
Abstract
Aims: Leaflet thrombosis (LT) has become increasingly recognised following transcatheter and surgical aortic bioprosthetic valve (ABV) replacement and can be reliably identified by multidetector computed tomography (MDCT). However, it is an ongoing debate whether MDCT-defined LT is associated with adverse cerebrovascular outcomes. We sought to perform a systematic review and meta-analysis in order to assess the incidence and clinical outcomes associated with MDCT-defined leaflet thrombosis following (ABV) replacement.
Methods and results: Electronic databases were searched for studies that performed mandatory MDCT imaging following ABV replacement. The primary endpoint was the incidence of cerebrovascular events, defined as a composite of stroke or transient ischaemic attack (TIA). Secondary endpoints included major adverse cerebrovascular and cardiovascular events (MACCE), stroke, TIA, death or myocardial infarction. In total, six studies met the inclusion criteria with 11.6% (198/1,704) of patients having MDCT-defined LT. The prevalence of LT following transcatheter and surgical ABV replacement was 13.2% and 3.6%, respectively. Cerebrovascular events were significantly increased in patients with LT (odds ratio [OR] 3.38, 95% CI: 1.78-6.41, p<0.001). The risk of MACCE (OR 2.10, 95% CI: 1.21-3.64, p<0.001) and TIA (OR 5.86, 95% CI: 2.05-16.75, p<0.001) was also increased in patients with LT, although there were no differences in the incidence of stroke (OR 2.43, 95% CI: 1.00-5.93, p=0.05), death (OR 0.92, 95% CI: 0.42-2.03, p=0.84) or myocardial infarction (OR 1.72, 95% CI: 0.34-9.78, p=0.54) between groups.
Conclusions: MDCT-defined LT following ABV replacement is associated with a significantly increased risk of adverse cerebrovascular events. Further prospective studies are required to ascertain whether LT can be prevented or treated with pharmacological strategies.
Abbreviations
ABV: aortic bioprosthetic valve
HALT: hypoattenuated leaflet thickening
LT: leaflet thrombosis
MACCE: major adverse cardiovascular and cerebrovascular events
MDCT: multidetector computed tomography
MI: myocardial infarction
OR: odds ratio
RELM: reduced leaflet motion
SAVR: surgical aortic valve replacement
TAVR: transcatheter aortic valve replacement
TIA: transient ischaemic attack
VARC-2: Valve Academic Research Consortium-2
Introduction
Aortic stenosis is the most prevalent form of valvular heart disease in developed countries and is estimated to be at a severity to require intervention in ~3% of patients aged >75 years1. Surgical aortic valve replacement (SAVR) remains an established therapeutic option for the majority of patients with symptomatic severe aortic stenosis, with transcatheter aortic valve replacement (TAVR) currently reserved for selected patients at intermediate and high surgical risk2,3. Transcatheter valves implanted have leaflets exclusively manufactured from biological material, while the use of aortic bioprosthetic valves (ABV) in surgical procedures is increasing and is often favoured in older patients with medical comorbidities4.
Leaflet thrombosis (LT) has been well documented in metallic heart valves, but is becoming increasingly recognised in surgical and transcatheter ABV. Although transoesophageal echocardiography is considered the gold standard for the evaluation of leaflet structure following ABV replacement, it is limited by its invasive nature and variability in image interpretation. Multidetector computed tomography (MDCT) has emerged as an alternative non-invasive imaging modality that allows assessment of post-procedure ABV geometry and leaflet function. Hallmarks of LT on MDCT include hypoattenuated leaflet thickening and/or reduced leaflet motion. Not all patients with MDCT-defined LT are symptomatic; recent observational studies have highlighted that subclinical LT may be relatively common5. There are also concerns that LT may be associated with an increased risk of adverse cerebrovascular events due to embolisation of thrombotic debris from the valve, although trial data are conflicting. We therefore sought to perform a systematic review and meta-analysis to determine both the prevalence of LT detected by MDCT following ABV replacement and the association between MDCT-defined LT and adverse cerebrovascular events.
Methods
DATA SOURCES AND SEARCH STRATEGY
An electronic literature review was performed in PubMed, MEDLINE and EMBASE databases covering the period from January 2000 until May 2017. There were no additional limits set to the search strategy. Keywords using medical subject heading (MeSH), where available, included “aortic valve”, “transcatheter aortic valve replacement”, “transcatheter aortic valve implantation”, “surgical aortic valve”, “bioprosthetic aortic valve”, “aortic valve stenosis”, “thrombosis”, “hypoattenuated leaflet thickening”, “valve thrombosis” and “leaflet thrombosis”. The study protocol was prospectively registered with PROSPERO (CRD42017069536) and adhered to PRISMA guidelines. An example search strategy for MEDLINE is provided in Supplementary Table 1.
STUDY SELECTION
Study criteria for inclusion were as follows: 1) reporting of subclinical or clinical LT in patients receiving a bioprosthetic aortic valve (BPAV), 2) MDCT performed on patients following valve implantation, 3) comparison of clinical outcomes between LT and non-LT patients, and 4) fully published status. Studies were excluded if they included patients receiving mechanical aortic valve replacement or if the diagnosis of LT was made on alternative imaging modalities, including echocardiography. Where multiple studies reported on the same cohort of patients, we prioritised the most recent articles with the largest cohort size. Study characteristics are provided in Supplementary Table 2. In this study, study-specific definitions of LT were used that encompassed either the presence of hypoattenuated leaflet thickening (HALT) or the reduction of leaflet motion (RELM). In four studies, LT was characterised by HALT, which was defined as the presence of hypoattenuation of at least one leaflet on MDCT6-9. Two studies characterised LT as RELM, described as at least moderate leaflet opening (≥50% systolic-phase maximal) on four-dimensional volume-rendered MDCT valve imaging5,10. Full details of the definitions of LT used within each study are presented in Supplementary Table 3.
DATA ITEMS AND COLLECTION PROCESS
Data items to be collected were specified before initiating database searches. Two investigators (H.N. Rashid and A.R. Ihdayhid) independently conducted the search and, later, data extraction for baseline patient demographics, type of BPAV implanted and clinical outcomes. Extracted data were verified by the senior author (A.J. Brown) and any discrepancies resolved by consensus agreement. Within each individual article, the risk of bias was assessed according to the Cochrane Collaboration Assessment (Supplementary Table 4).
CLINICAL ENDPOINTS
The primary endpoint for this study was the occurrence of any cerebrovascular event, defined as either stroke or transient ischaemic attack (TIA). Secondary outcomes included major adverse cardiovascular and cerebrovascular events (MACCE), which were defined as a composite of all-cause death, stroke, TIA and myocardial infarction (MI), as well as individual components of MACCE. Within studies, stroke was defined as either the loss of neurological function >24 hours or within 24 hours with neuroimaging evidence of an ischaemic or haemorrhagic event8, or following clinical assessment by a neurologist5,9,10. TIA was defined either as a transient neurological dysfunction <24 hours without neuroimaging evidence of a stroke8 or as diagnosed clinically by a neurologist5,9,10. Two studies did not define stroke or TIA but reported their outcomes6,7. MI was defined either 1) as elevation of cardiac biomarkers with ischaemic clinical symptoms or electrocardiogram changes8, or 2) as adjudicated after independent review by a clinical events committee5,10. Full details of individual clinical endpoints and definitions are presented in Supplementary Table 5.
STATISTICAL ANALYSIS
Outcomes were analysed using a random effects model and summary estimates reported as pooled odds ratios (OR) with 95% confidence intervals (CI) for the primary outcome of cerebrovascular events. Subgroup analysis was performed to evaluate summary statistics for various composites of MACCE (ischaemic cerebrovascular accident [CVA], TIA, death and MI). Sensitivity analysis was performed to assess pooled risk estimates of HALT vs. RELM as well as SAVR vs. TAVR. Statistical heterogeneity was quantified with the I2 statistic. Heterogeneity was quantified as low, moderate or high based on I2 values of 25%, 50% and 75%, respectively11. Publication bias was assessed by the Harbord test12. A two-sided p-value of <0.05 was considered significant. Statistical analysis was performed with Stata/MP 14.0 (StataCorp LP, College Station, TX, USA) and the metan suite of commands.
Results
SEARCH RESULTS
A total of 1,897 citations were reviewed and screened, with 24 studies identified for potential inclusion and further evaluation. Of these, 18 studies were excluded as either they included patients with aortic mechanical valve prostheses (six studies) or mandatory MDCT was not performed (three studies). Other reasons for study exclusion can be found in the PRISMA flow diagram provided in Figure 1.
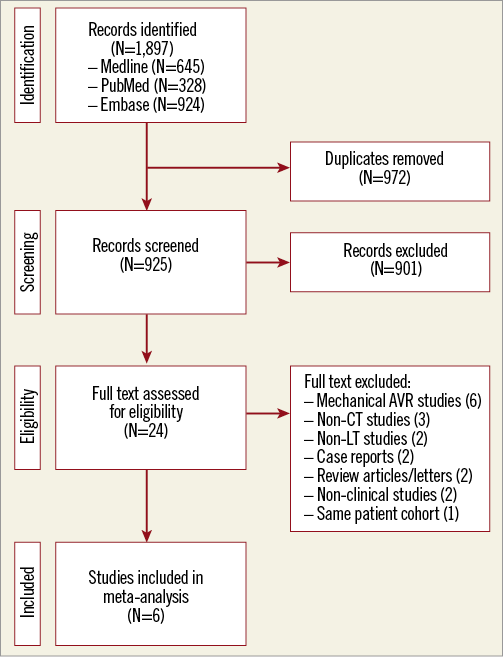
Figure 1. Study flow chart. Flow diagram illustrating the study selection process for the systematic review and meta-analysis. CT: computed tomography; LT: leaflet thrombosis
Six papers met the predefined inclusion criteria and were included in the final quantitative analysis. Eligible studies included combined data from the Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation (RESOLVE) and the Subclinical Aortic Valve Bioprosthesis Thrombosis Assessed with Four-Dimensional Computed Tomography (SAVORY) registries. Four other studies were single-centre registries, while one study reported specific outcome data from the PORTICO IDE trial.
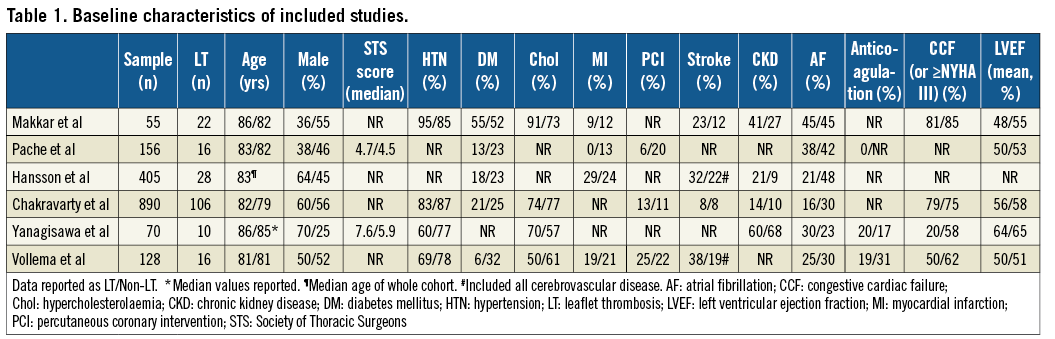
Overall, 1,704 patients were included in the analysis with 1,566 patients receiving a transcatheter and 138 receiving a surgical ABV. The prevalence of atrial fibrillation ranged from 28% to 45%, with between 8-23% of patients having a history of prior cerebrovascular disease. Full details of the clinical demographics for each study are presented in Table 1.
PREVALENCE OF LT
In total, 198 patients (11.6%) had evidence of LT following MDCT evaluation, with the weighted prevalence of LT in the cohort being 13.1% (95% CI: 8.7-17.5%) (Figure 2). The absolute prevalence of LT in transcatheter ABV was 13.2%, while in surgical ABV it was lower at 3.6%. For transcatheter ABV, the highest prevalence of LT was observed in Portico™ valves (St. Jude Medical, St. Paul, MN, USA) at 35.2% (37/108), but LT was also observed in LOTUS™ (Boston Scientific, Marlborough, MA, USA) at 14.5% (12/83), Edwards SAPIEN systems (Edwards Lifesciences, Irvine, CA, USA) at 10.2% (128/1,352) and Medtronic CoreValve®/Evolut™ R systems (Medtronic, Minneapolis, MN, USA) at 6.2% (9/145). With regard to surgical ABV, the highest prevalence of LT was observed in Magna valves (Edwards Lifesciences) at 10.8% (4/37), and there was one reported case of LT in a Perimount valve (Edwards Lifesciences) at 2.6% (1/39). The time from implantation to initial MDCT ranged between a median of 6 and 58 days (Table 2).
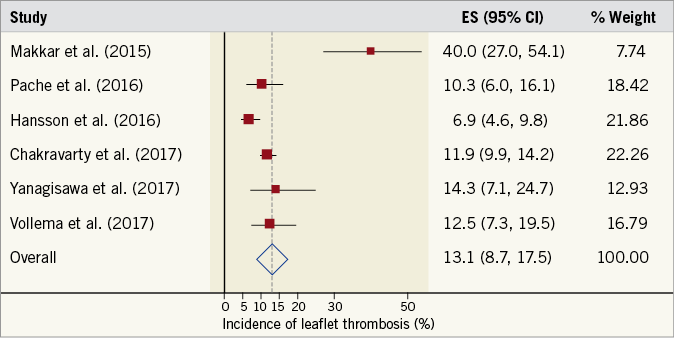
Figure 2. Prevalence of leaflet thrombosis. Forest plot displaying weighted prevalence and 95% confidence intervals (CI) for the occurrence of multidetector computed tomography-defined leaflet thrombosis. ES: effect size
CEREBROVASCULAR EVENTS
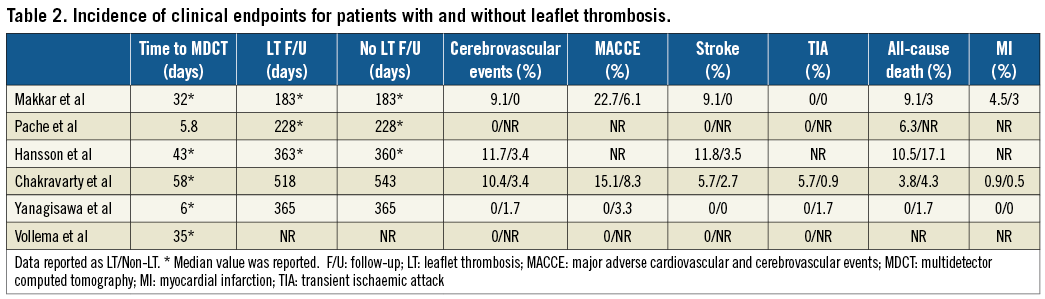
Four studies reported the incidence of cerebrovascular events in patients with and without LT. The summary OR for these studies was 3.38 (95% CI: 1.78-6.41, p<0.001), demonstrating an increased risk of cerebrovascular events in patients with evidence of LT (Figure 3). There was no evidence of statistical heterogeneity between studies (I2=0%, p=0.93). A pre-specified sensitivity analysis, stratifying outcomes by subcategory of LT, demonstrated that RELM was related to higher rates of cerebrovascular events (OR 3.41, 95% CI: 1.67-6.96, I2=0%, p<0.001) when compared with HALT (OR 3.23, 95% CI: 0.75-13.95, I2=0%, p=0.12) (p for interaction=0.95) (Supplementary Figure 1).
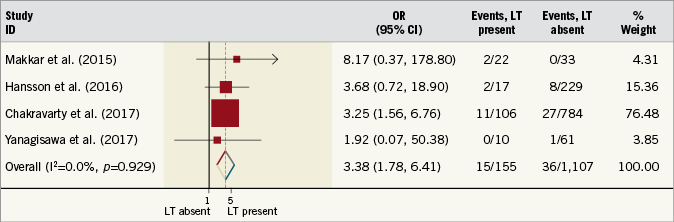
Figure 3. Risk estimates for cerebrovascular events. Forest plot displaying summary odds ratio (OR) and 95% confidence intervals (CI). LT: leaflet thrombosis
SECONDARY CLINICAL ENDPOINTS
Three trials reported the incidence of MACCE, with the same trials also reporting individual incidences of stroke, TIA and MI. The incidence of MACCE was increased in patients with LT (OR 2.10, 95% CI: 1.21-3.64, I2=0%, p<0.001) (Figure 4). Similar findings were also found for the incidence of TIA (OR 5.86, 95% CI: 2.05-16.75, I2=0%, p<0.001), although the incidence of stroke was not different between patient cohorts (OR 2.43, 95% CI: 1.00-5.93, I2=0%, p=0.05) (Figure 5). The incidence of MI was not significantly different between patients with or without LT (OR 1.72, 95% CI: 0.34-9.78, I2=0%, p=0.54). Four studies reported the incidence of all-cause death. Again, this was not significantly different between patient cohorts (OR 0.92, 95% CI: 0.42-2.03, I2=0%, p=0.84). Full details of the incidence of clinical endpoints for each study are presented in Table 2.
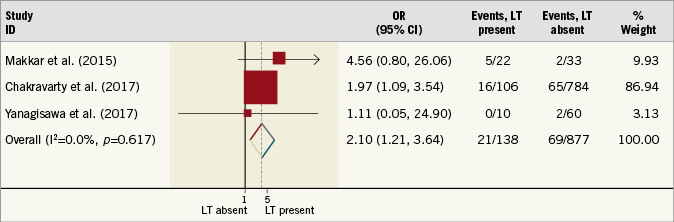
Figure 4. Risk estimates for major adverse cardiovascular and cerebrovascular events. Forest plot displaying summary odds ratio (OR) and 95% confidence intervals (CI). LT: leaflet thrombosis
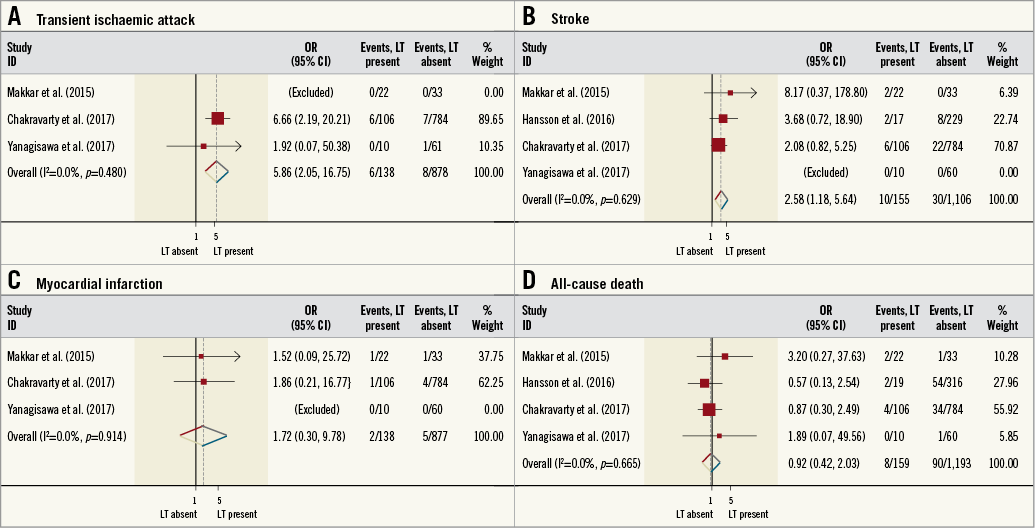
Figure 5. Risk estimates for individual clinical endpoints. Forest plot displaying summary estimates for transient ischaemic attack (A), stroke (B), myocardial infarction (C), and all-cause death (D). LT: leaflet thrombosis
PUBLICATION BIAS
The potential for publication bias was assessed statistically by the Harbord test, which demonstrated no evidence of small study effects (p=0.75).
Discussion
To our knowledge, this is the first meta-analysis investigating whether MDCT-defined LT following ABV replacement is associated with adverse clinical events. Our major finding is that the incidence of adverse cerebrovascular events is increased in patients with ABV who have MDCT evidence of LT, which was primarily driven by increased TIA events in patients with LT. Although the absolute number of strokes was increased in patients with LT, this did not reach statistical significance. We also found that the pooled incidence of MDCT-defined LT was ~13%, with the presence of LT also associated with increased risk of MACCE and TIA. Finally, there were no differences in the incidence of all-cause death and MI in patients with or without LT. These results suggest that the identification of LT is important and highlight that additional research is now warranted into whether antithrombotic strategies can be optimised to prevent the development of LT.
There is increasing recognition that the consequence of bioprosthetic valve thrombosis may not be an all or nothing event, and instead probably represents a continuum extending from leaflet thickening through to overt valve failure13. Importantly, recent studies have highlighted that many patients with imaging features consistent with early bioprosthetic valve thrombosis are asymptomatic, raising the question as to whether these findings are of clinical significance. Here, we demonstrate that patients with MDCT evidence of LT have significantly higher rates of cerebrovascular events, mainly driven by an increased risk of TIA. The pathological mechanism underlying these observations is yet to be determined, but could be explained by systemic embolisation of thrombotic material from the valve leaflets14. Support for this concept is also provided in our data, as we found that valves with RELM have a higher risk of cerebrovascular events when compared with HALT. These findings imply that the risk of cerebrovascular events is proportional to valve thrombotic burden and strategies that prevent LT have significant potential to improve outcomes. We have also shown that the reporting of cerebrovascular events was not standardised, as evident in our cerebrovascular events sub-analysis. One study in the sub-analysis did not provide a definition of stroke7, whilst only two studies had all neurological events evaluated by a neurologist5,10. As such, we strongly encourage future LT studies to incorporate mandatory review by a neurologist in the assessment of neurological events.
In our study we found that LT is relatively common following ABV replacement, occurring in >10% of patients having routine post-procedure MDCT imaging. In addition, the prevalence of LT appears to be higher following TAVR than conventional aortic valve surgery, though only one study reported the incidence of LT in SAVR and therefore conclusions should be drawn with caution. As the likelihood of bioprosthetic valve thrombosis is determined by leaflet/device surface, blood haemostatic and arterial haemodynamic factors, there are a number of plausible mechanisms that could explain our findings15. During TAVR preparation, the valve undergoes structural deformation from crimping to accommodate passage (increasingly) through sheath introducers16-18. Manipulation of the valve in this way therefore has potential to generate stress foci in leaflet collagen fibres inducing surface microdamage and predisposing to thrombus formation. TAVR prostheses are also expanded into position against heavily calcified native aortic valve cusps. Non-uniform valve delivery acts to alter transvalvular arterial flow dynamics and may lead to paravalvular malapposition against the fixed native valve leaflets and any abutting calcified nodules - both potential causes for thrombus activation19. Finally, previous series have shown that around 10-20% of TAVR patients undergo further post-dilatation after valve delivery, potentially precipitating further leaflet injury20,21. Further studies are now required in TAVR into the predictors of LT and whether changes to procedural strategy or device development could lower the risk of LT.
Although anticoagulation, fibrinolysis and repeat valve replacement are valid treatment strategies for patients with symptomatic valve thrombosis, the management of subclinical LT remains ambiguous. Formal anticoagulation with a vitamin-K antagonist would appear to be a logical therapeutic option, providing the bleeding risk is appropriately defined and tailored to individual patients. Support for this concept is provided by observational studies demonstrating lower rates of LT for patients on concurrent anticoagulation when compared with antiplatelet therapies5,10. In addition, valve thrombus load may be reduced and leaflet motion improved by administration of a vitamin-K antagonist22. Despite these observations, there is still debate as to whether the incidence of adverse cerebrovascular events can be reduced for patients with subclinical LT when anticoagulation is initiated. The conflict in opinion is illustrated in registry data from patients undergoing ABV replacement, with some studies reporting that anticoagulation either reduces23 or has no effect24 on the incidence of thromboembolic complications when compared with aspirin alone. The intensity of anticoagulation therapy also has to be delicately balanced against the risk of bleeding in an often frail and elderly patient cohort. Randomised trials such as the GALILEO [NCT02556203] and ATLANTIS [NCT02664649] trials will provide further insight into the optimal antithrombotic strategy following ABV replacement. Furthermore, both trials will be looking at leaflet thrombosis as a combined primary endpoint, which will provide further understanding on the optimal antithrombotic treatment post TAVR.
Study limitations
There are a few limitations to the current analysis. Firstly, there are currently few studies reporting the occurrence of MDCT-defined LT following ABV. The true prevalence, particularly for each valve type, may only be known with further research. However, we report the largest pooled analysis on MDCT-defined LT with ABV and the results provide a robust basis for future investigation. Secondly, all of the included studies were observational in design and thus have potential to have hidden biases that impacted on our results. Thirdly, the definition of LT varied slightly between studies, with either the presence of hypoattenuated leaflet thickening or restriction of leaflet motion required for diagnosis of LT. However, the definitions used by individual studies all relate to the early stages of ABV thrombosis, and the pathological mechanisms that underscore the development and clinical sequelae of LT are probably similar. Fourthly, the type of MDCT scanner and timing used to diagnose LT varied between studies, which may have influenced the diagnostic ability and the reported pooled prevalence of LT. Next, all cerebrovascular events were not routinely assessed by a neurologist, potentially impacting on the prevalence of stroke and TIA. Finally, antithrombotic strategy post ABV implantation was not clearly described in all studies and that strategy may have influenced both the development and sequelae of LT.
Conclusions
MDCT-defined LT following ABV replacement is associated with a significantly increased risk of adverse cerebrovascular events, particularly TIA. Further prospective studies are required to ascertain whether LT can be adequately prevented or treated through pharmacological strategies.
| Impact on daily practice Aortic bioprosthetic valve leaflet thrombosis defined with multidetector computed tomography is associated with an increased risk of adverse cardiovascular events, in particular transient ischaemic attacks. Leaflet thrombosis with the feature of reduced leaflet motion is associated with higher rates of cerebrovascular events when compared to hypoattenuated leaflet thickening alone. Further studies are warranted to determine the optimal antithrombotic strategy to prevent and treat leaflet thrombosis. |
Funding
R. Gooley and L. McCormick are both supported by a Robertson Family Research Cardiologist Fellowship. A. Brown is supported through a Monash University Early Career Practitioner Fellowship.
Conflict of interest statement
R. Gooley has received consultancy fees from Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Example search strategy for MEDLINE.
Supplementary Table 2. Study characteristics.
Supplementary Table 3. Leaflet thrombosis definitions.
Supplementary Table 4. Cochrane Review risk of bias assessment.
Supplementary Table 5. Definitions of clinical outcomes.
Supplementary Figure 1. Risk estimates by subcategory of leaflet thrombosis.
To read the full content of this article, please download the PDF.

