Abstract
BACKGROUND: There are limited data on the clinical characteristics and outcomes of patients who require prolonged mechanical circulatory support (MCS) after Impella-supported high-risk percutaneous coronary intervention (HR-PCI).
AIMS: The aim of this study is to describe the contemporary clinical characteristics, outcomes, and predictors associated with prolonged MCS support after assisted HR-PCI.
METHODS: Patients enrolled in the prospective, multicentre, clinical endpoint-adjudicated PROTECT III study who had undergone HR-PCI using Impella were evaluated. Patient and procedural characteristics and outcomes for those who received prolonged MCS beyond the duration of their index procedure were compared to those in whom MCS was successfully weaned and explanted at the conclusion of the index PCI.
RESULTS: Among 1,155 patients who underwent HR-PCI with Impella between 2017 and 2020 and had sufficient data to confirm the duration of Impella support, 16.5% received prolonged MCS (mean duration 25.2±31.1 hours compared with 1.8±5.8 hours for those who only received intraprocedural MCS). Patients receiving prolonged support presented with more urgent indications (e.g., acute coronary syndromes [ACS], lower ejection fraction [EF], elevated baseline heart rate and lower systolic blood pressure). Use of the Impella CP, intraprocedural complications, periprocedural complications and in-hospital mortality were all more common amongst the prolonged MCS group. Prolonged MCS was associated with increased rates of major adverse cardiovascular and cerebrovascular events, cardiovascular death, and all-cause mortality at 90-day follow-up.
CONCLUSIONS: Patients receiving prolonged MCS after Impella-supported HR-PCI presented with more ACS, reduced EF and less favourable haemodynamics. Additionally, they were more likely to experience intraprocedural and periprocedural complications as well as increased in-hospital and post-discharge mortality.
Advances in mechanical circulatory support (MCS) devices have led to improved outcomes in patients with severe comorbidities and complex coronary lesions undergoing high-risk percutaneous coronary interventions (HR-PCI)12345. The use of Impella (Abiomed) in HR-PCI has increased 27-fold from 2008 to 2018, particularly in small- and medium-sized hospitals6]]. Impella − a percutaneous, microaxial, left ventricular assist device − provides left ventricular unloading and enhances systemic and coronary perfusion, thus mitigating the risk of haemodynamic compromise commonly associated with HR-PCI7.
Patients undergoing HR-PCI with MCS may benefit from ongoing postprocedural haemodynamic support to promote myocardial recovery and systemic perfusion. This includes patients who experience extended periods of impaired myocardial perfusion, which can cause myocardial stunning, as well as those who experience procedural complications causing haemodynamic compromise8.
There are few data on characteristics associated with patients who require prolonged MCS after non-emergent HR-PCI or their clinical outcomes. In a prior analysis of 507 patients who underwent elective or urgent HR-PCI between 2007 and 2014, a minority of patients (8.5%) required MCS for ‘extended support’ with a mean duration of 11.4±16.8 hours9. The need for extended support was predicted only by revascularisation of a chronic total occlusion and was associated with higher risk of adverse events, including periprocedural bleeding and mortality.
Defining baseline and procedural characteristics of patients who require prolonged MCS after HR-PCI may be helpful to guide clinical decision-making. The aim of this study is therefore to describe the contemporary clinical characteristics, outcomes, and predictors associated with prolonged MCS after HR-PCI in the PROTECT III study.
Methods
STUDY DESIGN AND DATA COLLECTION
The PROTECT III study is a prospective, multicentre, single-arm U.S. Food and Drug Administration-audited postapproval study evaluating the safety and efficacy of the Impella 2.5 and the Impella CP in HR-PCI across 46 sites in the United States3. Primary indications for HR-PCI included acute coronary syndromes (ACS; including ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina)10, stable angina, chronic coronary artery disease, cardiomyopathy/heart failure, and refractory arrhythmia. The primary indication for Impella support was prevention of haemodynamic instability during HR-PCI; patients with cardiogenic shock as the primary indication for MCS were excluded from this registry. During the study, the index HR-PCI and the postprocedural care were conducted according to the discretion of the treating physicians, including the decision to implant Impella, the type of Impella utilised and the duration of MCS. Patients met enrolment criteria once the decision was made to use Impella, either before or during the index PCI.
Preprocedural baseline characteristics, echocardiographic data, and procedural data were collected from the time of admission up to discharge. Subjects were followed for 90 days for major adverse cardiovascular and cerebrovascular events (MACCE; defined as the composite of all-cause death, myocardial infarction, stroke/transient ischaemic attack, and repeat revascularisation) and for 1 year for all-cause mortality; as part of the global Catheter-based Ventricular Assist Device (cVAD) registry (ClinicalTrials.gov: NCT04136392), 90-day MACCE were adjudicated by an independent clinical endpoint committee, and echocardiographic and angiographic data were analysed by an independent core lab11. Other in-hospital adverse events were site-reported and are designated as such. Bleeding was defined using the Bleeding Academic Research Consortium (BARC) criteria, with major bleeding defined as those meeting BARC Type 3 or higher12.
The primary comparison of interest for this analysis was between patients who required continuation of MCS beyond the index PCI (“prolonged MCS”) versus those in whom MCS was weaned and explanted immediately after the index PCI (“intraprocedural MCS”). More specifically, patients were included in the prolonged MCS group if any of the following conditions were met: 1) the site reported that the Impella device was not explanted at the end of the procedure; 2) Impella time minus PCI stop time exceeded 60 minutes; 3) any additional mechanical support devices were implanted post-Impella explant. Patients were excluded from the analysis if information regarding items 1) and 3) above were missing, and the Impella time minus PCI time was less than 60 minutes. Outcomes of interest included periprocedural complications, in-hospital adverse events, intensive care unit and hospital length of stay. Post-discharge follow-up outcomes included 90-day MACCE, cardiovascular (CV) death at 90 days, and all-cause death at 1 year. Data on MACCE and CV death at 1 year were not collected.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the applicable institutional review boards or independent ethics committees at each centre prior to subject enrolment. An independent 12-member steering committee, including interventional cardiologists, cardiac surgeons, and heart failure specialists, oversaw the conduct of the cVAD study. The sponsor (Abiomed) oversaw study data management and source document verification and provided funding to the Cardiovascular Research Foundation (New York, NY, USA) for statistical analysis.
STATISTICAL ANALYSIS
Baseline characteristics were summarised with means and standard deviations or medians and interquartile ranges for continuous measures and proportions for categorical variables. Between the study groups, variables were compared with the t-test for continuous measures and the χ2 or Fisher's exact test for categorical variables. For time-to-first event analyses, event rates were estimated by the Kaplan-Meier method and compared with the log-rank test. Multivariable logistic regression models were used to examine the relationship between predictors and risk of prolonged MCS. The association between prolonged MCS and clinical outcomes was examined by multivariate Cox proportional hazard modelling. Multiple imputation was used to account for missing data for various covariates. All p-values are 2-tailed. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
BASELINE CHARACTERISTICS
Among 1,237 patients undergoing Impella-supported HR-PCI between 2017 and 2020 at 46 sites, 1,155 patients had sufficient data to confirm duration of Impella support and thus were included in this analysis. In 190 patients (16%), MCS was continued after HR-PCI for a mean duration of support of 25.2±31.1 hours compared to 1.8±5.8 hours for those who only received intraprocedural MCS. Baseline characteristics are shown in Table 1. Age, sex, and baseline medical history were similar between the two groups. The prolonged MCS group had a greater proportion of Black patients (17.9% vs 11.4%; p=0.01) and smaller proportion of White patients (59.5% vs 68.9%; p=0.01). The mean left ventricular ejection fraction (LVEF) in the entire analysis cohort was 33.9±15.3%, with lower LVEF in the prolonged MCS group (31.3% vs 34.4%; p=0.02). Those who received prolonged MCS had more severe valvular disease (including severe mitral regurgitation, mitral stenosis, aortic regurgitation, or aortic stenosis; 17.1% vs 10.2%; p=0.04) and atrial fibrillation (29.8% vs 15.1%; p=0.02).
There were no significant differences in baseline haemoglobin, white blood cell count, platelets, creatinine, or cardiac biomarkers (including creatine kinase-MB, troponin I, troponin T, and brain natriuretic peptide).
Table 1. Baseline characteristics.
| Patients with prolonged support (N=190) | Patients without prolonged support (N=965) | p-value | |
|---|---|---|---|
| Baseline demographics | |||
| Age, years | 69.6±11.8 (190) | 71.2±11.0 (965) | 0.08 |
| Sex, female | 26.3 (50/190) | 27.5 (265/965) | 0.75 |
| Race | |||
| White or Caucasian | 59.5 (113/190) | 68.9 (665/965) | 0.01 |
| Black or African American | 17.9 (34/190) | 11.4 (110/965) | 0.01 |
| Asian | 3.7 (7/190) | 2.8 (27/965) | 0.51 |
| American Indian or Alaska Native | 0 (0/190) | 0.6 (6/965) | 0.28 |
| Native Hawaiian or other Pacific Islander | 0 (0/190) | 0.1 (1/965) | 0.66 |
| Other race | 5.8 (11/190) | 2.7 (26/965) | 0.03 |
| Unknown race | 13.2 (25/190) | 13.5 (130/965) | 0.91 |
| Body mass index, kg/m2 | 28.8±6.8 (189) | 28.6±6.3 (962) | 0.65 |
| Medical history | |||
| History of tobacco use | 62.0 (116/187) | 62.6 (588/940) | 0.89 |
| Current | 31.6 (37/117) | 26.7 (157/588) | 0.28 |
| Hypertension | 92.6 (174/188) | 91.6 (878/958) | 0.68 |
| Dyslipidaemia | 75.3 (140/186) | 80.1 (764/954) | 0.14 |
| Diabetes mellitus | 61.7 (116/188) | 54.0 (518/959) | 0.053 |
| Anaemia | 15.9 (28/176) | 20.5 (186/907) | 0.16 |
| Peripheral vascular disease | 18.3 (34/186) | 22.9 (217/949) | 0.17 |
| Stroke/TIA | 15.0 (28/187) | 18.3 (174/953) | 0.28 |
| Renal insufficiency | 33.7 (63/187) | 30.5 (291/954) | 0.39 |
| Dialysis necessary | 34.9 (22/63) | 26.8 (78/291) | 0.19 |
| Prior myocardial infarction | 37.5 (66/176) | 41.0 (380/927) | 0.39 |
| Prior PCI | 36.4 (68/187) | 39.0 (370/948) | 0.49 |
| Prior CABG | 18.0 (34/189) | 13.9 (133/957) | 0.15 |
| Angina | 37.3 (66/177) | 44.8 (416/928) | 0.06 |
| Heart failure | 54.0 (101/187) | 61.8 (588/952) | 0.05 |
| Cardiomyopathy | 37.5 (66/176) | 43.1 (400/928) | 0.17 |
| Conduction disorder | 7.9 (14/177) | 9.8 (91/930) | 0.44 |
| Arrhythmia | 24.2 (43/178) | 30.5 (289/949) | 0.09 |
| Active implantable devices | 19.3 (35/181) | 17.1 (164/961) | 0.46 |
| LV ejection fraction, % | 31.3±14.8 (150) | 34.4±15.3 (740) | 0.02 |
| Severe valvular disease* | 17.1 (19/111) | 10.2 (56/548) | 0.04 |
| Atrial fibrillation | 29.8 (14/47) | 15.1 (27/179) | 0.02 |
| Baseline laboratory | |||
| Haemoglobin, g/dL | 12.1±2.3 (175) | 12.1±2.1 (834) | 0.96 |
| Platelets, K/µL | 219.2±86.4 (174) | 216.1±79.8 (822) | 0.65 |
| White blood cell, K/µL | 8.9±3.8 (171) | 8.3±5.3 (818) | 0.16 |
| Creatinine, mg/dL | 1.3±0.7 (148) | 1.2±0.5 (758) | 0.20 |
| Troponin I, ng/mL | 68.5±465.3 (71) | 130.8±946.7 (259) | 0.59 |
| BNP, pg/mL | 1,230.2±1,401.1 (29) | 1,374.1±1,425.3 (108) | 0.63 |
| Data are presented as mean±standard deviation (n) or % (n/N), where applicable. *Includes severe aortic stenosis/regurgitation and severe mitral stenosis/regurgitation. BNP: brain natriuretic peptide; CABG: coronary artery bypass graft; LV: left ventricular; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack | |||
ADMISSION AND PROCEDURAL CHARACTERISTICS
Patients requiring prolonged MCS were more likely to have undergone urgent rather than elective PCI (61.6% vs 48.5%; p=0.001) and the primary indication for PCI was more often for ACS (59.4% in the prolonged MCS group vs 49.8% in the intraprocedural MCS group; p=0.02). The vast majority of patients in both groups (97.8% in the prolonged MCS group vs 99.8% in the intraprocedural MCS group; p=0.001) had Impella placed upon arrival to the catheterisation laboratory, prior to HR-PCI (Table 2). Impella CP was used more frequently over Impella 2.5 in both groups but was used more frequently among those receiving prolonged MCS (75.3% vs 66.7%; p=0.02).
Intraprocedural haemodynamic data demonstrated higher heart rates and lower systolic blood pressure before, during, and after Impella placement in patients who required prolonged MCS (Supplementary Table 1). Invasive haemodynamics by right heart catheterisation were performed in 15.7% of patients overall pre-Impella placement, including 21.6% in the prolonged MCS group versus 14.5% in the intraprocedural MCS group. The median cardiac output and cardiac index were lower and the median left ventricular end-diastolic pressure was higher among patients requiring prolonged support, with no significant differences in pulmonary artery systolic pressure, pulmonary capillary wedge pressure, central venous pressure, or venous oxygen saturation (Supplementary Table 2).
No differences were observed between the two groups in terms of target vessel location, pre- and post-PCI SYNTAX scores or myocardial ischaemia jeopardy scores (MJS). Pre-PCI, the minimum Thrombolysis in Myocardial Infarction (TIMI) flow was lower in the prolonged MCS group (p=0.003), with no differences in TIMI flow between the groups post-PCI (p=0.81). Intraprocedural PCI-related complications were more common among patients requiring prolonged MCS (8.8% vs 3.9%; p=0.004) (Table 2).
Table 2. Indication and procedural characteristics.
| Patients with prolonged support (N=190) | Patients without prolonged support (N=965) | p-value | |
|---|---|---|---|
| Indication | |||
| PCI status: urgent | 61.6 (117/190) | 48.5 (468/965) | 0.001 |
| Primary indication for PCI: ACS | 59.4 (101/170) | 49.8 (423/849) | 0.02 |
| Staged PCI | 17.9 (34/190) | 23.5 (225/959) | 0.09 |
| Timing of Impella insertion | |||
| Prior to catheterisation lab arrival | 1.1 (2/180) | 0.2 (2/964) | 0.059 |
| Intraprocedural, prior to coronary intervention | 97.8 (176/180) | 99.8 (962/964) | 0.001 |
| Intraprocedural, during coronary intervention | 1.1 (2/180) | 0 (0/964) | 0.0006 |
| Impella type | |||
| Impella 2.5 | 24.7 (47/190) | 33.3 (321/965) | 0.02 |
| Impella CP | 75.3 (143/190) | 66.7 (644/965) | 0.02 |
| Number of vessels treated | 2.0 [1.0, 3.0] | 2.0 [2.0, 3.0] | 0.003 |
| Adjunctive therapy/diagnostics used | |||
| Atherectomy | 39.8 (74/186) | 39.5 (377/954) | 0.95 |
| FFR | 0 (0/186) | 2.3 (22/954) | 0.04 |
| OCT/IVUS | 50.0 (93/186) | 47.1 (449/954) | 0.55 |
| Temporary pacer | 9.7 (18/186) | 9.0 (86/954) | 0.77 |
| Vessel location | |||
| LM | 42.1 (80/190) | 46.7 (449/962) | 0.25 |
| LAD | 70.0 (133/190) | 73.7 (709/962) | 0.29 |
| LCx | 52.6 (100/190) | 54.9 (528/962) | 0.57 |
| RCA | 28.4 (54/190) | 30.1 (290/962) | 0.64 |
| Graft | 5.3 (10/190) | 3.6 (35/962) | 0.29 |
| Pre-PCI TIMI flow* | 0.003 | ||
| 0 | 18.5 (31/168) | 10.9 (84/774) | |
| 1 | 6.0 (10/168) | 2.7 (21/774) | |
| 2 | 5.4 (9/168) | 3.9 (30/774) | |
| 3 | 70.2 (118/168) | 82.6 (639/774) | |
| Post-PCI TIMI flow* | 0.81 | ||
| 0 | 0.6 (1/167) | 1.3 (10/782) | |
| 1 | 0.6 (1/167) | 0.4 (3/782) | |
| 2 | 1.8 (3/167) | 1.3 (10/782) | |
| 3 | 97.0 (162/167) | 97.1 (759/782) | |
| Pre-PCI SYNTAX score | 27.8±13.3 | 27.8±12.4 | 0.99 |
| Post-PCI SYNTAX score | 6.4±7.9 | 6.5±8.3 | 0.91 |
| Pre-PCI myocardial ischaemia jeopardy score | 8.6±2.3 | 8.9±2.1 | 0.14 |
| Post-PCI myocardial ischaemia jeopardy score | 2.0±2.3 | 1.9±2.1 | 0.76 |
| PCI-related complications during PCI procedure† | 8.8 (16/181) | 3.9 (36/934) | 0.004 |
| Coronary dissection | 1.7 (3/181) | 0.5 (5/934) | 0.10 |
| Coronary perforation | 2.2 (4/181) | 1.4 (13/934) | 0.41 |
| Acute (abrupt) closure | 0.6 (1/181) | 0.1 (1/934) | 0.19 |
| No reflow | 0.6 (1/181) | 0.2 (2/934) | 0.42 |
| Failure of stent deployment | 1.7 (3/181) | 0.2 (2/934) | 0.008 |
| Arrhythmia | 1.7 (3/181) | 0.1 (1/934) | 0.001 |
| Cardiac arrest | 1.7 (3/181) | 0.2 (2/934) | 0.008 |
| Data are presented as % (n/N), mean±standard deviation, or median [Q1, Q3], where applicable. *For patients with more than one lesion, the TIMI flow for the most severe lesion (i.e., the lowest TIMI flow measured) is reported. †Site-reported events. ACS: acute coronary syndrome (defined as ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina); FFR: fractional flow reserve; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main; NSTEMI: non-ST-elevation myocardial infarction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; Q: quartile; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction | |||
PREDICTORS OF NEED FOR PROLONGED MECHANICAL CIRCULATORY SUPPORT
In a univariate analysis, lower LVEF, higher heart rate, lower systolic blood pressure, urgent indication for PCI, ACS, and the use of Impella CP (vs Impella 2.5) were associated with an increased risk of prolonged MCS, while White race and history of stable angina were associated with a reduced risk (Table 3). In a multivariable regression, the use of Impella CP emerged as a variable independently associated with an increased risk of prolonged support after HR-PCI, while White race was associated with a decreased risk of prolonged support. There was also a trend suggestive of an association between history of angina and decreased risk of prolonged support, though this did not reach statistical significance (p=0.08).
Table 3. Predictors of prolonged support.
| Variable | Univariate model | Multivariable model | p-value | |
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | ||
| Age* | 0.95 (0.88-1.03) | 0.22 | 0.97 (0.89-1.07) | 0.60 |
| Sex, male | 1.03 (0.85-1.24) | 0.80 | 0.92 (0.73-1.15) | 0.47 |
| Race, White vs others | 0.76 (0.64-0.91) | 0.002 | 0.74 (0.59-0.92) | 0.006 |
| Diabetes mellitus | 1.16 (0.97-1.38) | 0.10 | 1.07 (0.86-1.32) | 0.55 |
| Coronary artery disease | 0.89 (0.72-1.11) | 0.31 | 1.04 (0.79-1.36) | 0.79 |
| Heart failure | 0.88 (0.74-1.05) | 0.15 | 0.85 (0.67-1.08) | 0.18 |
| History of stable angina | 0.79 (0.66-0.95) | 0.01 | 0.82 (0.66-1.02) | 0.08 |
| Left ventricular ejection fraction † | 0.88 (0.77-1.00) | 0.046 | 0.93 (0.80-1.09) | 0.36 |
| Heart rate pre-Impella implant ‡ | 1.12 (1.02-1.23) | 0.01 | 1.09 (0.97-1.21) | 0.14 |
| Systolic blood pressure pre-Impella implant ¶ | 0.91 (0.84-0.99) | 0.02 | 0.94 (0.85-1.04) | 0.23 |
| Urgent PCI status | 1.26 (1.06-1.50) | 0.008 | 1.11 (0.90-1.38) | 0.33 |
| Acute coronary syndrome | 1.19 (1.00-1.41) | 0.049 | 1.05 (0.85-1.31) | 0.65 |
| Impella CP (vs Impella 2.5) | 1.30 (1.06-1.59) | 0.01 | 1.28 (1.01-1.63) | 0.04 |
| *Per 5-year increments; † per 10% increments; ‡ per 10 beats per minute increments; ¶ per 10-mmHg increments. CI: confidence interval; OR: odds ratio; PCI: percutaneous coronary intervention | ||||
CLINICAL OUTCOMES OF PATIENTS REQUIRING PROLONGED MECHANICAL CIRCULATORY SUPPORT
In-hospital MACCE, all-cause death, CV death, and myocardial infarction were all more frequent in patients requiring prolonged MCS (Table 4). Among other site-reported adverse events (Table 5), haemolysis and in-hospital bleeding complications, including major bleeding, haematoma, and anaemia requiring transfusion were more common among the prolonged MCS group. There were no significant differences in vascular complications requiring intervention. Ventricular and supraventricular arrhythmias, acute kidney injury, and limb ischaemia were all more frequent among those with prolonged MCS. The duration of both intensive care unit stays (6.2±5.8 days vs 4.7±5.5 days; p=0.004) and overall hospital stays (12.8±28.9 days vs 8.1±18.3 days; p=0.004) were longer in those receiving prolonged MCS.
At 90-day follow-up, prolonged MCS was associated with increased MACCE, cardiovascular mortality, and all-cause mortality (Table 4, Figure 1). All-cause mortality remained higher among the prolonged MCS group (17.3% vs 9.2%; p=0.0008) up to 1-year follow-up (Figure 2), and Cox multivariable regression analysis revealed that prolonged MCS was independently associated with 90-day MACCE (hazard ratio [HR] 1.85, 95% confidence interval [CI]: 1.21-2.81), 90-day cardiovascular mortality (HR 2.49, 95% CI: 1.58-3.92), 90-day all-cause mortality (HR 2.21, 95% CI: 1.42-3.45), and 1-year all-cause mortality (HR 1.53, 95% CI: 1.02-2.27) (Central illustration, Table 6).
Table 4. In-hospital and 90-day major adverse cardiovascular and cerebrovascular events.
| Patients with prolonged support (N=190) | Patients without prolonged support (N=965) | p-value | |
|---|---|---|---|
| MACCE* at hospital discharge | 11.1 (21/190) | 3.8 (37/965) | <0.0001 |
| All-cause death | 10.0 (19/190) | 2.7 (26/965) | <0.0001 |
| Cardiovascular death | 10.0 (19/190) | 2.4 (23/965) | <0.0001 |
| Myocardial infarction | 2.6 (5/190) | 0.7 (7/965) | 0.02 |
| Neurological dysfunction (stroke/TIA) | 1.1 (2/190) | 1.1 (11/965) | 0.92 |
| Repeat revascularisation | 0.5 (1/190) | 0.1 (1/965) | 0.20 |
| MACCE* at 90 days | 18.9 (33) | 11.2 (88) | 0.004 |
| All-cause death | 17.3 (30) | 9.2 (70) | 0.0008 |
| Cardiovascular death | 17.3 (30) | 7.9 (60) | <0.0001 |
| Myocardial infarction | 6.1 (10) | 3.0 (22) | 0.03 |
| Neurological dysfunction (stroke/TIA) | 1.1 (2) | 1.6 (14) | 0.64 |
| Repeat revascularisation | 2.1 (3) | 1.9 (13) | 0.87 |
| *MACCE is defined as the composite of all-cause death, myocardial infarction, stroke/TIA, and repeat revascularisation. In-hospital events are reported as binary event rates (n/N) and were compared using the χ2 or Fisher’s exact test. Ninety-day events are reported as Kaplan-Meier time to event rates (n patients with event) and were compared using the log-rank test. Data are presented as % (n/N) or % (n). MACCE: major adverse cardiovascular and cerebrovascular events; TIA: transient ischaemic attack | |||
Table 5. Other in-hospital adverse events.
| Patients with prolonged support (N=190) | Patients without prolonged support (N=964) | p-value | |
|---|---|---|---|
| Any adverse event | 51.1 (97/190) | 22.5 (217/964) | <0.0001 |
| Cardiac arrest | 4.2 (8/190) | 1.8 (17/964) | 0.03 |
| Cardiogenic shock | 7.4 (14/190) | 1.7 (16/964) | <0.0001 |
| Hypotension during support | 12.6 (24/190) | 0.9 (9/964) | <0.0001 |
| Life-threatening, disabling, or major bleeding (BARC ≥3) | 6.3 (12/190) | 1.7 (16/964) | 0.0001 |
| Haemolysis | 5.8 (11/190) | 0.2 (2/964) | <0.0001 |
| Anaemia requiring transfusion | 15.8 (30/190) | 6.3 (61/964) | <0.0001 |
| Vascular/cardiac structural complication requiring surgery/reintervention | 1.6 (3/190) | 1.0 (10/964) | 0.52 |
| Vascular complication without surgery | 2.1 (4/190) | 1.5 (14/964) | 0.51 |
| Haematoma | 11.6 (22/190) | 6.5 (63/964) | 0.01 |
| Limb ischaemia | 5.8 (11/190) | 1.1 (11/964) | <0.0001 |
| Acute kidney injury stage 2 or 3 | 12.1 (23/190) | 2.7 (26/964) | <0.0001 |
| Ventricular arrhythmia | 4.7 (9/190) | 1.1 (11/964) | 0.0005 |
| Supraventricular arrhythmia | 3.7 (7/190) | 0.9 (9/964) | 0.003 |
| Deep venous thrombosis | 1.6 (3/190) | 0.2 (2/964) | 0.009 |
| Respiratory dysfunction/failure | 3.7 (7/190) | 1.3 (13/964) | 0.02 |
| Data are presented as % (n/N). Site-reported in-hospital adverse events. BARC: Bleeding Academic Research Consortium | |||
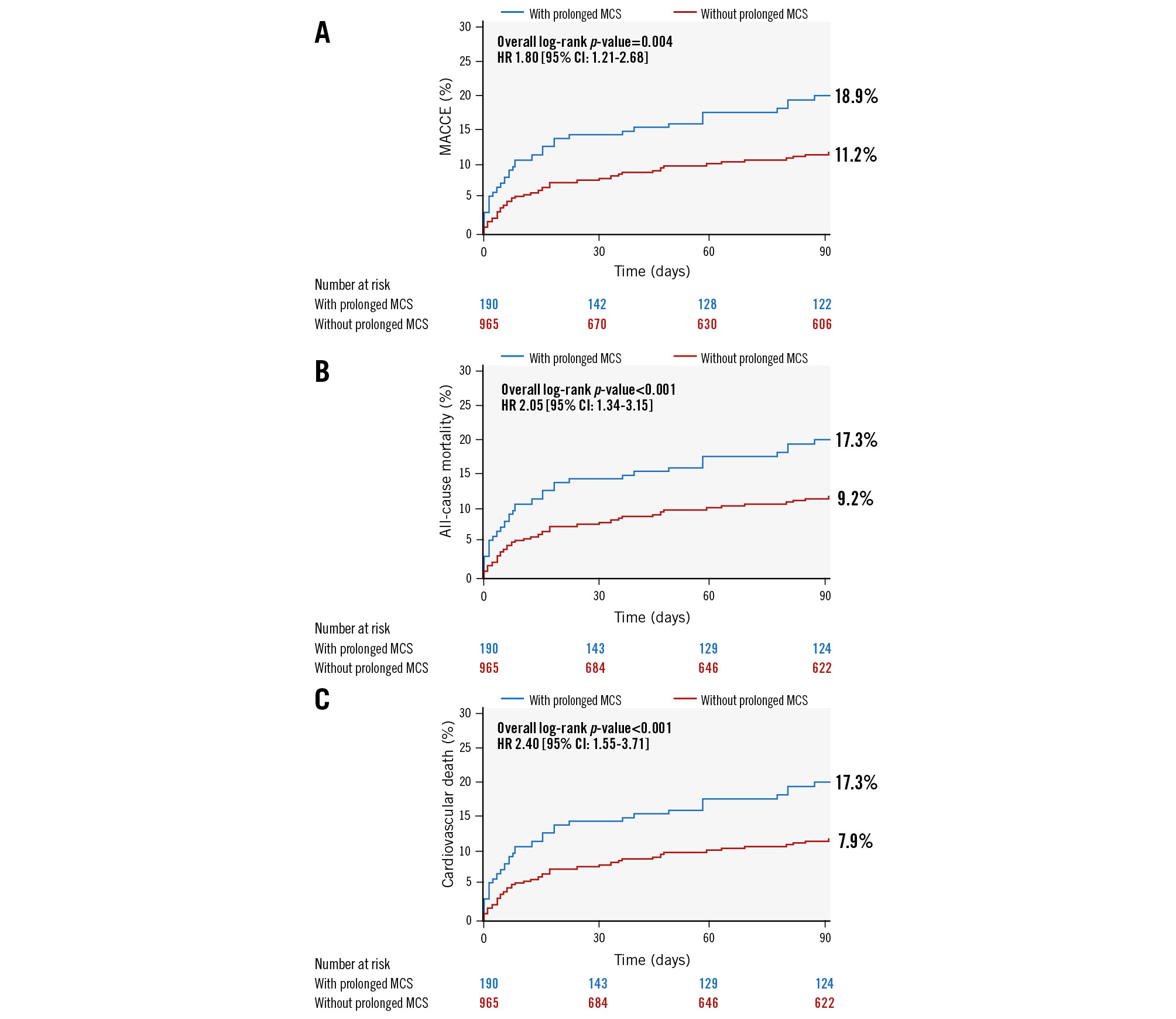
Figure 1. Kaplan-Meier curves for 90-day outcomes. A) MACCE, B) all-cause mortality, and C) cardiovascular death. CI: confidence interval; HR: hazard ratio; MACCE: major adverse cardiovascular and cerebrovascular events; MCS: mechanical circulatory support
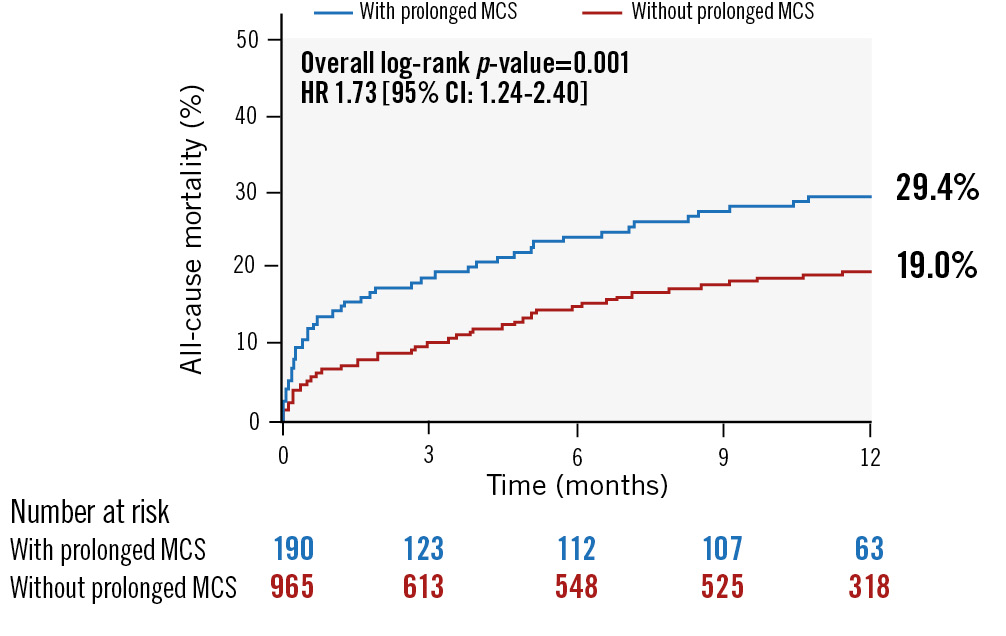
Figure 2. Kaplan-Meier curve for 1-year all-cause mortality. CI: confidence interval; HR: hazard ratio; MCS: mechanical circulatory support
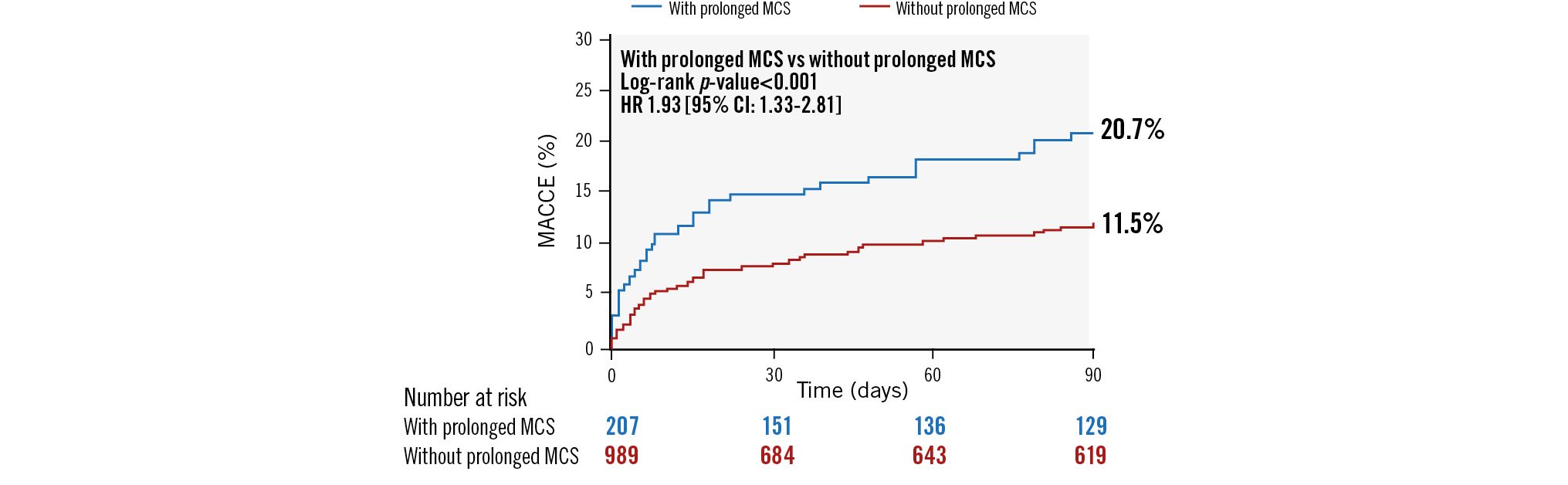
Central illustration. Ninety-day clinical outcomes of patients requiring prolonged mechanical circulatory support after high-risk percutaneous coronary intervention. CI: confidence interval; HR: hazard ratio; MACCE: major adverse cardiovascular and cerebrovascular events; MCS: mechanical circulatory support
Table 6. Relationship between prolonged support and clinical outcomes before and after adjustment for potential confounders*.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Endpoint | HR (95% CI) | p-value | HR (95% CI) | p-value |
| 90-day outcomes | ||||
| MACCE | 1.85 (1.21-2.81) | 0.004 | 1.86 (1.15-3.03) | 0.01 |
| Death | 2.21 (1.42-3.45) | 0.0005 | 2.13 (1.26-3.59) | 0.004 |
| CV death | 2.49 (1.58-3.92) | <0.0001 | 2.39 (1.40-4.08) | 0.001 |
| 1-year outcomes | ||||
| Death | 1.68 (1.19-2.38) | 0.003 | 1.53 (1.02-2.27) | 0.04 |
| *Adjusted for sex, age, race, dyslipidaemia, diabetes, heart failure, angina, left ventricular ejection fraction, heart rate pre-Impella implant, systolic blood pressure pre-Impella implant, and PCI status. CI: confidence interval; CV: cardiovascular; HR: hazard ratio; MACCE: major adverse cardiovascular and cerebrovascular event; PCI: percutaneous coronary intervention | ||||
Discussion
This analysis of patients enrolled in PROTECT III is the largest to date describing clinical characteristics and outcomes of patients who received prolonged MCS after Impella-supported HR-PCI. The key findings from this analysis are as follows: 1) 16% of patients undergoing Impella-supported HR-PCI received prolonged MCS, for a mean duration of 25.2±31.1 hours; 2) patients receiving prolonged MCS support were more likely to be non-White, presented with more urgent indications (e.g., ACS), had lower LVEF, higher baseline heart rate and lower systolic blood pressure, and experienced more periprocedural complications; and 3) prolonged MCS after the index HR-PCI was associated with higher rates of adverse events including MACCE and mortality at 90 days and mortality up to 1-year follow-up.
Overall, these findings demonstrate that the use of prolonged MCS following Impella-supported HR-PCI is common, and the need for prolonged support is driven by intraprocedural complications, acuity of presentation, and unfavourable haemodynamics. However, our multivariate model demonstrated that use of the Impella CP (over the Impella 2.5) was the only independent clinical variable associated with prolonged support. This is not surprising, as the Impella CP offers higher haemodynamic support compared to the Impella 2.5 and is likely to be preferentially selected when there is a higher index of suspicion for clinical decompensation. An operator’s decision to use the Impella CP over the Impella 2.5 likely captures a combination of higher-risk clinical features (e.g., urgent indication for procedure, reduced LVEF, higher heart rate and lower blood pressure), reflecting an overall clinical impression about the degree of risk in a given procedure which may not be captured in a single haemodynamic variable. This highlights the challenges of predicting which patients will require prolonged support after Impella-supported HR-PCI and emphasises the importance of operator interpretation of multiple different clinical variables in aggregate, along with the utilisation of invasive and non-invasive haemodynamic measurements, when deciding whether patients should remain on postprocedural MCS.
Compared with the earlier cVAD registry analysis of patients undergoing Impella-supported HR-PCI between 2007 and 20149, our contemporary cohort demonstrated an approximately 2-fold increase in the need for prolonged MCS and an approximately 2-fold increase in the mean duration of MCS. Important differences in study design and procedural characteristics likely explain these differences. The prior analysis excluded patients with ST-elevation myocardial infarction, while the PROTECT III study did not exclude these higher-risk patients. The PROTECT III study also included a greater number of lesions treated during the procedure (mean 2.6±1.4) as well as greater use of atherectomy (used in 39% of cases), compared to the earlier analysis (mean 1.71±0.78, and 16%, respectively), reflecting longer, more complex procedures performed in this cohort. However, while patients in this cohort had numerous higher-risk features, the 10% in-hospital mortality rate observed in our prolonged MCS group was comparable to the 11.6% in-hospital mortality rate in the earlier analysis; data on follow-up MACCE or mortality were not available for comparison. While differences in study design and time period limit direct comparisons (for instance, there was a greater use of the Impella CP in our study than the Impella 2.5), the current analysis suggests that contemporary advances in best practices for Impella-supported HR-PCI have likely resulted in more complete revascularisation during modern HR-PCI and have led to improved safety and clinical outcomes over time3. This is an important consideration for clinicians and their patients pursuing HR-PCI, as a recent analysis of the large IMP-IT registry from Italy showed improved survival associated with more extensive revascularisation in HR-PCI1314. Additionally, a subanalysis from the same Italian registry showed improved survival and decreased rates of complication when Impella was inserted prior to coronary intervention15, which was the timing strategy employed for the vast majority of patients in PROTECT III (Table 2).
For clinicians caring for patients receiving prolonged MCS after HR-PCI, weaning and the eventual explant of MCS devices is a critical consideration. While several recommendations have been proposed for the de-escalation of temporary MCS in cardiogenic shock patients1617, guidance for patients who have undergone HR-PCI is less robust. De-escalation and explantation of MCS devices after HR-PCI should be guided by the stability of invasive and non-invasive haemodynamic indices (i.e., blood pressure, cardiac index, cardiac power output and cardiac filling pressures), as well as clinical and laboratory markers of end-organ dysfunction (i.e., serum lactate, urine output, and liver function tests), while reducing device support. Frequent monitoring for device-related complications which may be related to long-term outcomes remains a critical part of the weaning strategy18. Importantly, our analysis demonstrates the high-risk characteristics of this patient population, highlighting the need for experienced, collaborative, multidisciplinary Heart Teams to tailor successful weaning strategies to individual patients and their clinical scenarios.
In the PROTECT III cohort, the use of right heart catheterisation to measure invasive haemodynamics was infrequent (21.6% in the prolonged MCS group, and 15.7% of patients overall), suggesting most decisions to extend or wean MCS were likely dictated by less invasive measures of haemodynamic compromise. Recent registry data have shown the value and possible survival benefit of invasive haemodynamic monitoring in cardiogenic shock, particularly in the assessment of right-sided and biventricular heart failure192021. Yet, in the absence of high-quality randomised data, it remains unclear how invasive haemodynamic parameters should be used in decision algorithms to prolong or wean MCS in instances of haemodynamic compromise after HR-PCI, and in cardiogenic shock generally. Given the paucity of data in this area, further investigation is needed to guide safe and timely weaning and explantation of MCS after HR-PCI, including the possible utility and clinical impact of invasive haemodynamics. The use of right heart catheterisation to inform MCS weaning after HR-PCI is among one of the prespecified substudies for the ongoing randomised PROTECT IV trial22, which could provide further guidance in the future.
Limitations
Limitations to our study include its post hoc, non-randomised design. As such, it is unable to address the question of which patients may benefit from prolonged MCS following HR-PCI. Additionally, PROTECT III did not collect data specifying the primary rationale or the underlying pathology prompting the need for prolonged MCS. Future studies investigating the various indications for MCS in HR-PCI and criteria for weaning and explantation are needed. Right heart catheterisation and invasive haemodynamic data, where available, were limited. Randomised controlled studies utilising haemodynamic and clinical assessments and exploring the outcomes and optimal duration of prolonged MCS after HR-PCI are needed.
Conclusions
In the prospective, multicentre PROTECT III study, patients who required prolonged MCS following Impella-supported HR-PCI were more likely to be non-White, present with more urgent indications (i.e., ACS), lower LVEF, less favourable haemodynamics and had experienced intraprocedural complications. Patients requiring prolonged support were more likely to experience periprocedural complications as well as increased in-hospital and post-discharge mortality. The management and device weaning of such patients should be done by experienced multidisciplinary Heart Teams to tailor successful weaning strategies to individual patients and their clinical scenarios.
Impact on daily practice
Contemporary characteristics and outcomes related to prolonged mechanical circulatory support (MCS) following high-risk percutaneous coronary interventions (HR-PCI) have not been well characterised. In PROTECT III, patients who underwent Impella-supported HR-PCI and required prolonged MCS were more likely to present with acute coronary syndrome, reduced ejection fraction, less favourable haemodynamics, and experience increased complications and mortality. Results from this study assist clinicians in early recognition of the high-risk characteristics associated with HR-PCI and highlight the need for safe practices for weaning MCS (including the potential utility of right heart catheterisation), while offering valuable insights for future investigations into the optimal use of MCS in this context.
Funding
The PROTECT III study, as part of The Global cVAD study, was sponsored by Abiomed.
Conflict of interest statement
M.B. Basir has been a consultant/speaker for Abiomed, Boston Scientific, Chiesi, Saranas, and Zoll. A.G. Truesdell has received consultant and speaker fees, paid to his institution, from Abiomed. A.S. Bharadwaj has been a consultant/speaker for Abiomed, Shockwave Medical, and CSI. A. Kaki receives speaker honoraria, consultant fees and research funding from Abiomed, Abbott, CSI, and Terumo. D.H. Wohns receives institutional research and educational funding as well as consulting/speaking honoraria from Abiomed. P.M. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. C.L. Grines reports participation on the advisory boards of Philips and Abiomed. W.W. O’Neill reports grant/research support from St. Jude Medical, Edwards Lifesciences, and Abiomed; consulting fees/honoraria from Medtronic and Abiomed; and is a major stock or shareholder/holds equity in Synecor, AccuMed, Neovasc, Tendyne (Abbott), and Mitralign. J.W. Moses holds equity in Orchestra BioMed. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

