In patients with acute coronary syndrome (ACS) activation and aggregation of platelets play a crucial role in the formation of intracoronary thrombus. Dual antiplatelet (DAPT) therapy, by combining the cyclooxygenase-1 blocker aspirin with an inhibitor of ADP-receptor P2Y12, represents the standard of care in this setting. While in the past, the second-generation thienopyridine clopidogrel had been the treatment of choice, recently the thienopyridine prasugrel and the triazolopyrimidine ticagrelor have emerged as potent alternatives for antiplatelet therapy.
All thienopyridines are normally rapidly absorbed in the intestine. The pro-drugs require bioactivation by cytochrome CYP450 in the liver to generate the active metabolites that irreversibly bind to the P2Y12 receptor and inhibit ADP-induced activation throughout the entire lifespan of a platelet. Clopidogrel is bioactivated in two CYP450-dependent steps. This pharmacokinetic feature leads to delayed onset of the antiplatelet effect and an intra- and inter-individual response variability1. Since the cytochrome enzymes participate in the metabolism of a wide variety of pharmacological compounds, interaction occurs. Co-administration of agents that share the metabolic pathways of clopidogrel, such as proton pump inhibitors, statins and calcium-channel-blockers, can decrease the antiplatelet effect of clopidogrel ex vivo2,3.
Variants in gene encoding for CYP450, intestinal transporters (e.g., ABCB1) or the P2Y12-receptor are known to modify the antiplatelet effect of clopidogrel3. These so-called genetic polymorphisms occur in at least 1% of the population, vary in different ethnic groups and cause either loss-of-function or gain-of-function effects on the corresponding gene product. Loss-of-function polymorphisms of the cytochrome isoenzymes, especially CYP3A4/5, CYP2C19 and CYP1A2, lead to lower levels of the active metabolite of clopidogrel and consequently poor-responder-status to the drug in 15%-40% of all patients1,3. This so-called “high-on treatment” platelet reactivity (HTPR) is associated with the occurrence of ischaemic events including coronary stent thrombosis4.
In this current issue of EuroIntervention, Liang et al describe the impact of various single nucleotide polymorphisms (SNPs) on the clinical outcome of patients with ACS after drug-eluting stent (DES) implantation during one-year follow-up5. There were 1,016 patients from the Chinese Han population presenting with ACS who were enrolled. After DES implantation, the post-procedure maximum platelet aggregation was tested, and cut-off values were determined for HTPR and bleeding. Not surprisingly, patients who responded poorly to clopidogrel had a significantly elevated risk of ischaemic events during the one-year follow-up. Furthermore, genotyping for 19 SNPs in CYP450 enzymes, P2Y12 receptor and ABCB1 transporter genes revealed an association between the carriage of two CYP2C19 loss-of-function alleles (CYP2C19*2 or CYP2C19*3) and the risk of ischaemic events and HTPR compared to carriage of none or only one allele.
These findings support the observation from several other trials of an association between HTPR, respectively, loss-of-function CYP450 SNPs and ischaemic events in patients after PCI treated with clopidogrel3,6. However, there are certain limitations of the trial that merit consideration: frequencies of the described alleles vary substantially in different populations. The CYP2C19*2 and CYP2C19*3 alleles represent 99% of loss-of-function alleles in Asians categorised as suboptimal responders, compared to 85% in Caucasians7. The gain-of-function allele CYP2C19*17 was markedly lower in the Chinese Han cohort (0.9%) than in the Western population (18-28%). Hence, the different frequencies of these alleles complicate the interpretation of the results of this trial for the Western population.
Mainly because of the complexity of its metabolism and the variability of the antiplatelet effect of clopidogrel, other more potent and consistent P2Y12 receptor inhibitors, such as the direct-acting ADP receptor inhibitor ticagrelor and the thienopyridine prasugrel, recently emerged as antiplatelet drugs of first choice in the setting of ACS. Ticagrelor requires no bioactivation and binds directly and reversibly to the P2Y12 receptor to inhibit ADP mediated platelet activation. Accordingly, the drug features a fast-onset effect, thereby lacking considerable variability of drug response as described for clopidogrel. The third-generation thienopyridine prasugrel is hydrolysed and subsequently bioactivated by hepatic CYP450 enzymes in a single step metabolism1. Hence, genetic polymorphisms of the cytochrome enzymes may also have a significant impact on platelet response to prasugrel8,9. Poor-response rates to prasugrel ranging from 0 to 25%, depending on the method and cut-off values used have been reported10.
In the clinical setting of ACS, the following questions have to be addressed at the present time:
– Which antiplatelet agent should be chosen?
– How can patients with HTPR be identified?
– When HTPR is suspected or proven, should increasing the dose or switching from clopidogrel to either one of the novel P2Y12 receptor inhibitors be considered?
According to the guidelines of the European Society of Cardiology (ESC) for the management of patients presenting with or without ST-elevation myocardial infarction, the use of either ticagrelor or prasugrel is a class I, level of evidence B recommendation in both settings in addition to aspirin. Clopidogrel is only recommended when the new antiplatelet agents are not available or contraindicated (recommendation class I, level of evidence C, respectively A)6,11. However, clopidogrel is still used in a considerable number of ACS patients.
So, how can patients be identified who might benefit the most from the administration of the novel drugs?
Clinically, certain conditions are linked to a suboptimal clopidogrel response: extent and severity of coronary artery disease, age, sex, diabetes mellitus, renal function and obesity3,5. Besides, the recurrence of atherothrombotic events including stent thrombosis arouses the suspicion of HTPR when drug-drug interactions and non-compliance have been ruled out. Basically, two methods of testing exist: platelet function assays preferably using aggregometry and genetic testing. However, it still remains unclear whether routine testing should be performed in those patients where we have the suspicion of HTPR or if clinical judgement is sufficient. For the moment, the ESC guidelines do not recommend either genetic or platelet activity testing in our clinical routine6 (Figure 1).
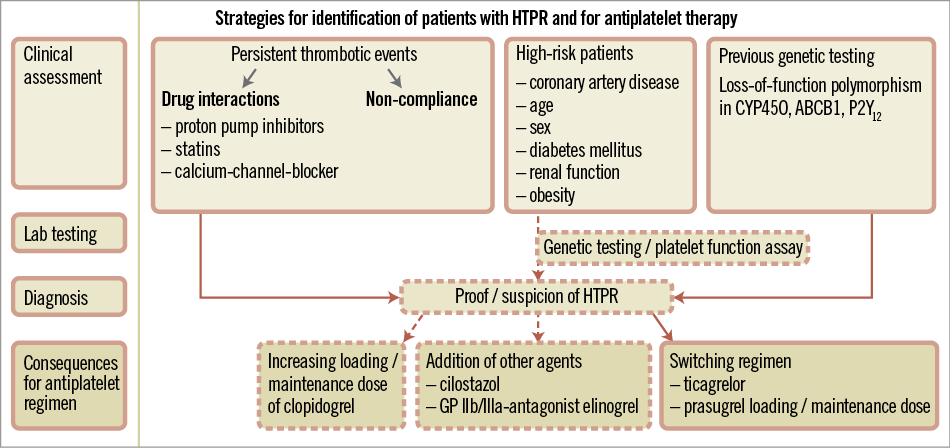
Figure 1. Strategies for identification of HTPR and antiplatelet regimen.
So, which antiplatelet regimen should be chosen in case of HTPR?
The benefit of doubling or even tripling the clopidogrel maintenance dose is unclear3,7. Small-scale trials have demonstrated that addition of cilostazol, elinogrel or glycoprotein receptor IIb/IIIa inhibitors, or switching to novel P2Y12 receptor inhibitors such as ticagrelor or prasugrel decreases the rate of HTPR7. Compared to prasugrel, treatment with ticagrelor results in even more pronounced platelet inhibition in those patients in vitro9.
Gradually we begin to understand the complexity of the clopidogrel metabolism. The one-fits-all-treatment strategy of platelet inhibition seems to be out-dated and replaced by a more selective administration of antiplatelet drugs. Nonetheless, the clinical benefit of a personalised approach, based on tailored antiplatelet therapy according to platelet reactivity or genetic testing has to be evaluated in large-scale clinical trials. Until then, as clinicians we have to rely on our clinical judgement.
Conflict of interest statement
The authors have no conflicts of interest to declare.

