Abstract
Background: Coronary computed tomography angiography (CCTA) has been proposed as an alternative to intravascular imaging for assessing plaque pathology.
Aims: We aimed to assess the efficacy of CCTA against near-infrared spectroscopy-intravascular ultrasound (NIRS-IVUS) in evaluating atheroma burden and composition and for guiding coronary interventions.
Methods: Seventy patients with a chronic coronary syndrome were recruited and underwent CCTA and NIRS-IVUS. The imaging data were matched, and the estimations of lumen, vessel wall and plaque dimensions and composition of the two modalities were compared. The primary endpoint of the study was the efficacy of CCTA in detecting lipid-rich plaques identified by NIRS-IVUS. Secondary endpoints included the performance of CCTA in evaluating coronary artery pathology in the studied segments and its value in stent sizing, using NIRS-IVUS as the reference standard.
Results: In total, 186 vessels were analysed. The attenuated plaque volume on CCTA had weak accuracy in detecting lipid-rich plaques (58%; p=0.029). Compared to NIRS-IVUS, CCTA underestimated the lumen volume (309.2 mm3 vs 420.4 mm3; p=0.001) and plaque dimensions (total atheroma volume 116.1 mm3 vs 292.8 mm3; p<0.001 and percentage atheroma volume 27.67% vs 41.06%; p<0.001) and overestimated the lipid component (lipid core burden index 48.6 vs 33.8; p=0.007). In the 86 lesions considered for revascularisation, CCTA underestimated the reference vessel area (8.16 mm2 vs 12.30 mm2; p<0.001) and overestimated the lesion length (23.5 mm vs 19.0 mm; p=0.029) compared to NIRS-IVUS.
Conclusions: CCTA has limited efficacy in assessing plaque composition and quantifying lumen and plaque dimensions and tissue types, which may potentially impact revascularisation planning.
Intravascular imaging enables the accurate assessment of coronary artery pathology, evaluates the implications of novel pharmacotherapies on atheroma burden and morphology and assesses post-percutaneous coronary intervention (PCI) results especially in high-risk patients and complex lesions1. However, intravascular imaging is invasive, is usually used in patients with advanced coronary artery disease, does not allow assessment of the entire coronary tree, and is associated with both an increased cost and a small, albeit non-negligible, risk of complications2.
Non-invasive imaging, specifically coronary computed tomography angiography (CCTA), has emerged as an alternative imaging modality for assessing plaque pathology3. Histological studies have shown that CCTA has value in characterising plaque composition and burden and has been increasingly used to examine the potency of emerging pharmacotherapies and their effects on plaque morphology4. In parallel, there is an increased interest in the value of CCTA in guiding PCI5. Nevertheless, so far there has been no prospective study specifically designed to assess the efficacy of CCTA in assessing plaque composition, quantifying lumen and vessel wall dimensions and guiding revascularisation against state-of-the-art intravascular imaging.
Methods
Study population
The Evaluation of the Efficacy of Computed Tomographic Coronary Angiography in Assessing Coronary Artery Morphology and Physiology study (ClinicalTrials.gov: NCT03556644) is a prospective, multivessel coronary imaging study that was designed to compare CCTA and NIRS-IVUS estimations. The study protocol has been published previously6. In brief, 70 patients with a chronic coronary syndrome and obstructive coronary artery disease on invasive coronary angiography undergoing further assessment (pressure wire or intravascular imaging) or treatment with PCI were recruited. All patients underwent a CCTA, followed by multivessel NIRS-IVUS imaging assessment. The patients were then treated according to their clinical indication. The recruited patients provided written consent prior to enrolment. The study was approved by the local ethics committee (Research Ethics Committee [REC] reference: 17/SC/0566) and was performed in accordance with the Declaration of Helsinki. The NIRS-IVUS and CCTA data acquisition, analysis and coregistration are described in Supplementary Appendix 1.
Data analysis and study endpoints
Primary endpoint
The primary endpoint of this study is the efficacy of plaque composition on CCTA in detecting lipid-rich plaque, using NIRS-IVUS as the reference standard. A plaque on NIRS-IVUS was defined as a segment with plaque burden (PB) ≥40% over three consecutive frames7. Plaques were considered separate if there was a segment with a length of >5 mm between them. In CCTA, there is no established PB cutoff to define plaques. Therefore, in this study, receiver operating characteristic (ROC) curve analysis was performed to identify the optimal PB cutoff that predicted a PB ≥40% on NIRS-IVUS. This was used to define plaques on CCTA similarly to NIRS-IVUS (i.e., a segment with at least three consecutive frames with increased PB). In total, 50 vessels from 15 patients were randomly selected to identify the optimal PB cutoff for predicting plaques. This test set was also used to identify plaque components on CCTA that corresponded to the presence of lipid tissue on the 2 mm block chemogram on NIRS-IVUS8, and these plaque components were used to build a model that enabled prediction of the presence of lipid tissue on CCTA. The performance of the model to detect lipid-rich plaques – defined as a plaque with at least one yellow block chemogram – was tested in the remaining dataset (136 vessels). A further analysis was performed to examine the efficacy of CCTA to identify lipid-rich plaques with and without increased calcific component (defined as the presence of calcific tissue with an arc >90 on NIRS-IVUS)9.
Secondary endpoints
The secondary endpoints of the study included the following:
1. The agreement between NIRS-IVUS and CCTA for quantifying the lumen, vessel wall and plaque dimensions, as well as plaque burden and composition at the segment level. For each segment of interest assessed by NIRS-IVUS and CCTA, the lumen, vessel, total atheroma (TAV) and percentage atheroma volumes (PAV) were estimated on NIRS-IVUS and CCTA and compared. Plaque composition comparison was performed using the lipid core burden index (LCBI), calcific burden index (CaBI) and maximum LCBI in a 4 mm segment (maxLCBI4mm).
2. The accuracy of CCTA in identifying high-risk lesions. Previous prospective NIRS-IVUS studies have shown that lesions with a large lipid content (maxLCBI4mm >325 and maxLCBI4mm >400)1011 on NIRS-IVUS are at risk of causing events. Therefore, we performed a further analysis to examine whether plaque components derived by CCTA can accurately predict these lesions.
3. The value of CCTA in guiding revascularisation. Intravascular imaging has an established role in identifying the optimal landing zone (based on the current consensus, segments with a PB <50%) and stent diameter defined by the external elastic membrane (EEM) diameter in the distal reference segment (rounded down by <0.5 mm)12. These criteria were used by the analyst who did the NIRS-IVUS analysis to determine the optimal stent size based on the NIRS-IVUS estimations for all lesions treated with PCI or that were considered for PCI and underwent functional assessment. In CCTA, there is no specific PB cutoff for identifying disease-free landing zones. Therefore, as before, ROC curve analysis was performed in the test set of 50 vessels to identify the optimal PB cutoff on CCTA that predicted a PB >50% on NIRS-IVUS. This cutoff was used by the analyst who performed the CCTA segmentation to define the optimal stent size and diameter based on the CCTA annotations in lesions that were treated with or considered for revascularisation. Tandem lesions on CCTA, with interpolated disease-free segments with a length <10 mm, that corresponded to the same lesion on NIRS-IVUS were treated as a single lesion, and the stent length on CCTA was estimated from the proximal reference segment of the most proximal lesion and the distal reference segment of the most distal lesion. Moreover, we examined the efficacy of CCTA to assess plaque composition and identify compositional predictors associated with periprocedural complications and worse prognosis and, in particular, the presence of maxLCBI4mm >600, which has been found to be a predictor of microvascular obstruction post-PCI; we also evaluated its efficacy in identifying the presence of a vessel diameter <3.5 mm in calcific lesions, of circumferential calcification, and of an arc of calcium >270° for a length >5 mm, all of which are established predictors of stent underexpansion1314.
Statistical analysis
Continuous variables are presented as median (interquartile range [IQR]) or mean±standard deviation (SD) – depending on their distribution – while categorical variables are presented as absolute values and percentages. The estimations of NIRS-IVUS and CCTA on the same subject were compared using Wilcoxon signed-rank tests for continuous variables or the McNemar test for categorical variables. The agreement of the two modalities for continuous variables was tested using intraclass correlation coefficients (ICC) under a two-way random-effects model, considering absolute agreement and average measurements. The kappa statistic was used for agreement between categorical variables. The Pearson correlation coefficient was implemented to examine the linear relationship between the two modalities, and Bland-Altman (BA) analysis was used to assess bias and determine the limits of agreement (LoA) between CCTA and NIRS-IVUS measurements. In parametric BA analysis, the bias was calculated as the mean or median difference between the two approaches depending on distribution, while the LoA were defined as a range centred around the mean bias±1.96 x SD. For non-parametric BA analysis, the median bias and the 2.5th-97.5th percentiles were computed using quantile regression. We chose non-parametric BA analysis for the lesion- and segment-level analyses due to the small sample size and the non-normal distribution of the differences1516. The association between two categorical variables was assessed by Cramer’s V value.
For the study's primary endpoint, the volumes of three tissue types (necrotic core, fibrofatty, necrotic core + fibrofatty) from 50 vesselsÂÂ were estimated and compared with the presence of lipid tissue on the 2 mm block chemogram from NIRS-IVUS. ROC curve analysis was performed, and the area under the curve (AUC) was used to assess the performance of these three tissue categories in identifying lipid on the block chemogram. The necrotic core volume was found to perform best, and the optimal cutoff (1 mm3; 95% confidence interval [CI]: 0.84-1.36 mm3) was applied to the remaining dataset. The primary endpoint of the study − the efficacy of CCTA in detecting fibroatheroma classified on NIRS-IVUS − was assessed by diagnostic performance measures (AUC, sensitivity, specificity, positive [PPV] and negative [NPV] predictive values).
All statistical tests were two-tailed, and statistical significance was set at p<0.05; analyses were performed using R software, v. 4.2.2 (R Foundation for Statistical Computing ) using “lme4”, “psych”, “dplyr”, “rms” and “ggplot2” packages.
Results
Patient baseline characteristics
The baseline characteristics of the 70 patients recruited in our study are summarised in Supplementary Table 1. CCTA images were reviewed by two independent experts who graded image quality. One patient was excluded because of poor image quality due to increased calcific burden. In addition, 9 vessels were excluded because of poor matching between CCTA and NIRS-IVUS. Therefore, in total 64 patients (186 vessels; 23,605 matched NIRS-IVUS and CCTA cross-sections) were included in the final analysis (Supplementary Figure 1).
Primary endpoint: efficacy of CCTA in detecting lipid-rich plaques
The necrotic core volume cutoff (≥1 mm3), found to best predict the presence of lipid-rich block chemogram in 50 vessels, was applied in the remaining dataset (49 patients, 136 vessels) to assess plaque phenotype. Of the 75 lipid-rich plaques detected by NIRS-IVUS, CCTA correctly detected 53 lipid-rich plaques but misclassified 44 others as lipid-rich. The sensitivity of CCTA was 70.7% (IQR 59-81%), specificity was 46.3% (IQR 35-58%), PPV was 55% (IQR 44-65%), NPV was 63% (IQR 50-75%) and accuracy was 58% (IQR 50-66%; p=0.010; AUC=0.585, 95% CI: 0.510-0.660; p=0.029).
CCTA showed a similar ability in detecting non-calcified lipid-rich plaques compared to calcified lipid-rich plaques with a sensitivity of 70.9% versus 66.7%, specificity 47.0% versus 40.6%, PPV 46.2% versus 9.0%, NPV 71.6% versus 93.0%, accuracy 56.4% versus 42.7% and AUC 0.590 versus 0.536; p=0.434, respectively.
Comparison of NIRS-IVUS and CCTA at the segment level
In total, 186 vessels were included in the final analysis. The mean length of the studied segments was 76.9±27.4 mm. An example of matched CCTA and NIRS-IVUS spread-out plots are shown in Figure 1. The ICC and the Pearson correlation coefficient were high for the lumen volume, vessel volume and TAV, moderate for the PAV and CaBI, and weak for the LCBI and maxLCBI4mm (Table 1). CCTA underestimated the lumen and vessel volumes, TAV and PAV, and overestimated the LCBI, but there was no difference between the two modalities for the CaBI or maxLCBI4mm (Figure 2). However, the LoA were wide for these latter two metrics (Figure 3).
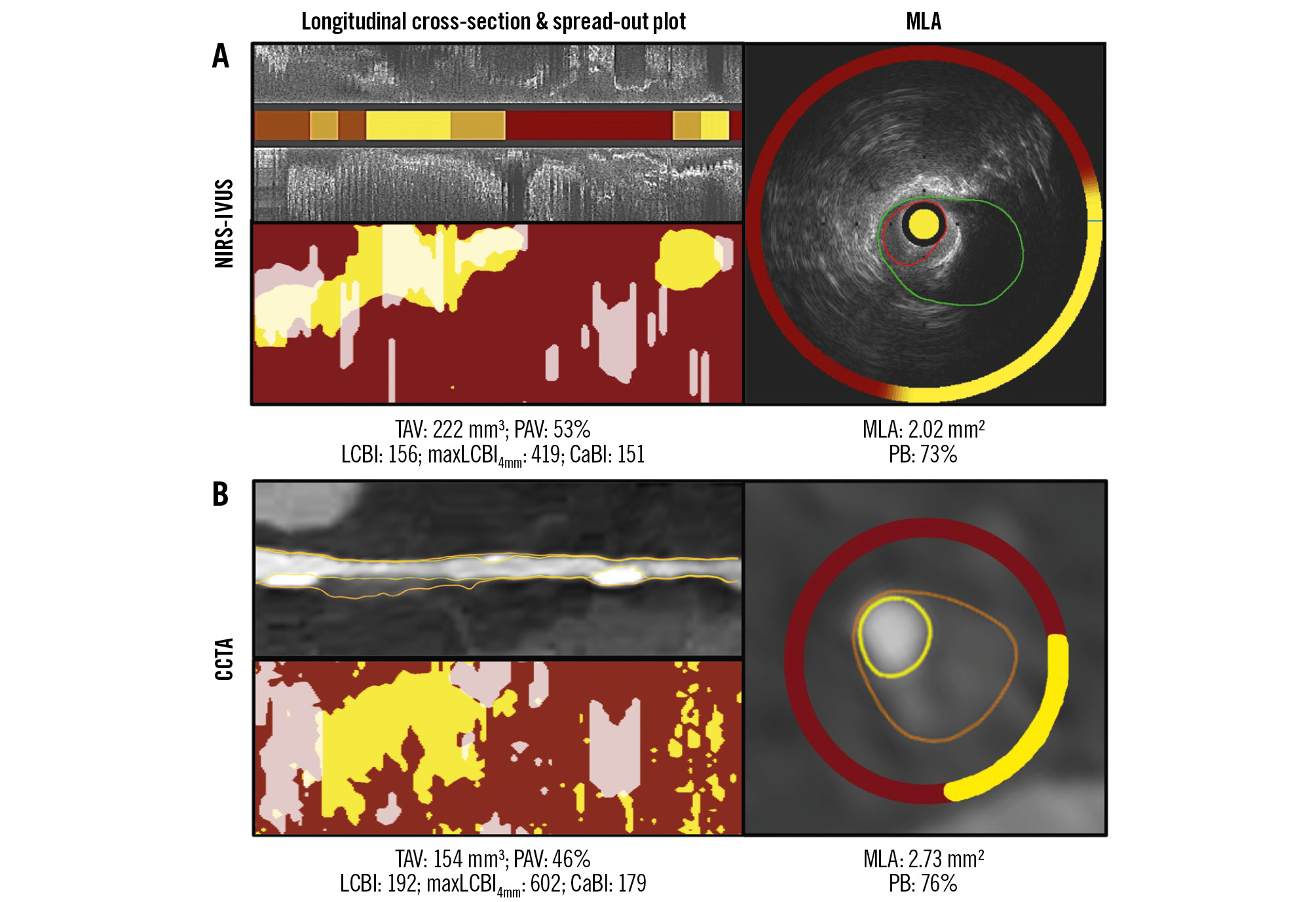
Figure 1. Lumen, vessel wall and spread-out plaque composition analysis of the NIRS-IVUS and CCTA images. A) portrays a longitudinal segment of interest in a left anterior descending artery on NIRS-IVUS, while (B) portrays the corresponding segment of interest on CCTA. The spread-out plots displaying lipid and calcific tissue distribution are also displayed. The TAV, PAV, LCBI, CaBI and maxLCBI4mm estimations of both modalities are shown at the bottom. The cross-sections showing the MLA with the annotated lumen and vessel wall borders and the circumferential distribution of tissue types on NIRS-IVUS and CCTA are portrayed in the images on the right in (A) and (B). CaBI: calcific burden index; CCTA: coronary computed tomography angiography; LCBI: lipid core burden index; maxLCBI4mm: maximum LCBI in a 4 mm segment; MLA: minimum lumen area; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PAV: percentage atheroma volume; PB: plaque burden; TAV: total atheroma volume
Table 1. Segment-level comparison of the estimations of NIRS-IVUS and CCTA imaging.
| Estimations | NIRS-IVUS | CCTA | p-value | Median bias (95% LoA) | r | p-value | ICC | p-value |
|---|---|---|---|---|---|---|---|---|
| Lumen volume, mm3 | 420.4 (223.8-612.8) | 309.2 (176.7-463.8) | 0.001 | 89.4 (–52.9, 411.8) | 0.98 (0.97-0.98) | <0.001 | 0.97 (0.96-0.98) | <0.001 |
| Vessel volume, mm3 | 735.0 (385.2-1,059.4) | 454.6 (247.5-672.1) | <0.001 | 251.3 (–7.2, 847.9) | 0.95 (0.93-0.96) | <0.001 | 0.95 (0.94-0.96) | <0.001 |
| TAV, mm3 | 292.8 (149.4-465.2) | 116.1 (64.9-229.7) | <0.001 | 139.9 (–61.1, 535.3) | 0.80 (0.74-0.84) | <0.001 | 0.86 (0.81-0.90) | <0.001 |
| PAV, % | 41.06 (33.75-47.86) | 27.67 (20.61-37.17) | <0.001 | 10.60 (–10.94, 31.60) | 0.48 (0.36-0.58) | <0.001 | 0.64 (0.52-0.73) | <0.001 |
| LCBI | 34 (5-91) | 49 (11-126) | 0.007 | –10 (–524, 175) | 0.18 (0.04-0.32) | 0.012 | 0.26 (0.14-0.45) | 0.020 |
| MaxLCBI4mm | 228 (60-407) | 240 (53-549) | 0.182 | –30 (–716, 510) | 0.33 (0.19-0.45) | <0.001 | 0.48 (0.30-0.61) | <0.001 |
| CaBI | 59 (17-131) | 61 (16-130) | 0.865 | 3.8 (–181.9, 150.3) | 0.66 (0.57-0.73) | <0.001 | 0.79 (0.73-0.85) | <0.001 |
| Values are median (interquartile range) unless otherwise stated. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; ICC: intraclass correlation coefficient; LCBI: lipid core burden index; LoA: limits of agreement; maxLCBI4mm: maximum LCBI in a 4 mm segment; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PAV: percentage atheroma volume; r: Pearson's correlation coefficient; TAV: total atheroma volume | ||||||||
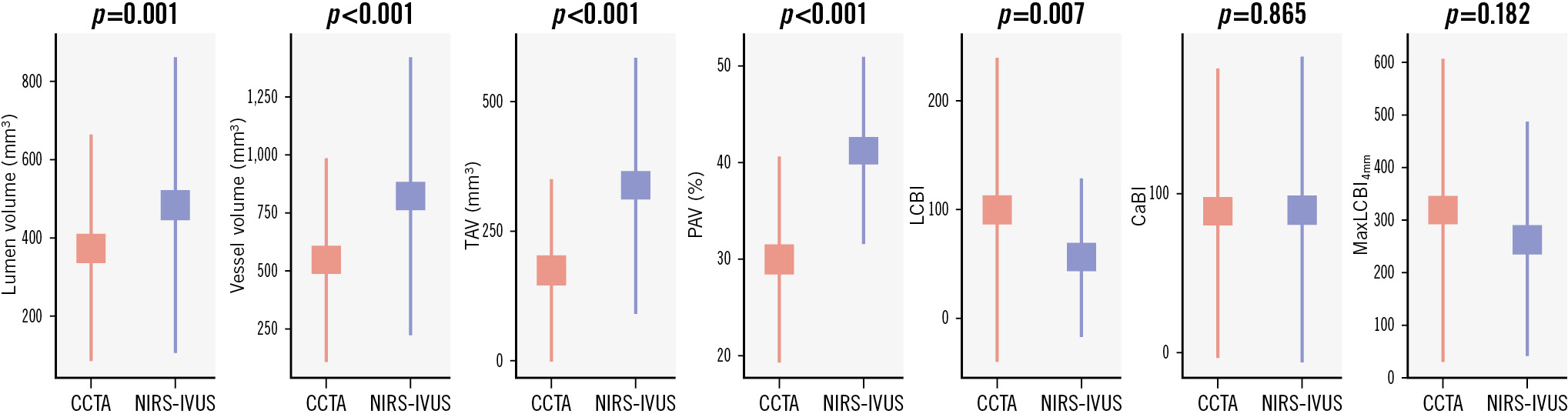
Figure 2. Segment-level analysis comparison of NIRS-IVUS and CCTA estimations for lumen volume, vessel volume, TAV, PAV, LCBI, CaBI and maxLCBI4mm. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; LCBI: lipid core burden index; maxLCBI4mm: maximum LCBI in a 4 mm segment; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PAV: percentage atheroma volume; TAV: total atheroma volume
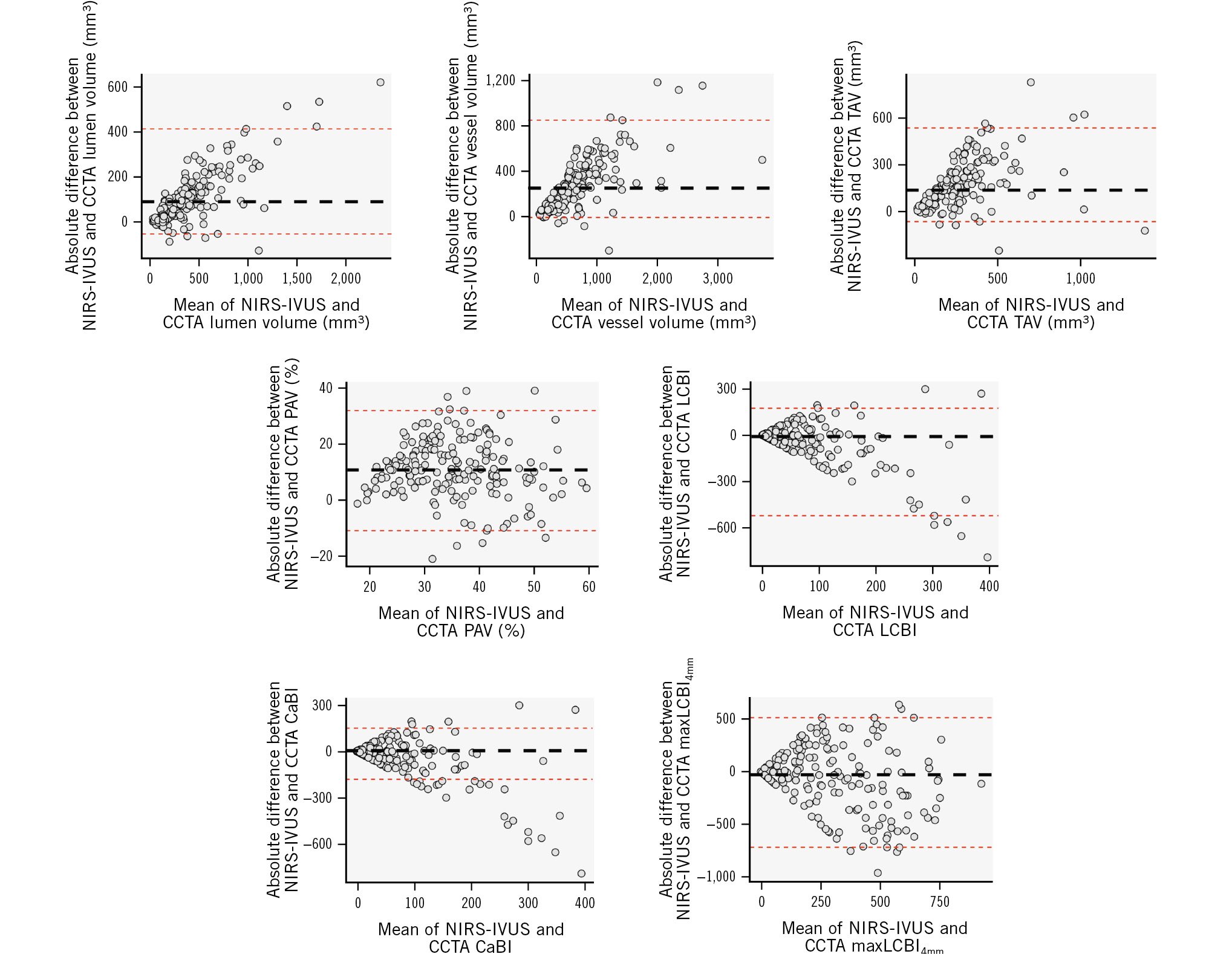
Figure 3. BA analyses of the mean differences between NIRS-IVUS and CCTA estimations at a segment level for lumen and vessel volumes, TAV, PAV, LCBI, CaBI and maxLCBI4mm. BA: Bland-Altman; CaBI: calcific burden index; CCTA: coronary computed tomography angiography; LCBI: lipid core burden index; maxLCBI4mm: maximum LCBI in a 4 mm segment; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PAV: percentage atheroma volume; TAV: total atheroma volume
CCTA for detecting high-risk lesions
In the 186 vessels that were assessed by NIRS-IVUS, 32 plaques had a maxLCBI4mm >400, and 40 plaques had a maxLCBI4mm >325. The accuracy of CCTA to detect high-risk plaques was moderate (Central illustration) and was not different for plaques with maxLCBI4mm >325 and maxLCBI4mm >400 (sensitivity: 63.5% and 68.1%; specificity: 67.4% and 65.6%; PPV: 47.1% and 37.6%; NPV: 80.2% and 87.1%; and AUC: 0.683 and 0.675; p=0.796, respectively).
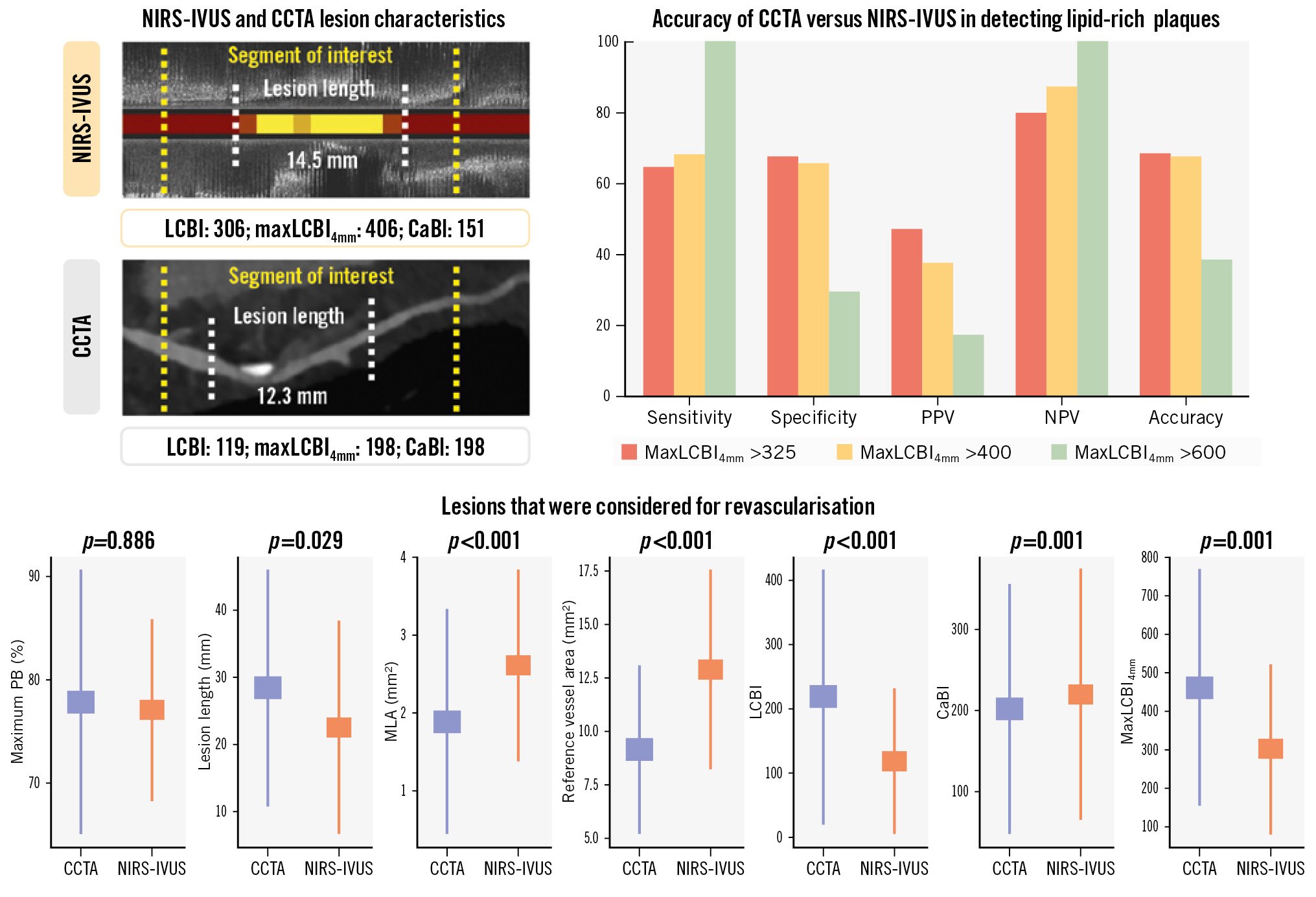
Central illustration. Efficacy of CCTA compared to NIRS-IVUS for assessing plaque pathology and guiding revascularisation. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; LCBI: lipid core burden index; maxLCBI4mm: maximum LCBI in a 4 mm segment; MLA: minimum lumen area; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; NPV: negative predictive value; PB: plaque burden; PPV: positive predictive value
CCTA for guiding revascularisation
Revascularisation with PCI or coronary artery bypass grafting (CABG) was performed in lesions with severe stenosis (angiographic diameter stenosis [DS] >90%) or in lesions with moderate stenosis (30-90%) and objective evidence of ischaemia on invasive or non-invasive imaging. In total, 86 lesions that were considered for revascularisation (Central illustration) were assessed by both NIRS-IVUS and CCTA (58 lesions treated with PCI, 7 lesions with CABG, and 21 lesions were found to be non-flow-limiting on functional assessment and were treated conservatively). In these lesions, the reference vessel area was larger on NIRS-IVUS (12.30 [IQR 9.17-16.0] mm2 vs 8.16 [IQR 6.55-11.0] mm2; p<0.001), while lesion length was longer on CCTA (23.5 [IQR 14.0-39.3] mm vs 19.0 [IQR 11.6-32.7] mm; p=0.029) (Table 2). There was no agreement between the two modalities for the LBCI, while for the CaBI, the median values were similar, but the bias and the LoA were large between NIRS-IVUS and CCTA. CCTA showed moderate accuracy in identifying lesions with maxLCBI4mm >600 (accuracy 0.583), for detecting vessel diameter <3.5 mm (accuracy 0.613) in calcified lesions and circumferential calcification (accuracy 0.750) and good accuracy for detecting >270° arc of calcium for a length of >5 mm (accuracy 0.885) (Supplementary Table 2).
The results were different when analysis focused on lesions that were treated with PCI; their mean length by NIRS-IVUS was 19.7 (IQR 14.8-32.9) mm and by CCTA was 21.0 (IQR 14.3-36.3) mm; p=0.415. Although there was no significant difference in the estimated stent length between the two modalities, the LoA were large between NIRS-IVUS and CCTA estimations (Table 3). In addition, the distal reference vessel area on NIRS-IVUS was 11.70 (IQR 9.01-15.60) mm2 and on CCTA was 7.83 (IQR 6.58-10.40) mm2; p<0.001. Based on these measurements, PCI guided by NIRS-IVUS would have resulted in the deployment of a stent with a mean diameter of 3.5 mm and post-dilatation with a 3.75 mm balloon, while using CCTA, the mean stent diameter would have been 3 mm, and post-dilatation would have been performed with a 3 mm balloon.
The location of the minimum lumen area (MLA) and the proximal and distal landing zones on NIRS-IVUS and CCTA in the 58 vessels treated with PCI are shown in Supplementary Figure 2. In 17 lesions, the difference in the distance of the proximal or distal landing zone between NIRS-IVUS and CCTA estimations was >5 mm; in 11 cases, CCTA indicated implantation of significantly longer stents, and in 8 cases, it indicated significantly shorter stents compared to NIRS-IVUS. The overestimation of the stent length with CCTA was mainly attributed to the presence of calcific plaques near the reference segment, resulting in an overestimation of the PB in these locations due to the blooming artefacts. Conversely, CCTA failed to detect fibrotic plaques seen on NIRS-IVUS, resulting in an underestimation of the stent length in fibrotic lesions with diffuse disease (Figure 4).
LCBI values derived from NIRS-IVUS were significantly lower than that of CCTA, but there was no significant difference in the CaBI values between the two modalities. The performance of CCTA in identifying a maxLCBI4mm >600 or calcific extent associated with stent underexpansion is shown in Supplementary Table 2.
Table 2. Comparison of the estimations of NIRS-IVUS and CCTA in lesions that were considered for revascularisation.
| Estimations | NIRS-IVUS | CCTA | p-value | Median bias (95% LoA) | r | p-value | ICC | p-value |
|---|---|---|---|---|---|---|---|---|
| Lesion length, mm | 19.0 (11.6, 32.7) | 23.5 (14.0, 39.3) | 0.029 | –2.6(–19.1, 4.3) | 0.81 (0.72, 0.88) | <0.001 | 0.89 (0.84, 0.93) | <0.001 |
| MLA*, mm2 | 2.22 (1.71, 3.21) | 1.49 (0.76, 2.38) | <0.001 | 0.77 (–0.70, 2.20) | 0.63 (0.47, 0.75) | <0.001 | 0.76 (0.64, 0.85) | <0.001 |
| Reference lumen area, mm2 | 6.81 (4.87, 9.10) | 6.30 (4.68, 9.02) | 0.279 | 0.70 (–2.24, 3.28) | 0.70 (0.56, 0.80) | <0.001 | 0.82 (0.71, 0.89) | <0.001 |
| Reference vessel area, mm2 | 12.30 (9.17, 16.0) | 8.16 (6.55, 11.0) | <0.001 | 4.01 (0.39, 8.60) | 0.63 (0.46, 0.75) | <0.001 | 0.77 (0.63, 0.86) | <0.001 |
| Maximum PB*, % | 77.9 (72.2, 82.6) | 78.0 (69.0, 87.3) | 0.886 | –0.3 (–15.4, 16.0) | 0.48 (0.28, 0.64) | <0.001 | 0.63 (0.42, 0.76) | <0.001 |
| LCBI | 79 (34, 179) | 149 (86, 283) | <0.001 | –39 (–445, 130) | 0.0 2 (–0.22, 0.25) | 0.897 | 0.03 (–0.56, 0.39) | 0.455 |
| MaxLCBI4mm | 286 (128, 437) | 449 (175, 721) | <0.001 | –124 (–616, 326) | 0.12 (–0.12, 0.34) | 0.335 | 0.20 (–0.28, 0.50) | 0.179 |
| CaBI | 195 (106, 310) | 192 (81.3, 289) | 0.001 | 26.0 (–83.0, 158) | 0.66 (0.50, 0.77) | <0.001 | 0.79 (0.67, 0.87) | <0.001 |
| Values are median (interquartile range) unless otherwise stated. *Lesions that were predilated before NIRS-IVUS (n=6) were excluded from the MLA and the maximum PB analyses. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; ICC: intraclass correlation coefficient; LCBI: lipid core burden index; LoA: limits of agreement; maxLCBI4mm: maximum LCBI in a 4 mm segment; MLA: minimum lumen area; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PB: plaque burden; r: Pearson's correlation coefficient | ||||||||
Table 3. Comparison of the estimations of NIRS-IVUS and CCTA in lesions that were treated with PCI.
| Estimations | NIRS-IVUS | CCTA | p-value | Median bias (95% LoA) | r | p-value | ICC | p-value |
|---|---|---|---|---|---|---|---|---|
| Lesion length, mm | 19.7 (14.8, 32.9) | 21.0 (14.3, 36.3) | 0.415 | –2.6 (–10.9, 4.3) | 0.90 (0.84, 0.94) | <0.001 | 0.95 (0.91, 0.97) | <0.001 |
| MLA*, mm2 | 1.84 (1.63, 2.38) | 1.20 (0.62, 2.20) | 0.001 | 0.66 (–0.92, 1.72) | 0.69 (0.50, 0.81) | <0.001 | 0.77 (0.61, 0.86) | <0.001 |
| Reference lumen area, mm2 | 6.26 (4.75, 8.63) | 5.87 (4.69, 8.41) | 0.344 | 0.70 (–2.24, 2.05) | 0.67 (0.48, 0.80) | <0.001 | 0.80 (0.65, 0.89) | <0.001 |
| Reference vessel area, mm2 | 11.7 (9.01, 15.6) | 7.83 (6.58, 10.4) | <0.001 | 3.86 (–0.53, 8.72) | 0.55 (0.32, 0.72) | <0.001 | 0.70 (0.47, 0.83) | <0.001 |
| Maximum PB*, % | 80.3 (76.6, 83.8) | 81.2 (70.5, 89.7) | 0.825 | –0.3 (–15.8, 16.2) | 0.61 (0.40, 0.76) | <0.001 | 0.64 (0.39, 0.79) | 0.002 |
| LCBI | 70 (29, 155) | 178 (53, 290) | 0.003 | –33 (–464, 130) | –0.06 (–0.34, 0.23) | 0.694 | 0.00 (–0.78, 0.44) | 0.500 |
| MaxLCBI4mm | 290 (105, 427) | 454 (148, 734) | 0.006 | –188 (–669, 335) | 0.07 (–0.22, 0.35) | 0.643 | 0.12 (–0.57, 0.51) | 0.335 |
| CaBI | 190 (112, 296) | 178 (58, 296) | 0.004 | 34 (–93, 162) | 0.66 (0.46, 0.79) | <0.001 | 0.79 (0.62, 0.88) | <0.001 |
| Values are median (interquartile range) unless otherwise stated. *Lesions that were predilated before NIRS-IVUS (n=6) were excluded from the MLA and the maximum PB analyses. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; ICC: intraclass correlation coefficient; LCBI: lipid core burden index; LoA: limits of agreement; maxLCBI4mm: maximum LCBI in a 4 mm segment; MLA: minimum lumen area; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PB: plaque burden; r: Pearson's correlation coefficient | ||||||||
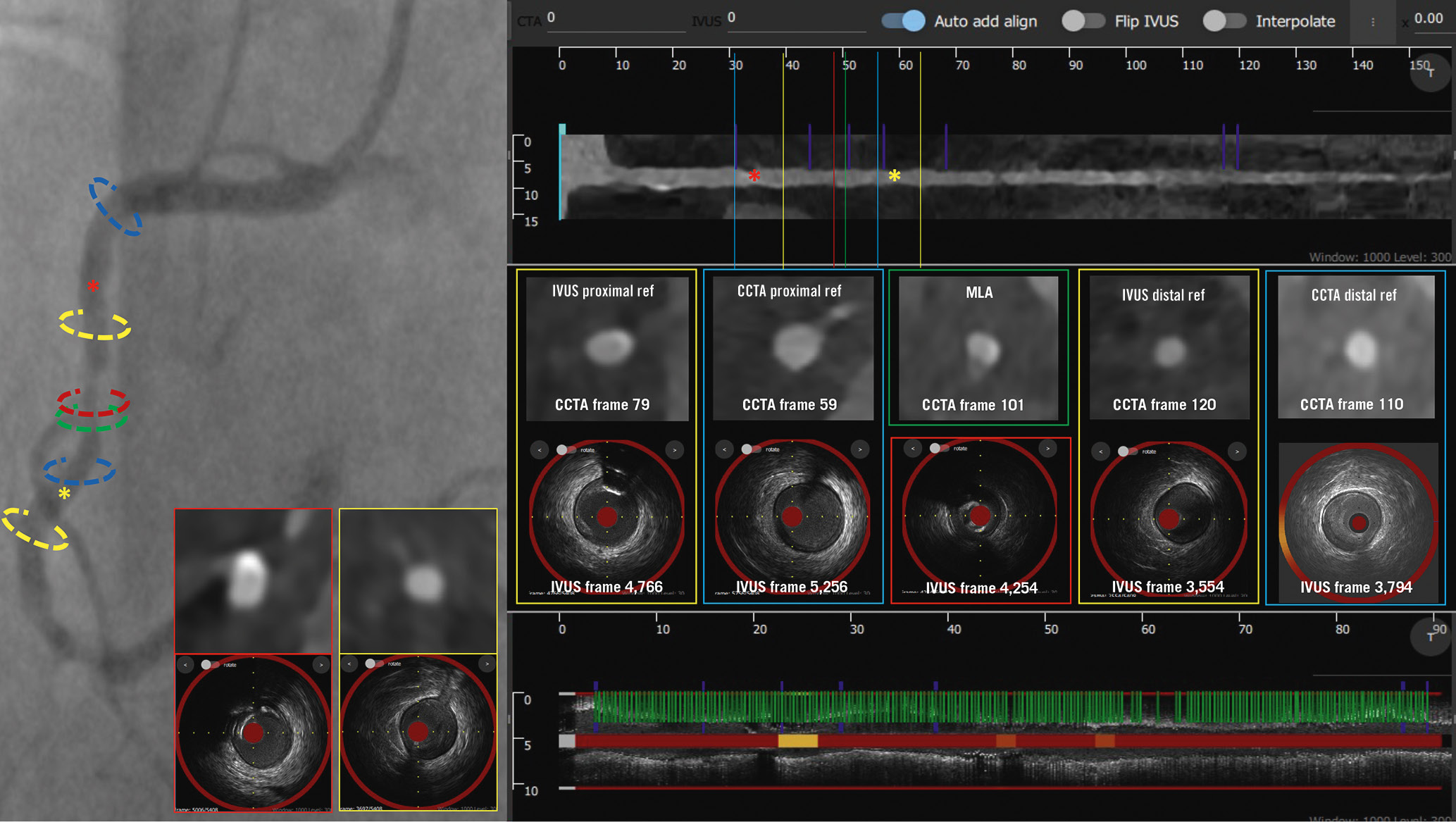
Figure 4. Case example of NIRS-IVUS- and CCTA-guided right coronary artery PCI. The treated lesion is shown on the coronary angiogram, along with CCTA and NIRS-IVUS longitudinal images. The location of the MLA on NIRS-IVUS (red) and CCTA (green) are different. The NIRS-IVUS proximal and distal reference areas are marked in yellow, while the CCTA proximal and distal reference areas are marked in blue. There was a significant difference in the landing zone estimations between NIRS-IVUS and CCTA; CCTA overestimated the PB in the calcific segment proximal to the lesion, while distally it missed a fibrotic plaque compared to NIRS-IVUS. CaBI: calcific burden index; CCTA: coronary computed tomography angiography; IVUS: intravascular ultrasound; MLA: minimum lumen area; NIRS-IVUS: near-infrared spectroscopy-intravascular ultrasound; PB: plaque burden; PCI: percutaneous coronary intervention
Discussion
This is the first prospective study to examine the efficacy of CCTA in assessing coronary artery pathology using state-of-the-art intravascular imaging as the reference standard. We found that (1) necrotic core volume measured by CCTA had a limited efficacy in detecting fibroatheromas, (2) CCTA had limitations in evaluating lumen and plaque dimensions and plaque composition, and (3) this resulted in suboptimal stent sizing in lesions considered for PCI.
Previous studies comparing NIRS-IVUS and CCTA have shown that CCTA has a value in assessing the lumen and vessel wall dimensions and quantifying plaque components17. The first studies showed that CCTA overestimated lumen dimensions18, but more recent reports have contradicted these findings, indicating that CCTA underestimates lumen area and PB19. Differences in CCTA scanners and reconstruction methods as well as limitations in image coregistration are likely to be responsible for the inconsistent results192021. The present analysis overcomes the above limitations as it has been prospectively designed to examine the performance of CCTA in assessing lumen and vessel wall dimensions at the segment level and characterise plaque composition. In contrast to previous reports, this analysis was appropriately powered for the primary endpoint, and it was conducted using a 3rd-generation CT scanner. Furthermore, it implemented a thorough protocol for comparing CCTA and NIRS-IVUS estimations that involved (1) assessment of the entire coronary artery tree by NIRS-IVUS rather than specific vessels or lesions so as to avoid bias; (2) administration of the same amount of nitrates during NIRS-IVUS and CCTA imaging to have the same vasodilatory effect22; (3) use of an optimal CCTA reconstruction algorithm for data reconstruction that appears to perform better than the algorithms used in clinical practice19; and (4) the implementation of a retrospective gating method to identify the end-diastolic NIRS-IVUS frames in order to analyse only frames acquired at the same phase of the cardiac cycle23 and avoid the effect of the change in coronary pressure during the cardiac cycle on the lumen and plaque dimensions and the backward-forward motion of the NIRS-IVUS probe during the cardiac cycle that can affect the quantification of the TAV and PAV and accurate CCTA coregistration24. In addition, all analyses were performed blindly, and we developed a dedicated software to match frame-by-frame the NIRS-IVUS and CCTA data (Supplementary Figure 3); we modified the output of the CCTA analysis and generated spread-out plots of plaque composition to compare this with the output of NIRS-IVUS.
We found a high agreement between CCTA and NIRS-IVUS for the lumen, vessel and plaque dimensions but also large biases, with the CCTA underestimating these metrics. Conversely, the correlation between the two modalities was weak for plaque components, with the CCTA overestimating both lipid and calcific components. BA analysis showed a large bias and wide LoA even for the calcific tissue, for which CCTA is regarded a reliable imaging modality. A careful examination of the spread-out plots showed that, in specific cases, the Hounsfield unit (HU) values in the lumen were high, resulting in a false classification of the defined plaque as calcific tissue. Replacing the tissue types in disease-free segments by the media – an option that is provided by the analysis software – resulted in an improvement in the correlation between the two modalities for the calcific tissue; however, the LoA were still large as this adjustment did not enable correction of tissue misclassification in diseased segments (Supplementary Figure 4). These results underscore the limitations of software that use HU cutoffs to assess tissue composition and highlight the need to develop advanced machine-learning methods that will be trained either by histology or high-resolution intravascular imaging and will enable more accurate plaque characterisation25.
The limited agreement between CCTA and NIRS-IVUS for assessing plaque composition influenced its performance in detecting fibroatheromas. The necrotic core volume had moderate accuracy in detecting lipid-rich plaques. These findings are in line with a recent study by Tanisawa et al which showed that 40% of the low-attenuation plaques on CCTA were classified as non-lipid-rich plaques on NIRS-IVUS26. In addition, several retrospective analyses have shown that the attenuated plaque volume and the morphological features on CCTA can detect vulnerable plaques but with limited accuracy2728. Radiomics analysis seems to provide an effective alternative to detect high-risk plaques29.
Finally, the limited efficacy of CCTA in assessing vessel dimensions affected its performance in guiding stent sizing. We found that CCTA would result in implantation of smaller stents and has limited efficacy in accurately determining stent length compared to NIRS-IVUS as the reference standard. Our results are in line with the findings of the P3 study30 that reported a moderate correlation and wide LoA between the estimations of optical coherence tomography and CCTA for lesion length and reference vessel diameter. In addition, CCTA was weak in identifying lipid-rich plaques that were likely to cause no-reflow during revascularisation and moderate in characterising calcium extent that has been associated with stent underexpansion. The above findings are clinically relevant as they underscore the advantages and limitations of CCTA in guiding revascularisation and should be taken into account by ongoing but also future studies assessing the potential of CCTA in guiding PCI (ClinicalTrials.gov: NCT05253677).
Limitations
Although this study is the first prospective clinical study to enable a thorough evaluation of CCTA in assessing coronary artery pathology against high-resolution NIRS-IVUS imaging – which is U.S. Food and Drug Administration-approved for detecting high-risk lesions and patients – it has several limitations. First, it is a single-centre study; however, this facilitated the implementation of a robust protocol for image comparison and enabled NIRS-IVUS imaging of long segments. Additionally, CCTA imaging was performed with a 3rd-generation scanner using a specific reconstruction protocol; therefore, it is unclear whether these findings apply to previous-generation, or photon-counting, CT scanners and different reconstruction protocols. Moreover, patients with stable angina were included in this analysis, so it is unclear whether our findings apply to patients with acute coronary syndrome that have more advanced atherosclerotic plaques. Furthermore, this study enabled evaluation of the performance of CCTA in detecting lesion characteristics related to stent underexpansion but did not allow comparison of the score, as CCTA cannot detect the presence of calcific nodules, which are included in the IVUS score14. Moreover, stent sizing in lesions considered for revascularisation was performed based on specific PB cutoffs – derived after performing ROC curve analysis to find the best value that predicted a PB of 50% on IVUS; it is unclear whether these results would have been different if a different PB cutoff had been used for defining the landing zones on CCTA. However, considering that CCTA tends to overestimate lesion length in calcific lesions and underestimate its length in fibrotic lesions, we believe that a different cutoff is unlikely to have resulted in a high agreement between the estimations of the two modalities. Finally, although it is the largest study in the field, including >23,000 matched cross-sections, the number of the studied segments and lesions considered for revascularisation remains relatively small.
Conclusions
Our head-to-head comparison of CCTA and NIRS-IVUS showed that CCTA has limitations in detecting the lipid component, measuring the lumen and plaque dimensions and characterising plaque components. This may have an impact on its value in guiding revascularisation.
Impact on daily practice
This is the first prospective study assessing the efficacy of optimal coronary computed tomography angiography (CCTA) in estimating the extent of coronary artery disease, quantifying plaque burden and composition and guiding percutaneous coronary interventions compared to state-of-the-art near-infrared spectroscopy-intravascular ultrasound (NIRS-IVUS) imaging. In our study, the necrotic core volume in CCTA had moderate efficacy in detecting lipid-rich plaques. CCTA also underestimated lumen and plaque dimensions in the segments that had been assessed by both modalities and had limited efficacy in assessing plaque composition. In lesions that were revascularised, CCTA underestimated the reference vessel area and was unable to accurately estimate lesion length and, thus, indicated implantation of smaller stents compared to NIRS-IVUS.
Funding
This study is jointly funded by the British Heart Foundation (PG/17/18/32883), University College London Biomedical Resource Centre (BRC492B) and Rosetrees Trust (A1773). There was additional funding from the project LSHM19028 PRAGMATICS, which is cofunded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships. A. Ramasamy, R. Parasa, A. Mathur, A. Baumbach and C.V. Bourantas are funded by Barts NIHR Biomedical Research Centre, London, United Kingdom.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

