
Abstract
Aims: We sought to investigate the clinical impact of chronic total occlusion (CTO) in a non-infarct-related artery (IRA) on long-term cardiovascular outcomes in patients with ST-elevation myocardial infarction (STEMI).
Methods and results: Among 5,429 patients enrolled in the CREDO-Kyoto AMI registry, the current study population consisted of 2,045 STEMI patients with multivessel disease (MVD) who underwent primary PCI within 24 hours after symptom onset. The cumulative five-year, 30-day and 30-day to five-year incidences of all-cause death were all significantly higher in the CTO group than in the non-CTO group (37.0% versus 22.0%, log-rank p<0.0001, 12.8% versus 6.3%, log-rank p<0.0001, and 28.2% versus 16.8%, log-rank p<0.0001, respectively). The adjusted risk for all-cause death in the CTO group was significantly higher during the entire five years, during the initial 30 days, and beyond 30 days and up to five years (hazard ratio [HR]: 1.47, 95% confidence interval [CI]: 1.18-1.84, p=0.0009; HR: 1.49, 95% CI: 1.04-2.13, p=0.03; and HR: 1.61, 95% CI: 1.23-2.07, p=0.0006, respectively).
Conclusions: CTO in a non-IRA was associated with increased five-year mortality in STEMI patients with MVD. This was consistently seen even after excluding early deaths within 30 days of the index STEMI event.
Abbreviations
AMI: acute myocardial infarction
CABG: coronary artery bypass grafting
CI: confidence interval
CTO: chronic total occlusion
HR: hazard ratio
MI: myocardial infarction
MVD: multivessel disease
PCI: percutaneous coronary intervention
RCT: randomised controlled trial
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Although primary percutaneous coronary intervention (PCI) is currently established as the first-line treatment of acute ST-elevation myocardial infarction (STEMI), there are still some unanswered questions such as the management of non-infarct-related artery (non-IRA) lesions. Several studies have already reported the presence of concurrent chronic total occlusion (CTO) in non-IRA as being an independent risk factor for early and late mortality in STEMI patients with multivessel disease1-3. However, these studies were confounded by several limitations such as a relatively small sample size, an insufficiently long follow-up period, and lack of information about the effect of PCI for CTO in non-IRA. In an attempt to overcome these limitations, we sought to investigate the clinical impact of CTO in non-IRA on long-term cardiovascular outcomes using detailed information from a large-scale observational database of AMI patients undergoing primary PCI in Japan.
Methods
STUDY POPULATION
The Coronary Revascularization Demonstrating Outcome study in Kyoto (CREDO-Kyoto) AMI registry is a physician-initiated, non-company sponsored, multicentre registry enrolling consecutive AMI patients undergoing coronary revascularisation within seven days of symptom onset among 26 centres in Japan between January 2005 and December 2007 (Appendix 1). The relevant review boards or ethics committees in all participating centres approved the research protocol. Because of retrospective enrolment, written informed consent from the patients was waived; however, we excluded those patients who refused to participate in the study when contacted at follow-up. This strategy is concordant with the guidelines for epidemiological studies issued by the Ministry of Health, Labor and Welfare of Japan.
Among 5,429 AMI patients enrolled in this registry, the current study population consisted of 2,045 STEMI patients with multivessel disease (MVD) who underwent primary PCI within 24 hours after symptom onset (Figure 1). The patients were classified into two groups according to the presence of CTO in a non-IRA (CTO group: 383 patients [18.7%], and non-CTO group: 1,662 patients [81.3%]).
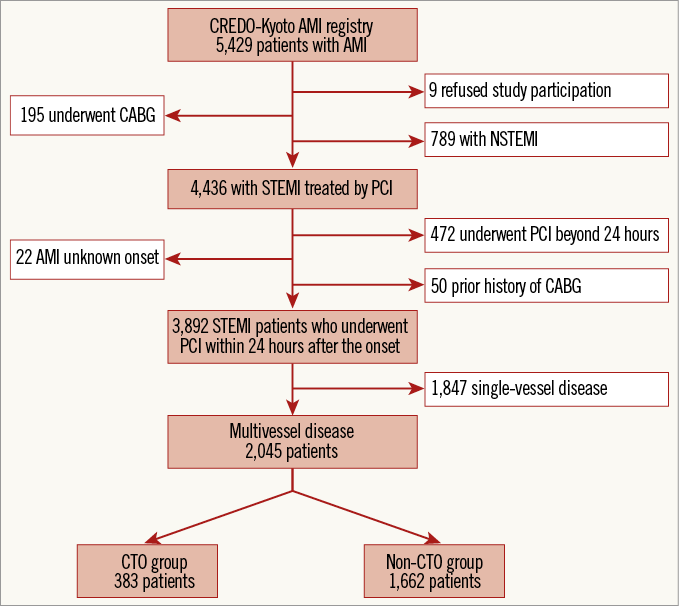
Figure 1. Study flow chart. CABG: coronary artery bypass grafting; CREDO-Kyoto AMI registry: Coronary Revascularization Demonstrating Outcome Study in Kyoto Acute Myocardial Infarction registry; CTO: chronic total occlusion; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: primary coronary intervention; STEMI: ST-segment elevation myocardial infarction
DEFINITIONS AND ENDPOINTS
Definitions of baseline clinical characteristics were previously described4. The initial perfusion status of the infarct-related artery was evaluated according to the Thrombolysis In Myocardial Infarction (TIMI) study classification. TIMI 0 referred to the absence of any antegrade flow beyond a coronary occlusion. CTO was defined as complete obstruction of the vessel with TIMI flow of 0 or 1 with an estimated duration of the occlusion >1 month or presence of collateral flow. The duration of occlusion was judged by the investigators in each participating centre on the basis of the interval from the last episode of MI in the target vessel territory, the previous coronary angiography, or changes in electrocardiographic findings. Multivessel disease was defined as the presence of at least one lesion with % diameter stenosis ≥50% by visual estimation in at least one major non-infarct-related epicardial coronary artery or left main coronary artery. The primary outcome measure for the current analysis was all-cause death. Secondary outcome measures included cardiac death, non-cardiac death, myocardial infarction (MI), heart failure hospitalisation, stroke, stent thrombosis (ST), any coronary revascularisation, and CABG. Death was regarded as cardiac in origin unless obvious non-cardiac causes could be identified. MI was defined according to the definition in the Arterial Revascularization Therapy Study5. ST was defined according to the Academic Research Consortium (ARC) definition6. Any coronary revascularisation was defined as either PCI or CABG for any reason.
DATA COLLECTION FOR BASELINE CHARACTERISTICS AND FOLLOW-UP EVENTS
Demographic, angiographic, and procedural data were collected from hospital charts or hospital databases according to the pre-specified definitions by experienced clinical research coordinators from the study management centre (Research Institute for Production Development, Kyoto, Japan) (Appendix 2). In this retrospective cohort study, data collection for follow-up events was performed in 2010 and 2012. Collection of follow-up information was mainly conducted through review of in-patient and out-patient hospital charts by the clinical research coordinators, and additional follow-up information was collected through contact with patients, relatives and/or referring physicians by sending mail with questions regarding vital status, subsequent hospitalisations, and status of antiplatelet therapy. Death, MI, ST, and stroke were adjudicated by the clinical events committee (Appendix 3). Median follow-up duration was 1,839 (interquartile range [IQR]: 1,494-2,156) days.
STATISTICAL ANALYSIS
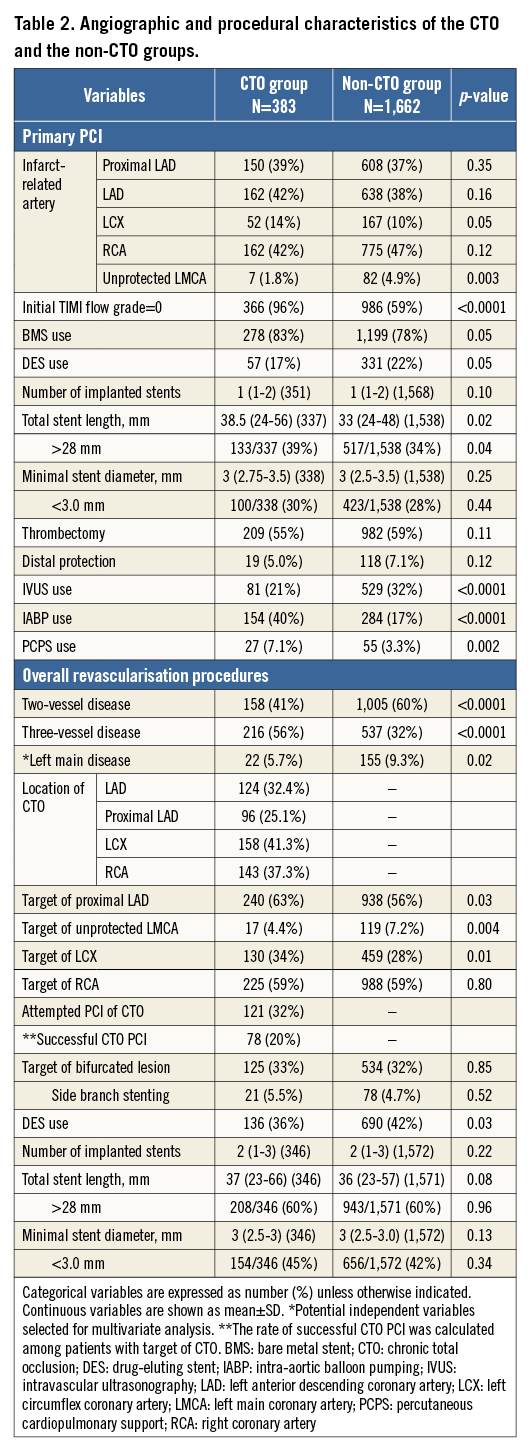
We expressed categorical variables as numbers and percentages, and continuous variables as mean±standard deviation when they followed a normal distribution or as median (interquartile range) when they did not. The distribution appearance was judged by illustrating the listed variables in a histogram. If it was a bell-shaped appearance, the variables were judged to follow a normal distribution. We compared categorical variables with the χ2 test when suitable; otherwise, we used Fisher’s exact test. We compared continuous variables with the Student’s t-test or the Wilcoxon rank-sum test based on their distributions. We performed a landmark analysis at 30 days and compared clinical outcomes in each group with early events excluded. We used the Kaplan-Meier method to estimate cumulative incidences of clinical events and evaluated the differences with the log-rank test. Consistent with our previous reports, we used a multivariable Cox proportional hazards model stratified by the participating centres to estimate the impact of CTO in non-IRA on the entire five-year, 30-day and 30-day to five-year all-cause deaths in the two groups by incorporating the presence of CTO in non-IRA together with 15 clinically relevant risk-adjusting variables (Table 1, Table 2). Proportional hazard assumptions for potential independent risk-adjusting variables were assessed on the plots of log (time) versus log (-log[survival]) stratified by the variable. It was confirmed that the assumptions were acceptable for all the variables. We calculated adjusted hazard ratios (HR) and their 95% confidence intervals (CI). In addition, we computed the adjusted event curves of the two groups using the methods described by Ghali et al7. Multivariable adjustment could not be performed for several endpoints due to the small number of events observed. As in our previous reports, we dichotomised some continuous variables by using clinically significant reference values or median values. Statistical analyses were performed by a physician (H. Watanabe) and a statistician (T. Morimoto) with the use of JMP 10.0 (SAS Institute Inc., Cary, NC, USA) software. All the statistical analyses were two-tailed. P-values <0.05 were considered statistically significant.
Results
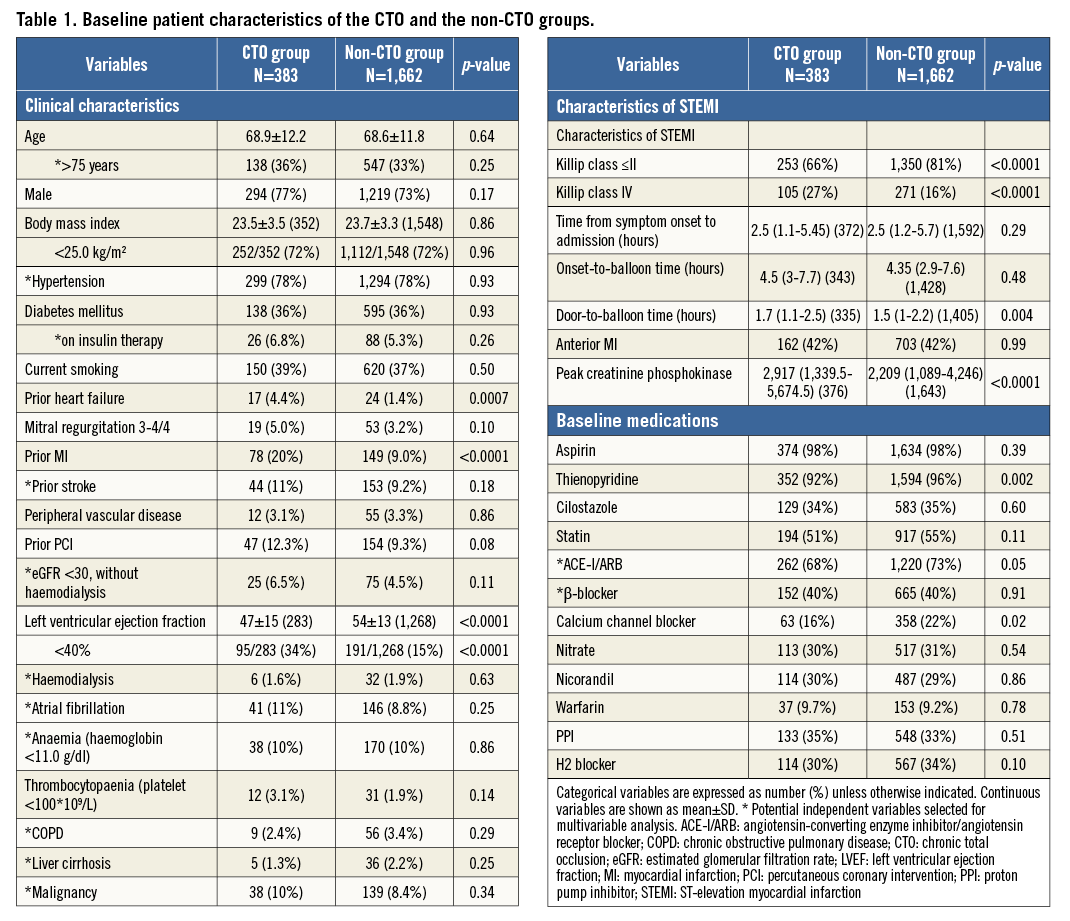
The baseline characteristics were significantly different between the CTO and non-CTO groups in a few important respects (Table 1). On the other hand, the baseline characteristics were not very different between the successful CTO-PCI and the failed CTO-PCI groups (Appendix Table 1, Appendix Table 2).
Throughout the entire five-year follow-up, the cumulative incidence of all-cause death was significantly higher in the CTO group than in the non-CTO group (37.0% versus 22.0%, log-rank p<0.0001). After adjusting for confounders, the excess risk of the CTO group relative to the non-CTO group for all-cause death during the entire five-year follow-up remained significant (HR: 1.47, 95% CI: 1.18-1.84, p=0.0009) (Table 3, Figure 2). During the initial 30 days, the cumulative incidence of all-cause death was significantly higher in the CTO group (12.8% versus 6.3%, log-rank p<0.0001). The adjusted risk of the CTO group relative to the non-CTO group for all-cause death also remained significant (HR: 1.49, 95% CI: 1.04-2.13, p=0.03) (Table 3, Figure 3). By the landmark analysis at 30 days, the cumulative incidence of and the adjusted risk for all-cause death beyond 30 days and up to five years were significantly higher in the CTO group than in the non-CTO group (28.2% versus 16.8%, log-rank p<0.0001, and HR: 1.61, 95% CI: 1.23-2.07, p=0.0006, respectively) (Table 3, Figure 3). The cumulative five-year incidence of and the adjusted risk for cardiac death, heart failure hospitalisation, any coronary revascularisation, and CABG were also significantly higher in the CTO group than in the non-CTO group (Table 3).
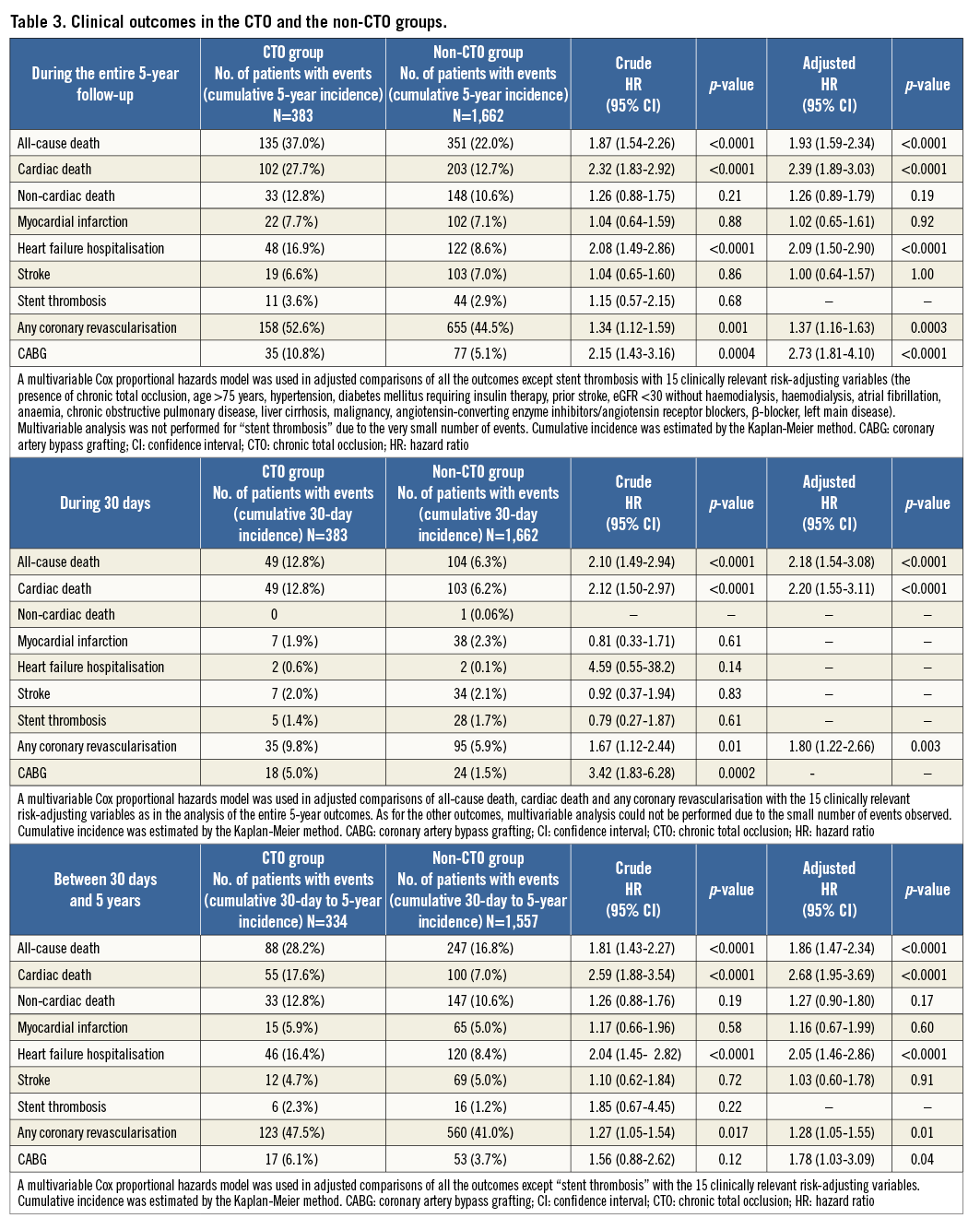
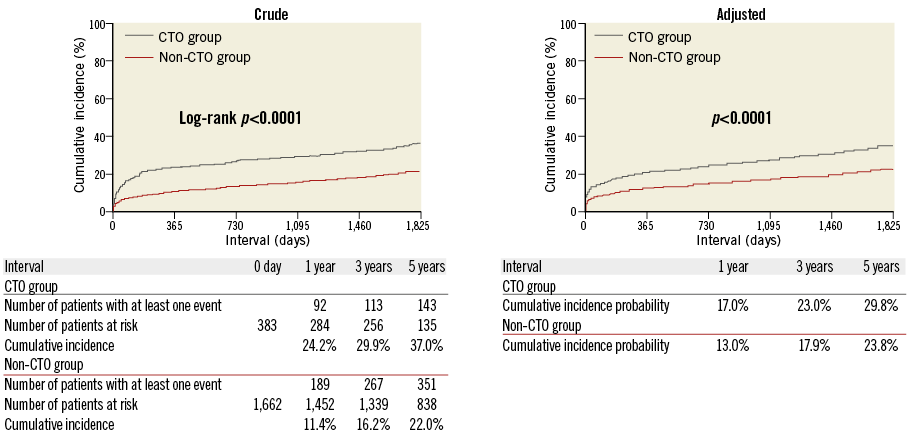
Figure 2. Crude and adjusted Kaplan-Meier curves for the cumulative incidence of all-cause death in the CTO and the non-CTO groups during the entire five-year follow-up. CTO: chronic total occlusion
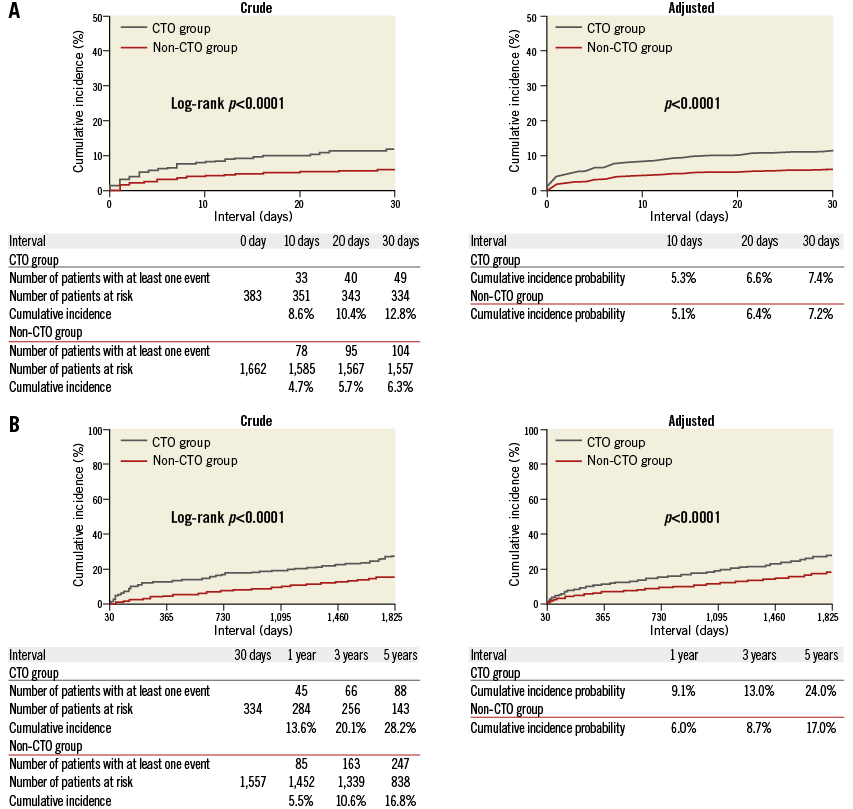
Figure 3. Crude and adjusted Kaplan-Meier curves for the cumulative incidence of all-cause death in the CTO and the non-CTO groups during the initial 30 days (A), and beyond 30 days and up to five years (B). CTO: chronic total occlusion
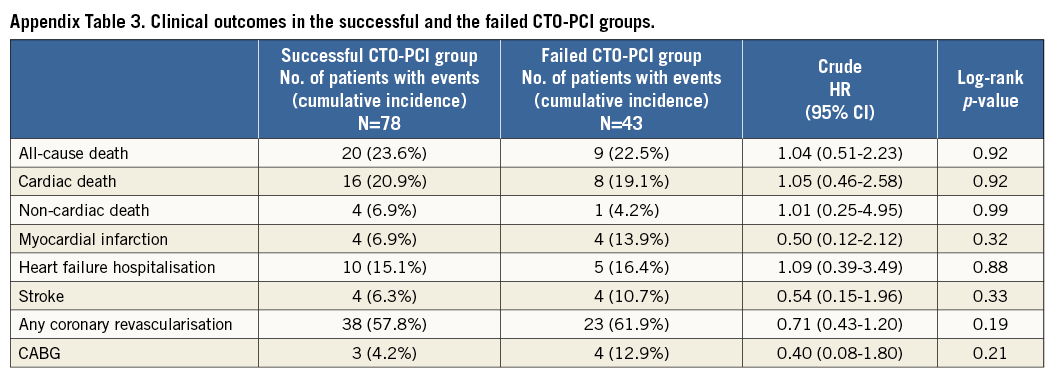
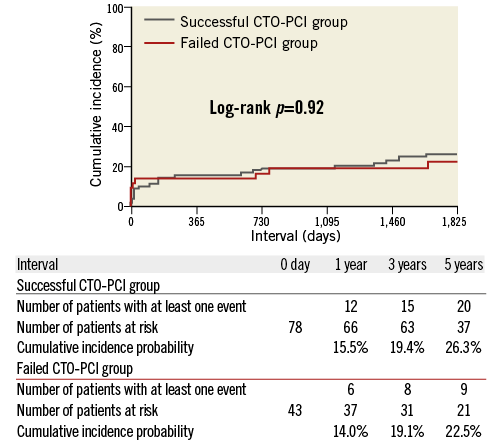
As for CTO-PCI groups, the cumulative five-year incidence of all-cause death was not significantly different between the successful CTO-PCI and the failed CTO-PCI groups (23.6% versus 22.5%, log-rank p=0.92) (Appendix Table 3, Appendix Figure 1).
Discussion
The main findings in the current analysis were as follows. 1) CTO in the non-IRA was independently associated with increased risk for mortality in STEMI patients with multivessel disease. 2) The independent increased mortality risk of CTO in the non-IRA was mainly observed beyond 30 days after the index STEMI event. 3) The five-year cumulative incidence of all-cause death was not significantly different between the successful CTO-PCI and the failed CTO-PCI groups.
The current analysis clearly demonstrated that the long-term mortality in STEMI patients with multivessel disease was higher in the presence of CTO in the non-IRA. Regrettably, this study could not provide the exact mechanisms of mortality deterioration by CTO, but some reasons might be inferred from several pieces of evidence. First of all, as many previous studies have suggested, STEMI patients with CTO had a higher prevalence of cardiovascular comorbidities such as prior MI, heart failure, and stroke, and more often presented in shock on admission. Therefore, the adjusted increased mortality of patients with CTO during the initial 30 days could mainly be explained by more unstable haemodynamic characteristics such as cardiogenic shock at the time of the index STEMI event. Hoebers et al reported that multivessel disease with CTO in a non-IRA was an independent risk factor for short-term mortality in STEMI patients3. However, the present multivariable analysis indicated an association of CTO in a non-IRA with increased long-term mortality beyond 30 days. One of the possible mechanisms of increased long-term mortality beyond 30 days in patients with CTO could be a larger infarct size due to the acute occlusion of a donor artery to CTO. The decreased flow of the donor artery might result in myocardial injury and necrosis in the myocardial area in which the myocardial perfusion was dependent on the collateral flow from the infarct-related artery. Lexis et al showed that the size of enzymatic infarct was significantly larger in the CTO group than in the non-CTO group8. In the present study, the peak creatinine phosphokinase value was higher in the CTO group than in the non-CTO group. As another explanation, a previous study by Claessen et al demonstrated that the presence of a CTO was associated with reduced residual left ventricular ejection fraction (LVEF) and with a further deterioration of LVEF during follow-up1. The CTO group included more patients with LVEF <40% in the present analysis, probably because patients with CTO had silent MI.
Indeed, it is challenging to discern whether CTO in a non-IRA is only a result of multiple cardiovascular comorbidities or whether it exacerbates mortality in STEMI patients, but it might potentially be a modifiable factor in the improvement of mortality. In the current analysis, the five-year cumulative incidence of all-cause death was not significantly different between the successful CTO-PCI and the failed CTO-PCI groups. However, the prevalence of CTO PCI was only around 32% and the success rate was 65%, both of which were relatively low in terms of current CTO PCI. Also, multivariable analysis could not be performed due to the small number of events in the analysis of successful CTO PCI. The robust assessment of the efficacy of successful non-IRA CTO revascularisation is beyond the scope of this study. As discussed previously, the next focus is whether or not the high rate of successful revascularisation of non-IRA CTO ameliorates mortality of STEMI patients. The way of dealing with non-IRA lesions in STEMI patients remains a target of controversy due to a paucity of randomised data and conflicting results in several observational studies. The EXPLORE trial, which randomised 304 STEMI patients to early CTO PCI versus conservative treatment without CTO PCI, assessed the impact of CTO PCI on LVEF and left ventricular end-diastolic volume (LVEDV) at four-month follow-up. The result was that staged PCI of non-IRA CTO within a week of primary PCI was not associated with improvement of LVEF or LVEDV at four-month follow-up9. A subgroup analysis suggested a clinical benefit in patients with LAD CTO, which should be investigated in further studies. Limitations in this trial included a lower success rate of CTO PCI and insufficient sample size for evaluation of clinical endpoints.
Concerning the treatment of non-culprit lesions in STEMI patients, two RCTs have suggested the clinical efficacy of preventive PCI of non-culprit lesions in STEMI patients10,11. PCI of non-IRA CTO assumes a critical role in the treatment of multivessel disease because the mastery of CTO PCI is imperative for complete revascularisation. The Complete Versus culprit-Lesion only PRimary PCI Trial (CvLPRIT) showed a significant reduction in the primary endpoint of mortality, recurrent MI, heart failure, or ischaemia-driven revascularisation at 12 months (10.0% vs. 21.2%; HR: 0.45; p=0.009)11. In these two RCTs, CTO in a non-IRA was part of the exclusion criteria. Thus, further prospective randomised trials concerning revascularisation of non-IRA lesions including CTO in STEMI patients are warranted to elucidate this unsolved question.
Limitations
The current study has several potential limitations. First, and most importantly, this is a retrospective observational, but not a prospective randomised study. As differences in baseline patient, angiographic and procedural characteristics between the groups exist, unmeasured confounders cannot be excluded despite robust multivariable analysis. The decision of PCI for non-IRA CTO or non-IRA non-CTO lesions was at the discretion of the operator. Onset-to-balloon time and/or door-to-balloon time were among the strongest factors that could influence mortality in AMI patients. We did not include these factors as adjusting variables in the Cox hazard models because of missing values. We should take into consideration the small but significant difference in door-to-balloon time (but not in onset-to-balloon time) between the two groups in interpreting the present study results. Secondly, we chose 15 clinically relevant adjusting variables according to the number of events for all-cause death during the initial 30 days in the multivariable analysis. Therefore, the models were inevitably overfitted with the 15 adjusters in several endpoints such as myocardial infarction, stroke and CABG. The results for these endpoints should be interpreted cautiously, although the rule of thumb of a minimum of 10 outcome events per predictor variable is not an absolute requirement and can sometimes be relaxed12. Finally, this study did not assess detailed angiographic or electrocardiographic findings, such as myocardial blush grade or ST-segment resolution.
Conclusions
CTO in a non-IRA was associated with increased five-year mortality in STEMI patients with MVD. This was consistently seen even after excluding early deaths within 30 days of the index STEMI event.
| Impact on daily practice As with previous studies, our robust analysis in a large cohort demonstrated that CTO in a non-IRA was independently associated with long-term clinical cardiovascular outcomes in STEMI patients with MVD. The assessment of the effect of successful non-IRA CTO PCI was also shown, though this involved many limitations such as small sample size and the lower patient-level CTO success rate. Whether more successful revascularisation of CTO in a non-IRA improves clinical outcomes is the next target of an investigation. |
Acknowledgements
We appreciate the collaboration of the co-investigators in the CREDO-Kyoto PCI/CABG Registry Cohort-2.
Funding
This study was supported by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
Conflict of interest statement
The authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix 1. List of participating centres and investigators for the CREDO-Kyoto PCI/CABG Registry Cohort-2
CARDIOLOGY
Kyoto University Hospital: Takeshi Kimura; Kishiwada City Hospital: Mitsuo Matsuda, Hirokazu Mitsuoka; Tenri Hospital: Yoshihisa Nakagawa; Hyogo Prefectural Amagasaki Hospital: Hisayoshi Fujiwara, Yoshiki Takatsu, Ryoji Taniguchi; Kitano Hospital: Ryuji Nohara; Koto Memorial Hospital: Tomoyuki Murakami, Teruki Takeda; Kokura Memorial Hospital: Masakiyo Nobuyoshi, Masashi Iwabuchi; Maizuru Kyosai Hospital: Ryozo Tatami; Nara Hospital, Kinki University Faculty of Medicine: Manabu Shirotani; Kobe City Medical Center General Hospital: Toru Kita, Yutaka Furukawa, Natsuhiko Ehara; Nishi-Kobe Medical Center: Hiroshi Kato, Hiroshi Eizawa; Kansai Denryoku Hospital: Katsuhisa Ishii; Osaka Red Cross Hospital: Masaru Tanaka; University of Fukui Hospital: Jong-Dae Lee, Akira Nakano; Shizuoka City Shizuoka Hospital: Akinori Takizawa; Hamamatsu Rosai Hospital: Masaaki Takahashi; Shiga University of Medical Science Hospital: Minoru Horie, Hiroyuki Takashima; Japanese Red Cross Wakayama Medical Center: Takashi Tamura; Shimabara Hospital: Mamoru Takahashi; Kagoshima University Medical and Dental Hospital: Chuwa Tei, Shuichi Hamasaki; Shizuoka General Hospital: Hirofumi Kambara, Osamu Doi, Satoshi Kaburagi; Kurashiki Central Hospital: Kazuaki Mitsudo, Kazushige Kadota; Mitsubishi Kyoto Hospital: Shinji Miki, Tetsu Mizoguchi; Kumamoto University Hospital: Hisao Ogawa, Seigo Sugiyama; Shimada Municipal Hospital: Ryuichi Hattori, Takeshi Aoyama, Makoto Araki; Juntendo University Shizuoka Hospital: Satoru Suwa.
CARDIOVASCULAR SURGERY
Kyoto University Hospital: Ryuzo Sakata, Tadashi Ikeda, Akira Marui; Kishiwada City Hospital: Masahiko Onoe; Tenri Hospital: Kazuo Yamanaka; Hyogo Prefectural Amagasaki Hospital: Keiichi Fujiwara, Nobuhisa Ohno; Kokura Memorial Hospital: Michiya Hanyu; Maizuru Kyosai Hospital: Tsutomu Matsushita; Nara Hospital, Kinki University Faculty of Medicine: Noboru Nishiwaki, Yuichi Yoshida; Kobe City Medical Center General Hospital: Yukikatsu Okada, Michihiro Nasu; Osaka Red Cross Hospital: Shogo Nakayama; University of Fukui Hospital: Kuniyoshi Tanaka, Takaaki Koshiji, Koichi Morioka; Shizuoka City Shizuoka Hospital: Mitsuomi Shimamoto, Fumio Yamazaki; Hamamatsu Rosai Hospital: Junichiro Nishizawa; Japanese Red Cross Wakayama Medical Center: Masaki Aota; Shimabara Hospital: Takafumi Tabata; Kagoshima University Medical and Dental Hospital: Yutaka Imoto, Hiroyuki Yamamoto; Shizuoka General Hospital: Katsuhiko Matsuda, Masafumi Nara; Kurashiki Central Hospital: Tatsuhiko Komiya; Mitsubishi Kyoto Hospital: Hiroyuki Nakajima; Kumamoto University Hospital: Michio Kawasuji, Syuji Moriyama; Juntendo University Shizuoka Hospital: Keiichi Tanbara.
Appendix 2. List of clinical research coordinators
RESEARCH INSTITUTE FOR PRODUCTION DEVELOPMENT
Kumiko Kitagawa, Misato Yamauchi, Naoko Okamoto, Yumika Fujino, Saori Tezuka, Asuka Saeki, Miya Hanazawa, Yuki Sato, Chikako Hibi, Hitomi Sasae, Emi Takinami, Yuriko Uchida, Yuko Yamamoto, Satoko Nishida, Mai Yoshimoto, Sachiko Maeda, Izumi Miki, Saeko Minematsu.
Appendix 3. List of clinical events committee members
Mitsuru Abe (Kyoto Medical Center), Hiroki Shiomi (Kyoto University Hospital), Tomohisa Tada (Kyoto University Hospital), Junichi Tazaki (Kyoto University Hospital), Yoshihiro Kato (Kyoto University Hospital), Mamoru Hayano (Kyoto University Hospital), Akihiro Tokushige (Kyoto University Hospital), Masahiro Natsuaki (Kyoto University Hospital), Tetsu Nakajima (Kyoto University Hospital).
Appendix Figure 1. A crude Kaplan-Meier curve for the cumulative incidence of all-cause death in the successful and the failed CTO-PCI groups during the entire five-year follow-up.

