
Abstract
An original in vitro/in silico method was developed to estimate the local and global mechanical stress applied on the bioprosthetic valve leaflet, which can be important for better understanding of the valve durability. A non-contact system based on stereophotogammetry and digital image correlation enabled filming and studying the valve leaflet movement frame by frame and performing three-dimensional analysis. The deformation was applied in a finite element model in order to calculate the local mechanical stress applied. High stress regions were primarily observed in the upper leaflet edge and belly and to a lesser extent at the free leaflet edge.
Introduction
The main limitation of bioprosthetic valves used for surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI) is their limited durability due to structural valve degeneration (SVD), which implies a risk of valve reintervention. The main determinant of SVD is the repetitive mechanical stress imposed on the bioprosthetic valve leaflets1. The objective of this in vitro/in silico study was to propose a novel method to assess the strain and mechanical stress applied on the bioprosthetic valve leaflets during the cardiac cycle.
Methods
Stress computation is based on coupling an experimental method based on non-contact digital image correlation (DIC) and finite element (FE) modelling (Figure 1).
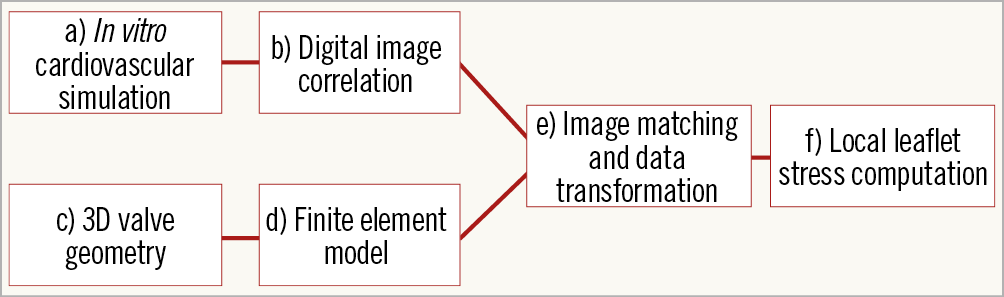
Figure 1. Implementation of experimental results into the FE model. Schematic diagram of a novel method for calculation and a preliminary evaluation of mechanical stress.
IN VITRO CARDIOVASCULAR SIMULATION
For the purpose of this study, a double activation left heart duplicator system2 was used. This system includes anatomically shaped, deformable silicone moulds of left heart cavities and a glass aorta that enables optimal visualisation of valve leaflets (Supplementary Appendix 1, Supplementary Figure 1-Supplementary Figure 3). The aortic valve used in this feasibility study was a surgical stent pericardial bioprosthesis (Trifecta™ 25 mm; St. Jude Medical, St. Paul, MN, USA).
DIGITAL IMAGE CORRELATION
To obtain a high contrast stochastic pattern for the use of DIC, a fine speckle pattern using a black tissue dye (Shandon™ Tissue Marking Dye; Thermo Fisher Scientific, Waltham, MA, USA) was applied to the leaflet surface (Figure 2). The leaflet motion was recorded with two high-speed cameras (2,000 img/sec) (FASTCAM SA3; Photron, Inc., San Diego, CA, USA), equipped with 105 mm lenses (EF 24 Reflex lenses; Sigma Corporation, Kanagawa, Japan) (Supplementary Figure 4).
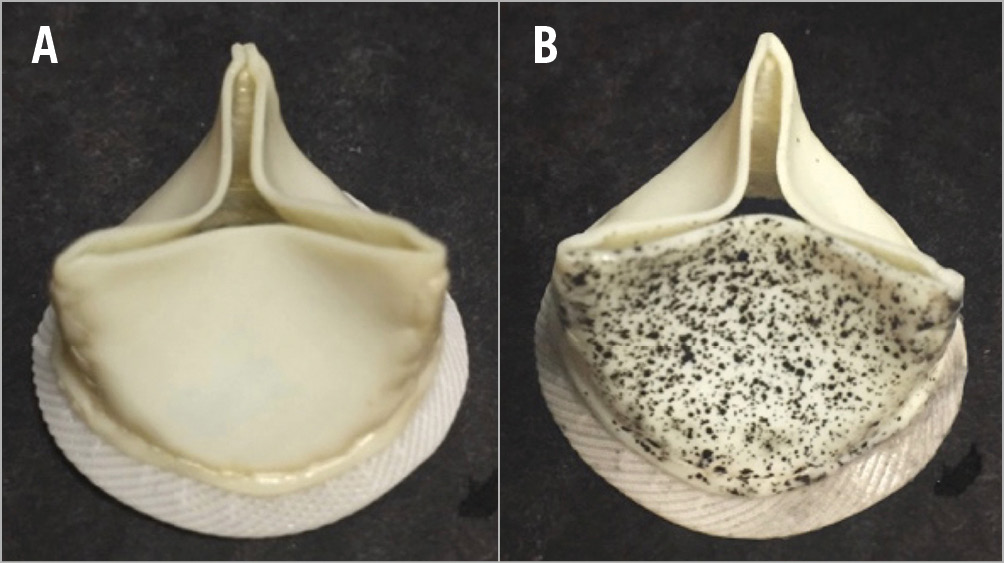
Figure 2. The surgical bioprosthesis before (A) and after (B) the speckle pattern application. Approximately 1,500 speckle points are applied on the surface of the leaflet.
Measurement of point displacement was performed using a non-contact optical three-dimensional (3D) DIC method conducted with a commercial system (VIC-3D; Correlated Solutions, Inc., Irmo, SC, USA). This technique allowed direct assessment of the deformation field of the valve leaflet (Figure 3). Local strain was assessed through a section line following the curved surface of the leaflet, and the global strain was computed over the whole surface of the leaflet.
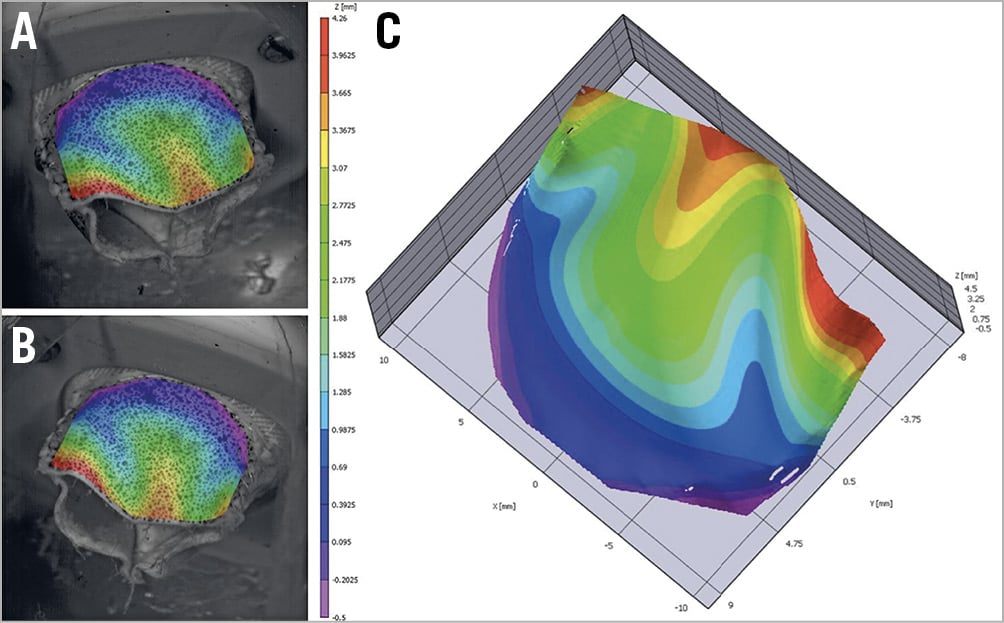
Figure 3. Creation of three-dimensional surface. A) Left camera view. B) Right camera view. C) 3D surface reconstruction.
LEAFLET NUMERICAL MODEL
The Trifecta valve was imaged with a desktop micro-computed tomography scanner (microCT-40; Scanco Medical AG, Wangen-Brüttisellen, Switzerland) (Figure 4A). The high-resolution images were imported into a 3D Slicer (open platform for subject-specific image analysis) to create a 3D valve model in stereolithography format (Figure 4B). The finalised surfaces were imported into HyperMesh™ (Altair HyperWorks; Altair Engineering, Troy, MI, USA) to generate a surface mesh (Figure 4C) with accurate size ex vivo.
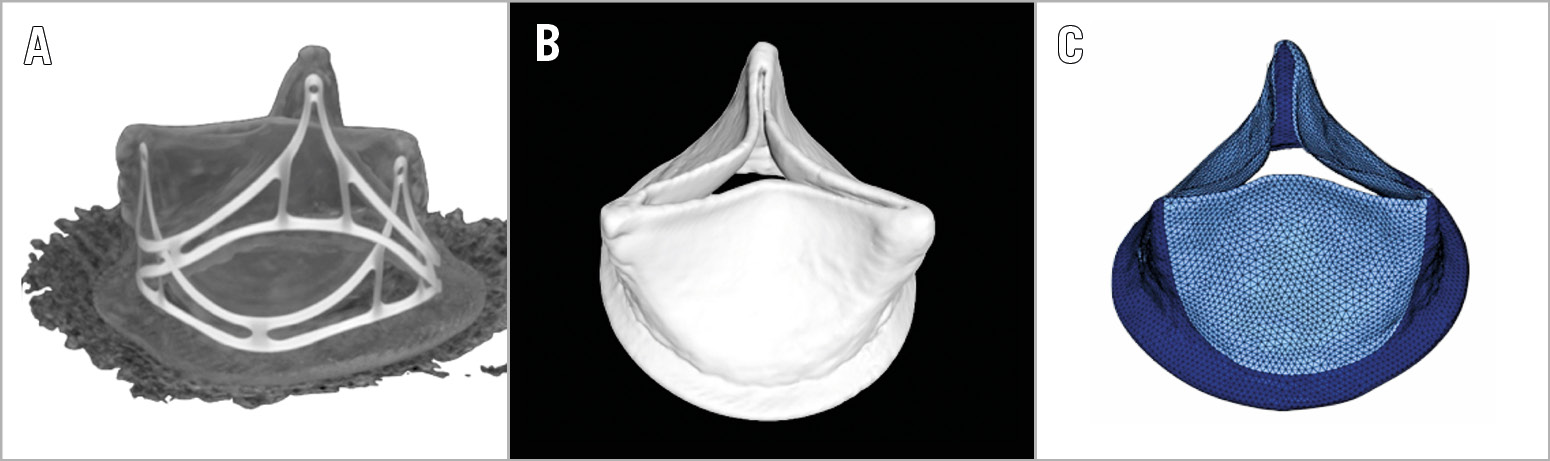
Figure 4. Creation of finite element model. There are three steps. A) DICOM image. B) Creation of 3D model/.stl file after 3D Slicer reconstruction. C) Final surface mesh created in HyperMesh.
Stent and skirt geometry were considered a rigid body. Leaflet geometry was modelled using two-dimensional (2D) shell elements. The symmetric impact contact definition was chosen for leaflet-to-leaflet contact to avoid leaflet penetration. FE simulations were performed using RADIOSS™ (Altair Engineering).
A biaxial stretch testing was performed on a homemade rake-based planar biaxial tensile testing device3 to determine the material properties of the Trifecta valve. Leaflet thickness was measured using SmartScope® MVP (OGP, Rochester, NY, USA) with a resolution <5 µm. The properties data set used in the FE model is shown in Supplementary Table 1.
IMAGE MATCHING AND DATA TRANSFORMATION
The 3D data alignment was performed using MeshLab (open-source mesh processing tool, 2008) by matching the point cloud extracted from the first moment of analysed VIC-3D data to numerical surface mesh (HyperMesh). A custom-made Matlab (MATLAB R2015a; The MathWorks, Inc., Natick, MA, USA) program was coded to apply the data transformation (Supplementary Figure 5) and create a file to contain all displacements applied on each chosen node in x,y,z direction as a function over time. This 3D data set was then used to perform FE simulations.
Results
DIGITAL IMAGE CORRELATION
The accuracy for the DIC was 0.0011 mm. The maximum major principal strain was observed during leaflet closing near the leaflet commissures (35%) and at the upper part of the belly (27%) (Figure 5). The average value of maximum major principal strain across the leaflet was 4%.
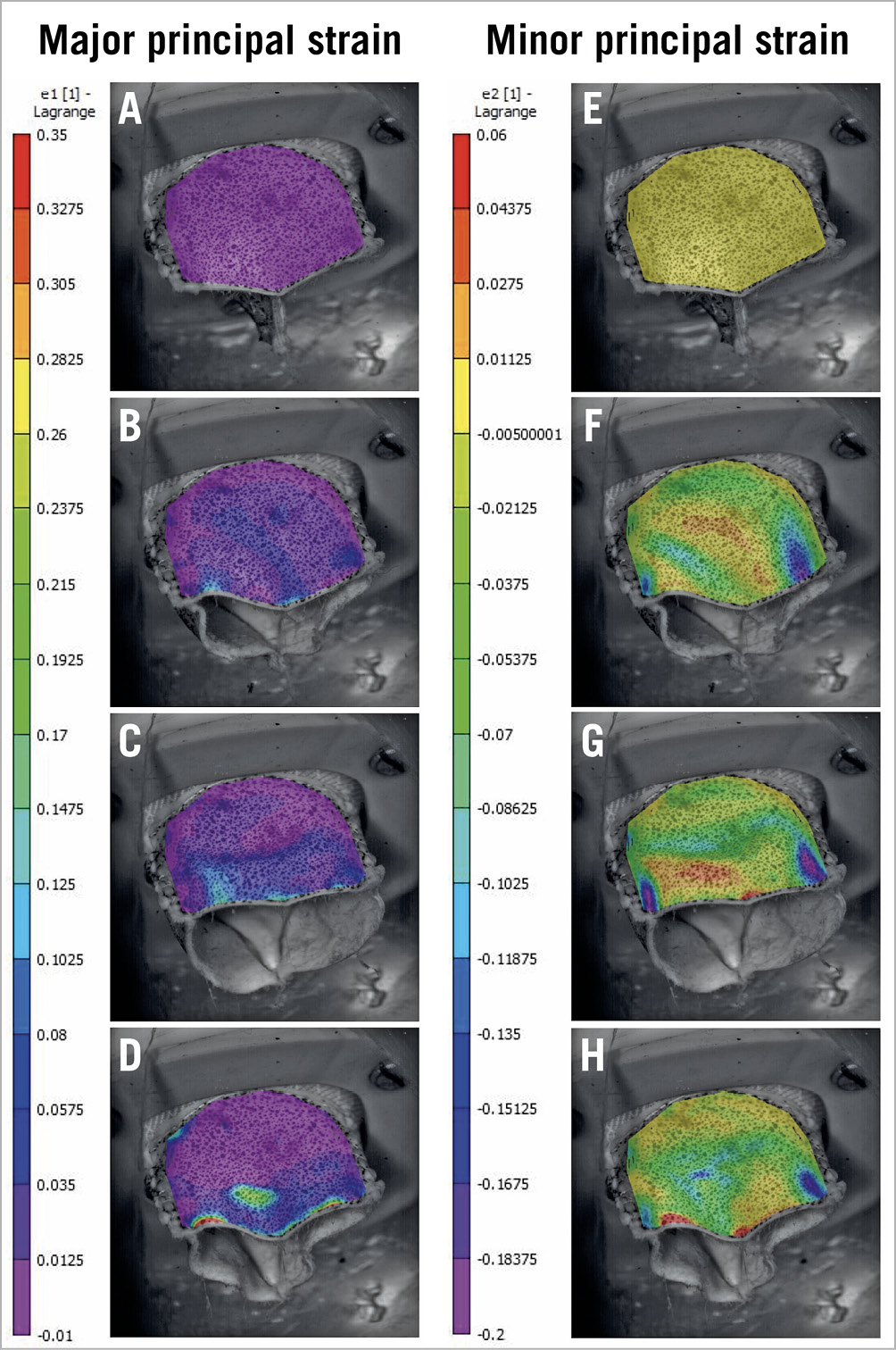
Figure 5. Development of major and minor principal strain during valve leaflet opening and closing. A) & E) Strain distribution in the initial reference image (closed valve). B) & F) Leaflet opening. C) & G) Leaflet at fully open position. D) & H) Leaflet closing. Positive signs in strain describe tensions and negative signs the compression.
FINITE ELEMENT ANALYSIS
The displacement of the leaflet during opening and closing is displayed in Figure 6.
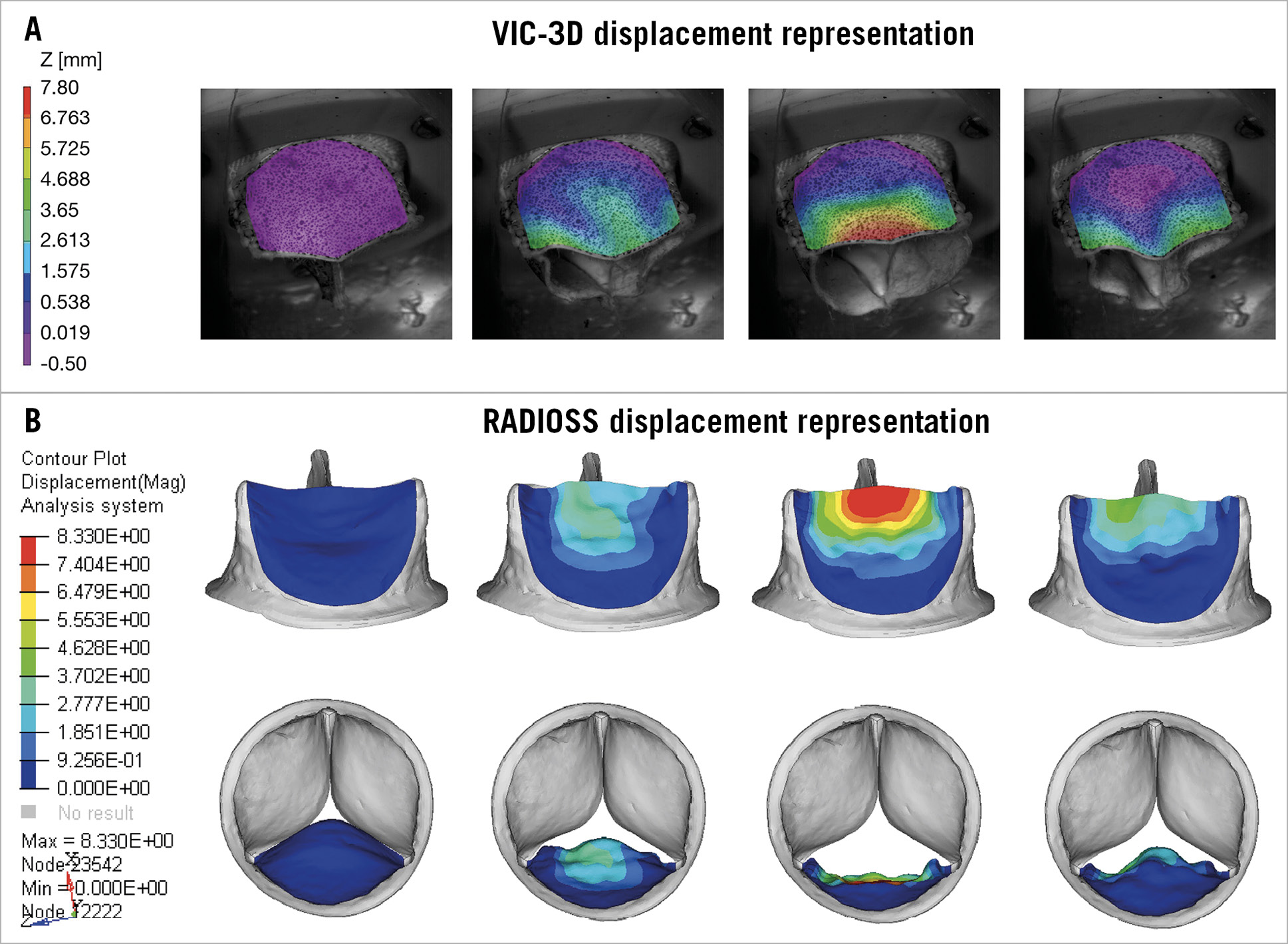
Figure 6. Displacement representation of opening and closing of the leaflet. A) VIC-3D. B) HyperMesh. The red and orange areas represent high values, whereas the violet and blue areas represent low values.
During opening, the higher stress occurs at the commissures and, to a lesser extent, in the upper part of the belly of the leaflet (Figure 7). When the valve is fully open, the maximum stress is observed at the commissures and at the belly of the leaflet and to a lesser extent at the free edge. The maximum principal stress reached 1.97 MPa. During closing, high stress regions were primarily observed at the commissures and in the middle of the belly of the leaflet (1.06 MPa).
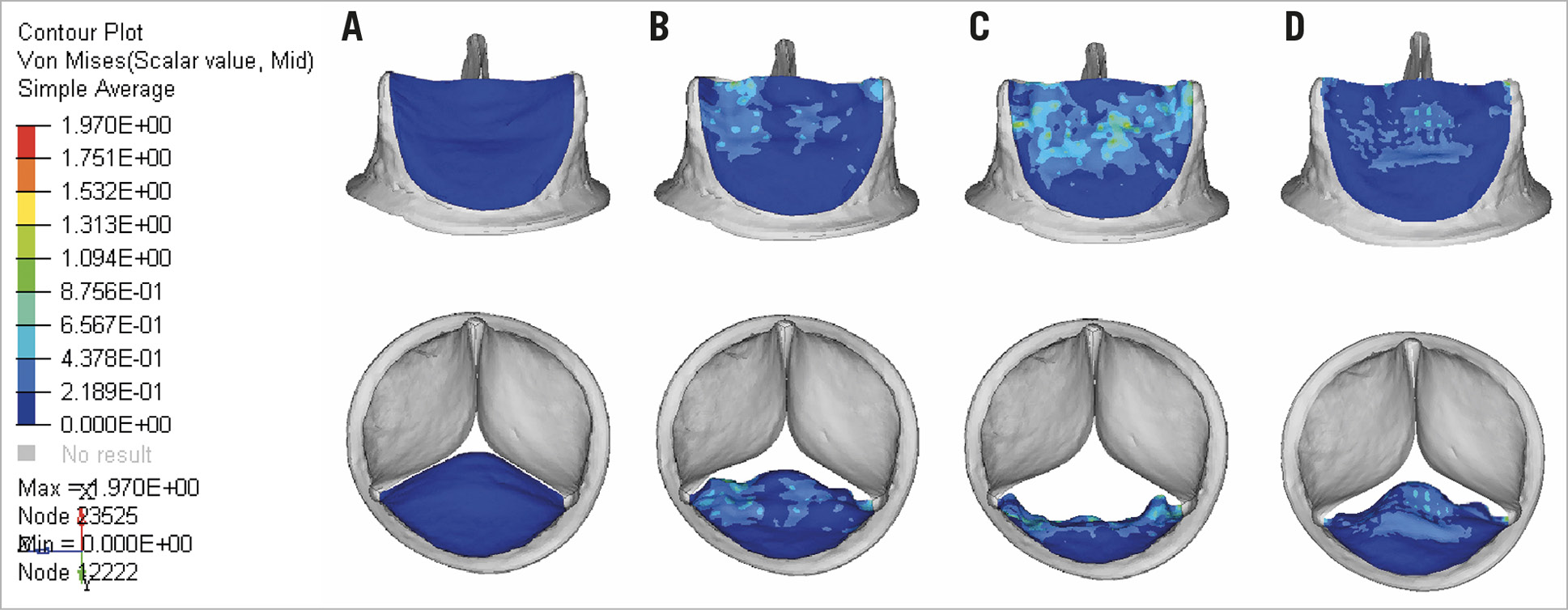
Figure 7. FE model for leaflet stress distribution. A) Leaflet coaptation. B) Leaflet opening. C) Leaflet at fully open position. D) Leaflet closing. The red and orange areas represent high values, whereas the blue areas represent low values.
Discussion
The novel method that we proposed by coupling in vitro experiments and numerical simulation allows accurate assessment of the magnitude and distribution of the leaflet stress during the different phases of the cardiac cycle. Compared to previously proposed methods, our method measures the real displacement of the leaflet tissue and thus has the potential advantage of being more accurate in estimating the magnitude and distribution of the leaflet mechanical stress. In this study, we assessed the feasibility of this new method in one leaflet of one model and size of SAVR valve. The results show that the higher leaflet strain and stress are observed at and near the commissures during the opening phase, at the commissures and belly when the valve is fully open, and at the belly during the closing phase.
Limitations
We present the results of only one leaflet (one model and size of surgical valve). The next step is to extend the analysis to the three leaflets to: i) be able to simulate the full leaflet closure during diastole, ii) determine the heterogeneity in the magnitude and distribution of the stress among the three leaflets.
Conclusion
Recent studies using a definition of SVD based on the evidence of valve morphologic and haemodynamic deterioration during echocardiographic follow-up revealed an incidence of SVD >30% at 10 years following SAVR4. For TAVI valves, the results of midterm (five to seven years) durability are encouraging but long-term data are not available. The novel in vitro/in silico method can provide a useful tool to determine the magnitude and distribution of the leaflet strain and stress during the cardiac cycle.
|
Impact on daily practice This method provides useful information that may help to predict the durability of a given model and size of bioprosthetic valve in different conditions relevant to the clinical context. In particular, the measurement of leaflet strain and stress in the context of TAVI oversizing and thus underdeployment or undersizing and overexpansion would inform on which conditions may worsen the leaflet stress and thus impair the long-term valve durability. |
Conflict of interest statement
P. Pibarot reports receiving research grants from Edwards Lifesciences and Medtronic. R. Rieu reports receiving research grants from Medtronic. The other authors have no conflicts of interest to declare.

