worldwide call for investigators was conducted through www.crtonline.org and www.pcronline.com homepages over a predetermined enrolment time frame of 1.5 months. An electronic data capture system was designed and employed such that a data monitoring system ensured that >50% of the data collected were verified. The baseline patient and angiographic characteristics, as well as in-hospital outcomes, were entered into the database within one month of the procedure. Clinical follow-up was conducted at six to nine months. Angiographic follow-up was also performed for a subset of patients. This study was performed according to international healthcare guidelines, as well as local laws and regulations. The results were first presented at Cardiovascular Research Technologies (CRT) 2012 in Washington, DC, USA, and at EuroPCR 2012, Paris, France.
Patient enrolment
From 14/02/2011 to 31/03/2011, patients suffering from de novo lesions were enrolled by 38 investigators from 16 countries. Included patients were >18 years of age, and presented with stable or unstable angina, and/or evidence of ischaemia on stress testing. The treated lesions were in native coronary arteries, and angiographically had ≥50% diameter stenosis, with up to two lesions treated per patient. Excluded patients were those with acute myocardial infarction (MI) within the previous 48 hours, a life expectancy <12 months, lesions in the left main coronary artery, lesion length >24 mm, heavily calcified, thrombotic or bifurcation lesions, and lesions requiring additional stenting with either a BMS or DES prior to the DCB treatment.
DIOR drug-coated balloon
The DCB being evaluated in this study is the second-generation DIOR coronary angioplasty balloon catheter (available lengths: 15, 20, 25, and 30 mm; available diameters: 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, and 4.0 mm). The DIOR balloon is coated with three micrograms of paclitaxel per square millimetre of balloon surface using shellac as its excipient. The balloon is folded thrice to prevent early drug wash-off during insertion and tracking. The recommended inflation time is 30-45 seconds to achieve adequate drug delivery to the vessel wall.
Interventional procedure
Enrolled patients were treated with acetylsalicylic acid (80-325 mg per day) and clopidogrel (300-600 mg loading dose, followed by 75 mg maintenance). Intravenous heparin, with a targeted activated clotting time of ≥250 seconds, or bivalirudin was used for procedural anticoagulation. The use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. Post procedure, dual antiplatelet therapy (DAPT) was recommended for ≥3 months, followed by acetylsalicylic acid indefinitely.
After routine coronary angiography, regular balloon predilatation of the target lesion was recommended. If a good angiographic result was obtained (i.e., Thrombolysis In Myocardial Infarction [TIMI] 3 flow and residual stenosis <30%), the DIOR balloon was then inflated for ≥30 seconds with an overlap of ≥2 mm on each edge of the predilatation balloon-treated segment. The DIOR balloon was sized to the vessel diameter in a 1:1 ratio. Additional bail-out stenting using a BMS in case of suboptimal angiographic result (TIMI flow grade <3 and/or residual stenosis >30%) was left to the operator’s discretion.
Follow-up and study endpoints
Clinical follow-up was performed six to nine months after the index procedure. Clinical events were site-reported based on definitions included in the study protocol. The primary endpoint was the occurrence of major adverse cardiac events (MACE), defined as all-cause death, MI, target vessel revascularisation (TVR) and vessel thrombosis. The definition for vessel thrombosis follows the Academic Research Consortium criteria for stent thrombosis. Spontaneous MI was defined as any elevation of troponin (or other cardiac enzymes if troponin was not recorded) in combination with ischaemic chest pain. Periprocedural MI was defined as an elevation of any cardiac biomarkers (troponin, creatinine kinase-MB or creatinine kinase) >3 times the upper limit of normal. The relationship of the MI to the target vessel was based on electrocardiography localisation. Target lesion revascularisation (TLR) was defined as any repeat revascularisation (percutaneous or surgical) due to a restenosis in the DCB-treated segment (which included 5 mm beyond the treated segments proximally and distally). TVR was defined as any repeat revascularisation of the DCB-treated vessel. Device success was defined as without bail-out stenting and/or without device complications; procedural success was defined as TIMI 3 flow and final residual stenosis of <30% after DCB treatment and possible bail-out stenting.
Angiographic follow-up was performed in a subset of patients at six to nine months. Quantitative coronary angiography was performed according to standard procedures, using dedicated software (CAAS 5.9.2; Pie Medical Imaging, Maastricht, The Netherlands). Images were analysed by an independent core laboratory (MedStar Health Research Institute Angiographic Core Laboratory, Washington, DC, USA). Binary restenosis was defined as a diameter stenosis of ≥50% at angiographic follow-up. Late luminal loss (LLL) was defined as the difference between post-procedural minimum luminal diameter and the minimum luminal diameter at follow-up in the same vessel segment analysed. In-balloon analysis refers to the DCB-treated vessel segment. In-segment analysis includes the vessel segments within 5 mm beyond the proximal and distal edge of the DCB-treated vessel segment.
Statistical analysis
Due to the study design, only descriptive statistics are presented. Continuous variables are presented as means±standard deviations, while categorical variables are presented as counts and percentages. The diabetic substudy was pre-specified and analysed for descriptive purposes.
Results
PATIENT AND PROCEDURAL CHARACTERISTICS
A total of 122 patients with de novo coronary lesions were enrolled and treated with the DIOR DCB from February 14 to March 31, 2011. Of the initial patients enrolled, 19 were excluded because of inclusion/exclusion criteria violations (n=13), and unanswered procedural and/or discharge queries (n=6). Hence, the clinical and procedural characteristics of 103 patients were included and entered into the database for analysis (Table 1, Table 2, Figure 1). The rate of lesion predilatation was high at 85%, although it did not reach the recommended 100%. Overall procedural success was achieved in 102 patients (99.0%), with one failure (0.9%) due to dissection and significant residual stenosis. Bail-out stenting was required in 13 lesions (11.9%): 10 for dissections, one for dissection with flow reduction, one for residual stenosis, and one for an unspecified indication. Device success with a DCB-only strategy was 87.2%.
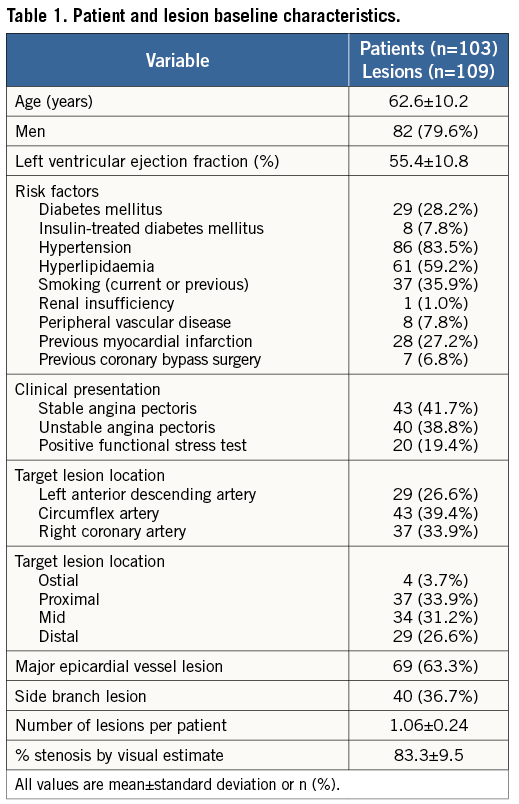
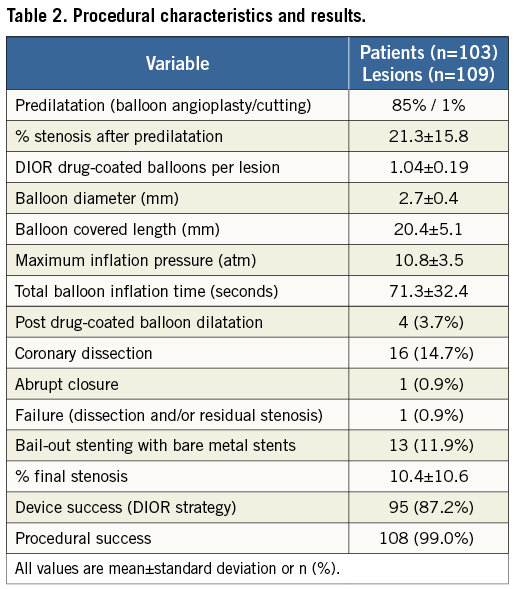
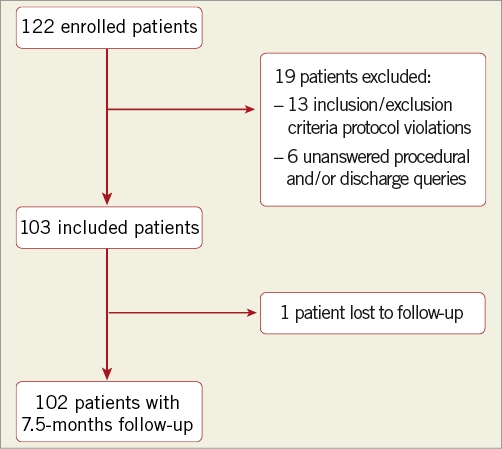
Figure 1. Patient enrolment.
CLINICAL OUTCOMES
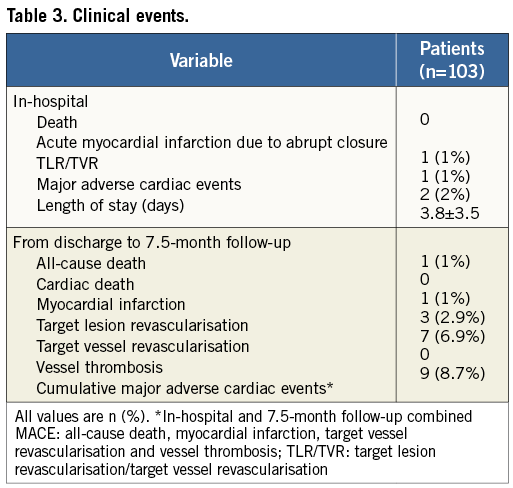
Clinical events are shown in Table 3. In-hospital MACE was 2%, with one patient suffering a periprocedural MI due to abrupt closure, and another patient undergoing TLR due to planned finalisation of the index treatment. There were no in-hospital deaths. Information was available in 102 patients with a mean follow-up of 227±40 days. During this period, overall MACE occurred in 8.7%, with 2.9% TLR and 6.9% TVR. Combined death and MI was 2%. There were no instances of cardiac death or vessel thrombosis.
OUTCOMES OF DIABETIC AND NON-DIABETIC PATIENTS
Clinical outcomes were recorded for all of the diabetic patients (n=29) and for 98.6% of the non-diabetic patients (n=74) at follow-up. Although not statistically significant, overall MACE trended to be higher in the diabetic population (13.8% vs. 6.8%, respectively, p=NS). Likewise, TVR (13.8% vs. 4.1%, p=NS) and TLR (6.9% vs. 1.4%, p=NS) showed similar trends.
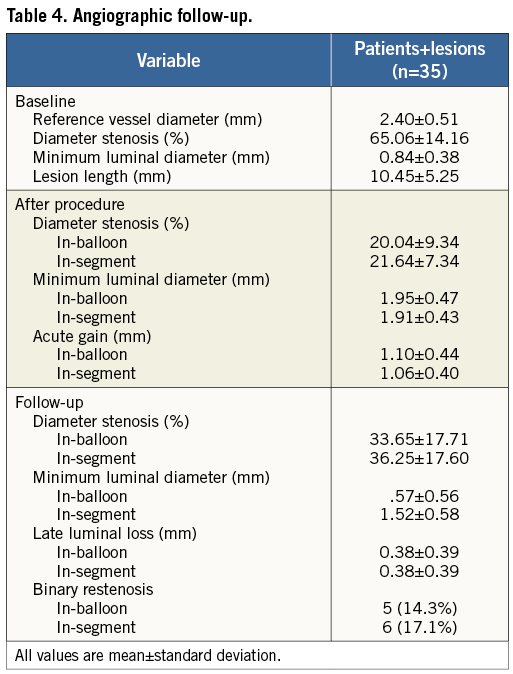
ANGIOGRAPHIC FOLLOW-UP ANALYSIS
An angiographic cohort analysis was obtained for 35 patients (35 lesions) consisting of 34% of the study cohort (Table 4). Angiographic follow-up was research-driven and site-selected. The mean reference vessel diameter was 2.40±0.51 mm, with mean pre-diameter stenosis of 65.06±14.16%. Post procedure, the mean final diameter stenosis was 20.04±9.34 mm, which increased to 33.65±17.71 mm at follow-up. The follow-up LLL was 0.38±0.39 mm, and the binary restenosis rate was 14.3%.
Discussion
The main findings of the Valentines II trial are: 1) high procedural success with an acceptable rate of bail-out stenting; 2) low overall MACE at follow-up; 3) low overall TLR and TVR rates at follow-up; and 4) successful short-term, web-based worldwide enrolment of study patients.
DCBs have been successful in treating ISR; currently BMS ISR is the only approved indication for DCB use in the European guidelines18. Much less is understood of their applicability in de novo coronary lesions, although the use of a local drug delivery system without a metallic stent or durable polymer sounds attractive in complex lesions where restenosis risk remains high.
The application of a DCB-only approach in treating de novo coronary lesions has been limited to a few small studies, mainly in small vessel disease. Paclitaxel Eluting PTCA balloon in Coronary Artery Disease (PEPCAD) I assessed the SeQuent® Please DCB (B. Braun Melsungen AG, Berlin, Germany) in small vessels10. Despite a higher LLL demonstrated in the Valentines II angiographic subset (0.38 mm) against the DCB-only arm of PEPCAD I (LLL 0.18 mm), TLR rates were actually comparable in the Valentines II trial (2.9% at 7.5 months) and in the DCB-only arm of PEPCAD I (4.9% at 12 months). The TLR rate in Valentines II is much better compared to that seen in Paclitaxel-Coated Balloon Versus Drug-Eluting Stent During Percutaneous Coronary intervention of Small Coronary vessels (PICCOLETTO) (32.1% at nine months), in which the less effective first-generation DIOR DCB was used19.
The Valentines II results are similar to another study, the Spanish DIOR registry for small vessels11. Both first- and second-generation DIOR DCBs were used in vessels with mean diameters of 1.9 mm. Overall rates of MACE and TLR were 5.8% and 2.9%, respectively, at 12-month follow-up. In 50% of the cohort with angiographic follow-up, LLL was 0.34±0.23 mm, similar to that obtained in the Valentines II angiographic subset.
Diabetics are particularly prone to restenosis, and the results in this trial suggest a trend towards higher repeat revascularisation rates (both TLR and TVR) compared to non-diabetics. Nevertheless, DCB use does not pose an additional safety hazard in diabetics. No death, MI or vessel thrombosis was recorded. These trends are consistent with those observed in the Valentines I study. In the Diabetic Argentina Registry (DEAR), the DIOR DCB was found to be comparable to historic controls using PESs, and superior to BMSs in TVR (8.3% vs. 14.0% vs. 22.9%, respectively) and MACE20. SeQuent® Please DCB use in diabetics was evaluated in PEPCAD IV, showing no difference in LLL (0.51 mm vs. 0.53 mm) and comparable rates of TLR (7.7% vs. 8.3%) at nine months when used in combination with a BMS, as compared with the PES. The results approximate to the TLR rate of 6.9% found in our diabetic population16. Although the patient numbers in this study are too small to draw any definitive conclusions, the findings suggest the feasibility of DCB use in a diabetic subpopulation, comparable in efficacy to a PES.
Recent data on DCB use suggest a DCB-alone strategy is superior in efficacy to a DCB+BMS strategy. From the PEPCAD series, which evaluated a SeQuent Please DCB + BMS, the LLL for in-stent analysis was observed to be numerically higher than the in-segment analysis (defined as within 5 mm of the stented edges, treated with a DCB)10,14-16. New data from the Balloon Elution and Late Loss Optimisation (BELLO) study, which evaluated the use of the IN.PACT Falcon DCB (Medtronic-Invatec, Frauenfeld, Switzerland) in small coronary vessels, demonstrated superior LLL in non-stented vessels treated with a DCB, compared to those treated with a PES21. In the Valentines II trial, DCBs were used as an adjunct to POBA without the need for stenting. This approach proved to be effective with low TLR rates and angiographic LLL at six months.
The LLL obtained after this approach is similar to that seen in the POBA arms of historical trials comparing balloon angioplasty to stenting (Belgium Netherlands Stent [Benestent]: 0.32±0.47 mm; Stent Restenosis Study [SRS]: 0.38±0.66 mm); however, the TVR rate in Valentines II (6.9%) appears much lower than is seen with POBA alone (about 15-20%) at mid-term follow-up22,23. Brachytherapy as an adjunct to POBA, however, demonstrated an unexpectedly low LLL of 0.05 mm and a restenosis rate of 15% in an early feasibility study of 23 patients with de novo coronary lesions; nevertheless, this approach has not been further tested specifically in de novo lesions24.
Moreover, DCB use without stenting, as seen in Valentines II, confers the added advantage of safety without the need for prolonged DAPT. Here, DAPT was prescribed to all patients for three months; no episodes of vessel thrombosis were recorded on follow-up. Studies using a DCB-only approach to revascularisation show minimal risk of vessel thrombosis even with only one month of DAPT6,7. This risk increases, however, when DCBs are used in combination with a BMS, with instances of late stent thrombosis occurring beyond six months10,14,15. Additional research is needed to determine the optimal duration of DAPT in DCB use. If used in combination with a BMS, perhaps the duration should be the same as prescribed with DES use.
Stents are still needed to overcome the risk of vessel dissections, abrupt closure and elastic recoil from balloon angioplasty. Our approach in the Valentines II trial was to use bare metal stenting as a bail-out strategy only. The 11.9% bail-out stenting rate seen here reflects the expected result if one employs a balloon angioplasty-alone strategy. This rate is numerically lower than the bail-out stenting rates seen in studies using a DCB in small vessels (PEPCAD I: 28%; PICCOLETTO: 36%; BELLO: 20%). It may be reasoned that the threshold for accepting a suboptimal angiographic result is much higher in small-calibre (vs. normal-sized) vessels. The exception, however, was seen in the Spanish DIOR registry where only 6.7% bail-out stenting was performed although the average vessel diameter was 1.98 mm11.
In the DES era of revascularisation, the role of DCB use in de novo lesions is contraindicated when placing a DES. These scenarios include patients intolerant of prolonged DAPT due to bleeding or pending non-cardiac surgery, or in patients who have failed stenting and present with a de novo lesion requiring revascularisation. The “DCB as an adjunct to POBA approach” provides an effective and safe alternative to DES implantation without disruption to patient selection.
In some settings (which the Valentines II trial did not evaluate), stenting may be more beneficial than the DCB as an adjunct to the POBA approach. These scenarios include: acute MI, thrombotic lesions, bifurcations, heavily calcified lesions, and lesions involving the left main coronary artery. Moreover, patients who developed acute vessel complications requiring stenting following initial POBA (with the regular balloon) were not treated with a DCB in this trial. Hence, this approach is applicable in non-complex lesions which demonstrate a satisfactory angiographic result post POBA. Our trial was conducted only in the setting of elective percutaneous coronary intervention. In the setting of acute MI, the use of second-generation DIOR DCBs+bare metal stenting did not demonstrate any added advantage over a BMS-alone strategy, and is shown to be inferior to DES in angiographic and clinical outcomes in the Drug-Eluting Balloon in Acute ST-segment Elevation Myocardial Infarction (DEB-AMI) trial25.
The DIOR technology
The first-generation DIOR DCB failed to meet the angiographic criteria for non-inferiority against PESs in the PICCOLETTO study of small vessels, and did not prove superior against BMSs in the Drug Eluting Balloon In BIfUrcations Trial (DEBIUT)11,12. This is most likely due to the lower paclitaxel delivery dose in the first-generation DIOR DCB. The second-generation DIOR DCB uses shellac as its excipient, as compared to dimethyl sulphoxide, which was used in the first-generation DIOR DCB. Although the drug dose has not been changed (3.0 mcg/mm2), the second-generation DIOR DCB delivers up to 50x more paclitaxel than its first-generation counterpart, and is similar to that of SeQuent Please26. This updated DCB technology, as used exclusively in the Valentines I and II trials, confirms the effect of a higher delivery dose in the second-generation DIOR DCB in terms of safety and efficacy.
Study design
Valentines II continued the success seen in the novel study design employed in Valentines I27. Briefly, we used a broad-based enrolment approach to include patients across several worldwide centres, instead of enrolment from only a few high-volume centres. Only sites that participated in Valentines I (which did not preselect their sites) were invited to participate in this trial, and investigators volunteered suitable cases to be screened and approved through an on-line web survey. This perhaps allowed for a better reflection of the patients and cases in contemporary practice, and minimised treatment bias from the better-skilled centres. Moreover, we were able to recruit patients relatively quickly using a fixed enrolment time frame rather than a predetermined number of patients. Compared to other large, multicentre registry studies, a relatively high rate (50.5%) of on-site data monitoring was also achieved.
Implications
Valentines II demonstrates that, in elective percutaneous coronary intervention, the adjunctive use of DCBs may be a feasible alternative to stenting in lesions that respond favourably to POBA. This may prove valuable in the subset of patients who are unsuitable for DES implantation.
Study limitations
The inherent limitations of a registry apply here. The enrolment design allowed investigators to volunteer cases, hence the possibility of selection bias. This was a single-arm treatment group analysis without a comparative reference technique. Angiographic analysis was performed in a subset of patients only, which may not fully represent the treatment effects of the population being studied. Although a high rate of clinical follow-up was achieved, angiographic follow-up was not mandatory, which could have led to an underestimation of silent restenoses. Detailed information on the type of de novo coronary lesions, which would have helped determine the relative treatment success in differing lesion complexities, was not available. Longer-term follow-up to study the durability of DCB effects was also not available. The low patient numbers in this study prevent us from drawing conclusions on events with low occurrences (i.e., vessel thrombosis).
Conclusion
Second-generation DIOR DCB use as an adjunct to POBA is feasible in patients with de novo coronary lesions. High procedural success and acceptable bail-out stenting rates were achieved with low revascularisation and MACE rates at mid-term follow-up. This method of short-term, web-based, multicentre study enrolment continues to prove successful in providing efficiency in enrolment and in analysing outcomes.
Conflict of interest statement
S. Stahnke and R. Pogge von Strandmann are employees of Eurocor. The other authors have no conflicts of interest to declare.

