Abstract
Background: Reperfusion therapy is challenging in the elderly. Catheter-directed therapies are an alternative for higher-risk pulmonary embolism (PE) patients if systemic thrombolysis (ST) is contraindicated or has failed. Their safety has not been evaluated in specific vulnerable populations.
Aims: We aimed to assess the safety of reperfusion therapies in elderly and frail patients in the real world.
Methods: In the US Nationwide Inpatient Sample from 2016 to 2020, we identified hospitalisations of patients ≥65 years with PE and defined a frailty subgroup using the Johns Hopkins Adjusted Clinical Groups frailty-defining diagnosis indicator. We investigated reperfusion therapies (ST, catheter-directed thrombolysis [CDT], catheter-based thrombectomy [CBT], surgical embolectomy [SE]) and their associated safety outcomes (overall and major bleeding).
Results: Among 980,245 hospitalisations of patients ≥65 years with PE (28.0% frail), reperfusion therapies were used in 4.9% (17.6% among high-risk PE). ST utilisation remained stable, while the use of catheter-directed therapies increased from 1.7% in 2016 to 3.2% in 2020. Among all hospitalisations with reperfusion, CDT, compared to ST, was associated with reduced major bleeding (5.8% vs 12.2%, odds ratio [OR] 0.58, 95% confidence interval [CI]: 0.49-0.70); these results also applied to frail patients. CBT, compared to SE, was also associated with reduced major bleeding (11.0% vs 22.4%, OR 0.63, 95% CI: 0.43-0.91), but not among frail patients. These differences were particularly significant in patients with non-high-risk PE. Differences persisted for overall bleeding as well.
Conclusions: Catheter-directed therapies may be a safer alternative to classical reperfusion therapies for elderly and frail patients with PE requiring reperfusion treatment.
Introduction
Acute pulmonary embolism (PE) is a common cardiovascular disease whose incidence rises exponentially with age12. Although the annual PE-related mortality rates have decreased over the past two decades, especially among the elderly, PE is still a main contributor to overall mortality in the elderly34. Reperfusion therapies, primarily systemic (intravenous) thrombolysis (ST) or surgical embolectomy (SE), have traditionally been the treatment of choice in patients with haemodynamic instability or risk of imminent decompensation56. However, age >75 is often mentioned as a relative contraindication for ST since the rate of major extracranial bleeding reached 11.1% in aged patients in the Pulmonary Embolism Thrombolysis (PEITHO) Study7. On the other hand, patients with advanced age and frailty often have a high surgical risk, and SE may not be an ideal choice89. Therefore, these patients are in need of alternative reperfusion strategies.
Catheter-directed therapies, including catheter-directed thrombolysis (CDT) and catheter-based thrombectomy (CBT), can swiftly restore the haemodynamic parameters of right ventricular function in high- and intermediate-high-risk PE patients and are suggested for those in whom thrombolysis has failed or is contraindicated6. Standard or ultrasound-assisted CDT consists of a local infusion of lower-dose thrombolytics directly into the pulmonary arteries. CBT may refer either to thrombus fragmentation with the use of rotational pigtail catheters or thrombus aspiration by applying suction with the use of large-bore catheters. Pharmacomechanical catheter-directed techniques have also been used. A recent meta-analysis evaluated the safety profile of these endovascular therapy approaches, reporting major bleeding rates of 6.7% and 1.4% for high-risk and intermediate-risk PE, respectively10. However, this evidence is derived mainly from smaller single-arm studies, whereas major controlled randomised trials investigating the efficacy and safety of CDT are still ongoing11. Moreover, frail older patients are usually underrepresented in randomised trials, as they fulfil many exclusion criteria.
In the present study, we aimed to assess the contemporary use of reperfusion therapies for the management of elderly and frail hospitalised patients with acute PE and to specifically investigate the safety with regard to bleeding of different reperfusion treatment approaches in a large nationwide sample.
Methods
The Nationwide Inpatients Sample (NIS), provided by the Agency for Healthcare Research and Quality, is the largest publicly accessible, all-payer, de-identified, inpatient healthcare database in the USA and was developed for the Healthcare Cost and Utilization Project (HCUP). The database represents roughly 97% of the US population, and it contains a 20% stratified sample of discharges from US hospitals that take part in the HCUP, excluding rehabilitation and long-term acute care facilities. For this analysis, we used data from the NIS from 2016 to 2020. The database includes only de-identified data, and this analysis was exempt of institutional review board approval or informed consent. Additional information on the NIS is provided in the HCUP NIS Database Documentation website (www.hcup-us.ahrq.gov).
STUDY POPULATION AND DATA DEFINITION
The International Classification of Diseases, 10th version (ICD-10-CM for diagnoses and ICD-10-PCS for procedures) is used to code the diagnoses and procedures in the NIS database. The database contains up to 40 diagnoses and 25 procedures for each hospital discharge record. For the purpose of the present study, all hospitalisations of elderly (≥65 years) patients were selected with a discharge diagnosis code of acute PE (primary and secondary diagnosis). Frail patients were defined applying the Johns Hopkins Adjusted Clinical Groups (ACG) Frailty Assessment Calculator121314. This indicator consists of a total of 10 frailty-defining unweighted diagnoses: malnutrition, dementia, severe vision impairment, decubitus ulcer, urinary incontinence, weight loss, poverty, barriers to access to care, difficulty in walking, and falls; at least one of 10 diagnoses (in conjunction with age more than 65 years old) was required to define frailty. Hospitalisations with an additional diagnosis of shock, vasopressor use, cardiac arrest, or need for cardiopulmonary resuscitation were classified as high-risk acute PE.
The reperfusion therapeutic strategies during hospitalisation were classified as ST, CDT, CBT, and SE. If two or more reperfusion therapies were used during the same hospitalisation, we considered the reperfusion therapy performed first in our descriptive analysis, whereas those cases were excluded in the inferential analysis. Safety outcomes and complications during the index PE hospitalisation primarily included overall bleeding rates and major bleeding rates (defined as the presence of intracranial bleeding, haemopericardium, haemoperitoneum, haemarthrosis; all with or without transfusion). We also assessed in-hospital mortality, length of stay, shock, and acute renal failure.
Supplementary Table 1 lists the ICD-10-CM and ICD-10-PCS codes used to define the aforementioned variables, as well as comorbidities.
STATISTICAL ANALYSIS
Since the percentage of missing data was below 3% for all variables, we assumed that they were missing at random and conducted a complete case analysis. For categorical data, descriptive statistics comprised counts and percentages, and for continuous variables, medians and interquartile ranges (IQR). Logistic regression was used to calculate odds ratios (OR) of overall bleeding, major bleeding, and all-cause in-hospital mortality and make comparisons of (i) ST versus CDT and (ii) CBT versus SE. Comparisons were made among patients who received only one reperfusion therapy, excluding those who received multiple therapies. The regression models were adjusted for age, sex, presence of high-risk PE, and comorbidities (arterial hypertension, diabetes, obesity, congestive heart failure, chronic pulmonary disease, and cancer). We performed a subgroup analysis according to frailty status. All analyses were conducted by application of discharge-level weights provided in the HCUP database and by taking into account the NIS stratification and hospital clustering. A 2-sided p-value<0.05 was set as the level of statistical significance. The statistical analysis was conducted in R version 4.1.3 (R Project for Statistical Computing).
Results
Out of a total of 1,942,505 patients hospitalised with a diagnosis of PE between 2016 and 2020, 980,245 (50.5%) were ≥65 years of age and were included in the present analysis. In these elderly patients, the median age was 75 (IQR 70-82) years; 54% were women (Table 1); and 274,175 (28.0%) patients were considered frail, based on the Johns Hopkins ACG frailty-defining indicator as explained in the Methods, and were included in the predefined subgroup analysis.
In this study population of patients ≥65 years, reperfusion therapies were used in 4.0% of PE hospitalisations in 2016 with an increase up to 5.6% in 2020 (Figure 1). Interestingly in high-risk PE, the rates of reperfusion use remained largely unchanged with 16.9% in 2016 and 16.5% in 2020. Reperfusion rates by age group and high-risk PE are presented in Supplementary Figure 1. ST was the most used reperfusion therapy (2.5% overall, 12.3% for high-risk PE), followed by catheter-directed thrombolysis (1.8% overall, 3.1% for high-risk PE), CBT (0.7% overall, 2.5% for high-risk PE), and SE (0.1% overall, 0.9% for high-risk PE). Overall, use of CDT increased from 1.7% in 2016 to 3.2% in 2020, and both CDT and CBT showed an increasing trend throughout the study period (Figure 1). The use of reperfusion therapies was more frequent in non-frail patients (5.6%, compared to 3.1% in those with frailty), and this was also true among patients with high-risk PE (non-frail, 19.9%; frail, 12.6%).
Bleeding complications were documented in 15.6% of elderly patients; of those, major bleeding was found in 7.6% and intracranial haemorrhage in 1.0%. Since transfusions may also be procedure-related, by not taking them into account, major bleeding rates were i) 2% among patients not receiving any reperfusion treatment, ii) comparable for CDT and CBT at 2% and 3%, respectively, and iii) observed to be higher at 4% and 7% for ST and SE, respectively. Intracranial haemorrhage, which is the most feared complication of lytic treatment, was low at 1% in all groups, except for the ST group, where it reached 3%. Bleeding events stratified by type of reperfusion treatment are shown in Figure 2. The in-hospital fatality rate was 8.4% across the entire study population, while among patients with high-risk PE it was as high as 55.3%. Patients without frailty had 7.4% in-hospital mortality (56.3% in high-risk PE), while those with frailty had considerably higher overall in-hospital mortality of 11.2%. In-hospital outcomes and complications in elderly patients who received at least one reperfusion therapy are presented in Figure 2 and, according to frailty status, in Table 2.
Table 1. Baseline characteristics of pulmonary embolism hospitalisations among patients aged ≥65 years according to frailty status.
| Characteristic | Overall N=980,245 | Non-frail N=706,070 | Frail N=274,175 | |
|---|---|---|---|---|
| Age, years | 75 [70, 82] | 74 [69, 81] | 79 [72, 86] | |
| Women | 527,935 (54) | 370,825 (53) | 157,110 (57) | |
| Race | White | 728,660 (77) | 533,450 (78) | 195,210 (73) |
| Black | 133,585 (14) | 89,985 (13) | 43,600 (16) | |
| Hispanic | 51,790 (5.4) | 36,070 (5.3) | 15,720 (5.9) | |
| Asian/Pacific Islander | 13,000 (1.4) | 8,585 (1.3) | 4,415 (1.7) | |
| Native American | 3,200 (0.3) | 2,315 (0.3) | 885 (0.3) | |
| Other | 20,815 (2.2) | 14,305 (2.1) | 6,510 (2.4) | |
| Deep vein thrombosis | 347,255 (35) | 252,975 (36) | 94,280 (34) | |
| Diabetes mellitus | 279,535 (29) | 203,870 (29) | 75,665 (28) | |
| Myocardial infarction | 124,065 (13) | 89,435 (13) | 34,630 (13) | |
| Congestive heart failure | 273,895 (28) | 191,575 (27) | 82,320 (30) | |
| Chronic pulmonary disease | 301,865 (31) | 219,830 (31) | 82,035 (30) | |
| Renal disease | 202,230 (21) | 143,595 (20) | 58,635 (21) | |
| Cancer | 347,235 (35) | 252,675 (36) | 94,560 (34) | |
| Cerebrovascular disease | 78,555 (8.0) | 47,530 (6.7) | 31,025 (11.0) | |
| Dementia | 116,840 (12) | 0 (0) | 116,840 (43) | |
| Hypertension | 725,275 (74) | 526,870 (75) | 198,405 (72) | |
| Obesity | 175,470 (18) | 144,500 (20) | 30,970 (11) | |
| Renal impairment | 140,310 (14) | 100,080 (14) | 40,230 (15) | |
| High-risk PE | 63,840 (6.5) | 43,800 (6.2) | 20,040 (7.3) | |
| Data are expressed as median [IQR] or n/N (%). IQR: interquartile range; PE: pulmonary embolism | ||||
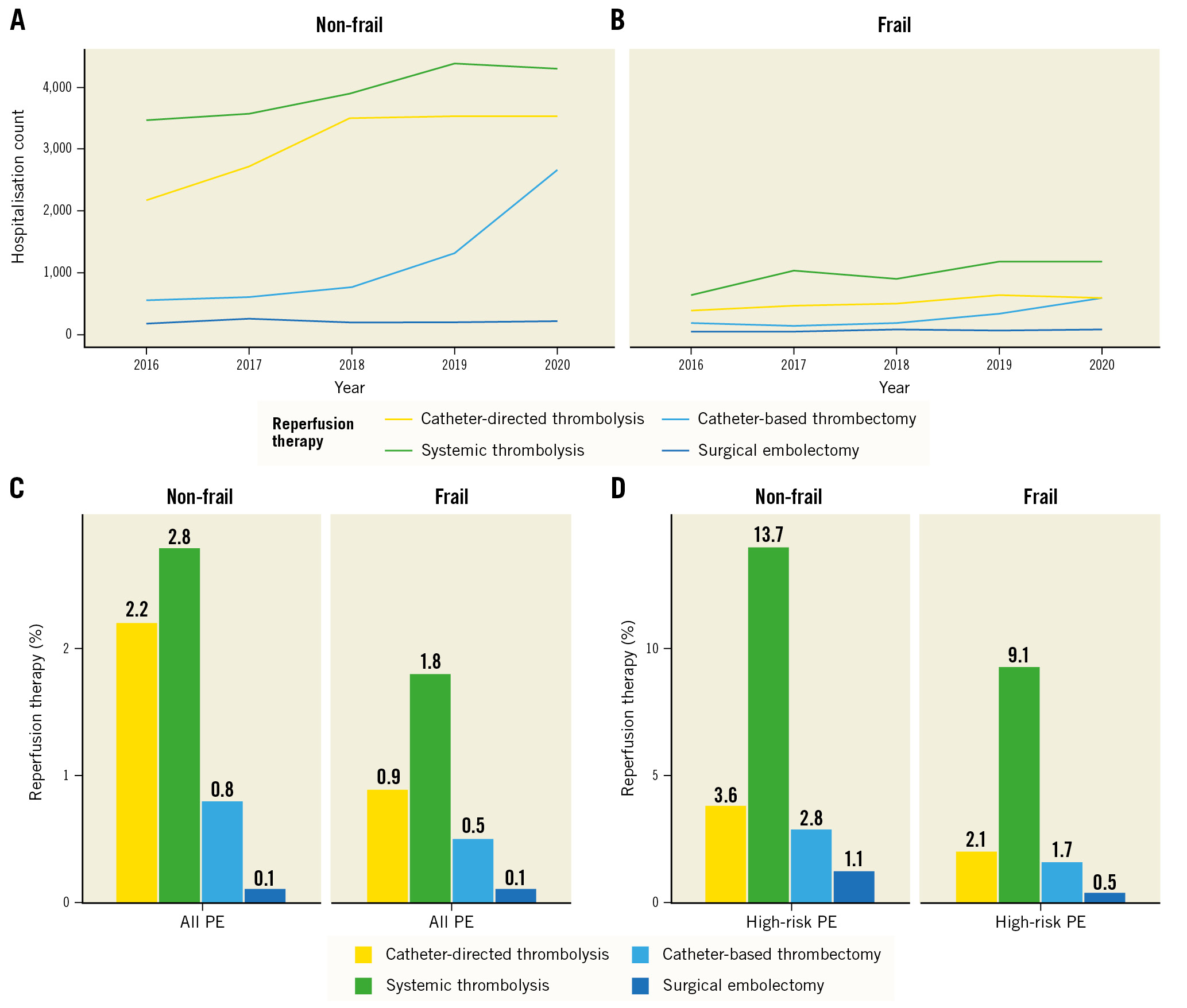
Figure 1. Counts of hospitalisations of elderly patients with PE requiring reperfusion therapies from 2016-2020 in the Nationwide Inpatient Sample and rates of reperfusion therapies. A) Hospitalisations for patients without frailty. B) Hospitalisations for frail patients. Rates of reperfusion therapies in all patients with PE (C) and patients with high-risk PE (D) according to frailty status. PE: pulmonary embolism
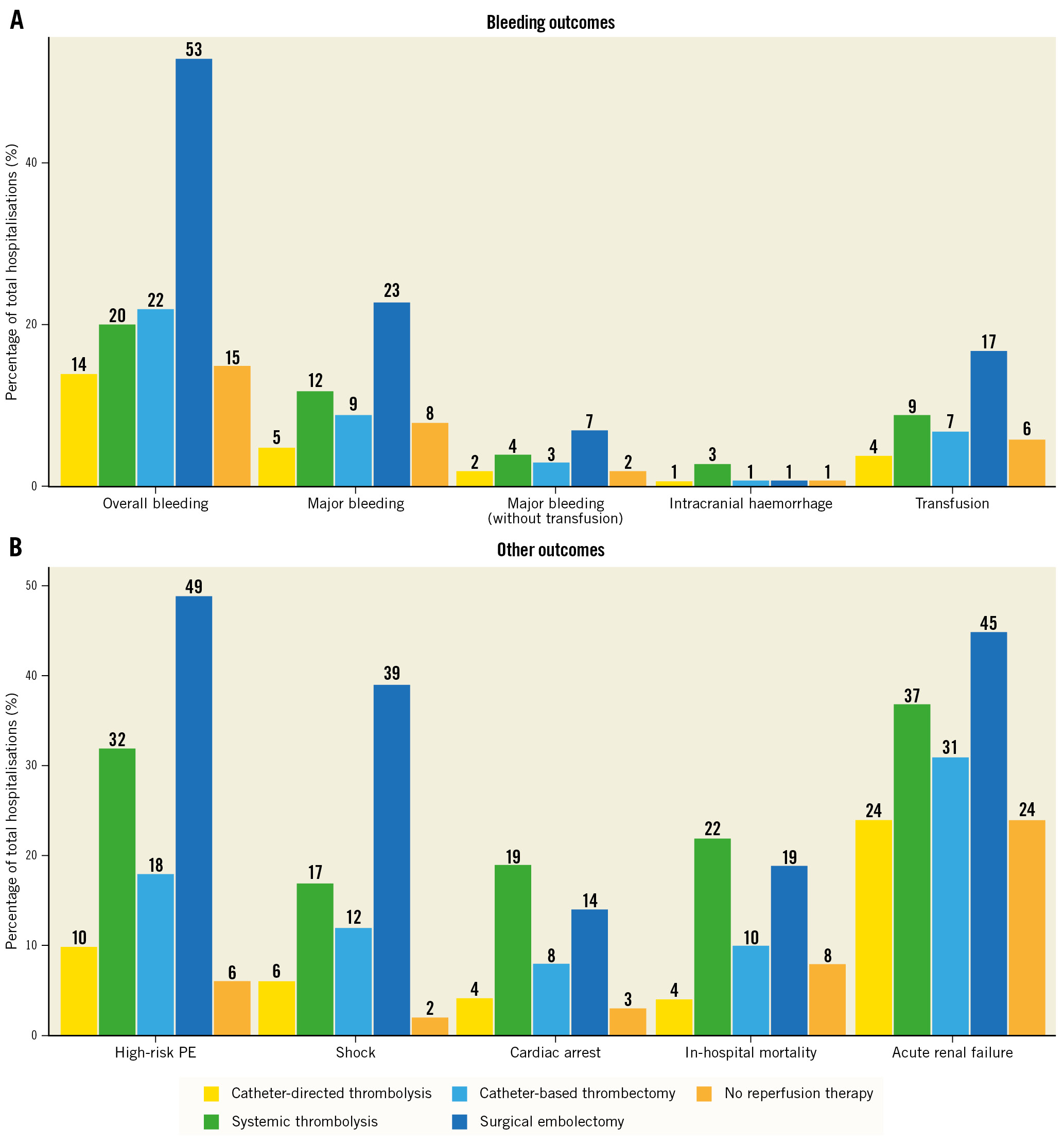
Figure 2. Hospitalisation rates according to bleeding and other outcomes for reperfusion therapies. A) Hospitalisation rates of bleeding outcomes, including overall bleeding, major bleeding, transfusions, intracranial bleeding, and other outcomes (B) across reperfusion treatments (systemic thrombolysis, catheter-directed thrombolysis, catheter-based thrombectomy, and surgical embolectomy).
Table 2. Severity of pulmonary embolism, therapeutic procedures, complications, and outcomes of the study patients stratified by frailty status and type of reperfusion therapy.
| Characteristic | Non-frail | Frail | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No reperfusion, N=666,295 | Catheter-based thrombectomy, N=4,200 | Catheter-directed thrombolysis, N=13,965 | Surgical embolectomy, N=870 | Systemic thrombolysis, N=18,535 | No reperfusion, N=265,795 | Catheter-based thrombectomy, N=975 | Catheter-directed thrombolysis, N=2,155 | Surgical embolectomy, N=175 | Systemic thrombolysis, N=4,640 | |
| High-risk PE | 35,085 (5.3) | 740 (18) | 1,260 (9.0) | 420 (48) | 5,720 (31) | 17,520 (6.6) | 210 (22) | 300 (14) | 90 (51) | 1,760 (38) |
| VA-ECMO | 415 (<0.1) | 60 (1.4) | 30 (0.2) | 80 (9.2) | 105 (0.6) | 65 (<0.1) | NR | NR | 25 (14) | 15 (0.3) |
| Inferior vena cava filter | 42,880 (6.4) | 930 (22) | 2,365 (17) | 315 (36) | 2,955 (16) | 21,700 (8.2) | 275 (28) | 470 (22) | 60 (34) | 810 (17) |
| Bleeding | 94,775 (14) | 890 (21) | 1,825 (13) | 460 (53) | 3,460 (19) | 48,695 (18) | 260 (27) | 355 (16) | 90 (51) | 1,195 (26) |
| Major bleeding | 44,635 (6.7) | 365 (8.7) | 685 (4.9) | 200 (23) | 2,035 (11) | 25,395 (9.6) | 120 (12) | 150 (7.0) | 45 (26) | 755 (16) |
| Major bleeding (without transfusion) | 11,280 (1.7) | 110 (2.6) | 285 (2.0) | 65 (7.5) | 770 (4.2) | 5,230 (2.0) | 20 (2.1) | 35 (1.6) | NR | 235 (5.1) |
| Intracranial haemorrhage | 6,035 (0.9) | 50 (1.2) | 130 (0.9) | 10 (1.1) | 485 (2.6) | 3,150 (1.2) | NR | 20 (0.9) | 0 (0) | 125 (2.7) |
| Transfusion | 35,130 (5.3) | 270 (6.4) | 460 (3.3) | 140 (16) | 1,435 (7.7) | 21,020 (7.9) | 110 (11) | 120 (5.6) | 40 (23) | 555 (12) |
| Acute renal failure | 147,525 (22) | 1,240 (30) | 3,240 (23) | 370 (43) | 6,510 (35) | 72,490 (27) | 360 (37) | 690 (32) | 100 (57) | 2,000 (43) |
| Shock | 14,225 (2.1) | 475 (11) | 840 (6.0) | 330 (38) | 3,015 (16) | 6,585 (2.5) | 125 (13) | 180 (8.4) | 75 (43) | 875 (19) |
| Cardiac arrest | 18,985 (2.8) | 370 (8.8) | 495 (3.5) | 125 (14) | 3,525 (19) | 8,530 (3.2) | 55 (5.6) | 125 (5.8) | 25 (14) | 940 (20) |
| In-hospital mortality | 46,505 (7.0) | 415 (9.9) | 580 (4.2) | 165 (19) | 4,040 (22) | 29,285 (11) | 105 (11) | 145 (6.7) | 35 (20) | 1,115 (24) |
| Length of stay | 4.0 [2.0, 7.0] | 5.0 [3.0, 8.0] | 4.0 [3.0, 7.0] | 9.0 [6.0, 16.5] | 5.0 [3.0, 8.0] | 6.0 [3.0, 10.0] | 7.0 [4.0, 11.0] | 6.0 [4.0, 10.0] | 13.0 [7.0, 18.0] | 7.0 [4.0, 12.0] |
| Patients receiving more than one reperfusion therapy were excluded. Data are expressed as n/N (%) or median [IQR]. AHRQ: Agency for Healthcare Research and Quality; HCUP: Healthcare Cost and Utilization Project; IQR: interquartile range; NR: not reported, due to data privacy as per HCUP AHRQ regulations; PE: pulmonary embolism; VA-ECMO: veno-arterial extracorporeal membrane oxygenation | ||||||||||
CATHETER-DIRECTED THROMBOLYSIS VERSUS SYSTEMIC THROMBOLYSIS
Among patients ≥65 years who received thrombolysis, CDT was associated with less major bleeding compared to systemic (intravenous) thrombolytic treatment (5.8% vs 12.2%; OR 0.58, 95% CI: 0.49-0.70), as well as among the subgroup of frail patients (8.6% vs 16.2%, OR 0.60, 95% CI: 0.41-0.88) (Figure 3A). Overall bleeding was also significantly less frequent in the patients treated with CDT compared to those receiving ST (14.4% vs 20.4%; OR 0.79, 95% CI: 0.70-0.90); this difference was observed also in the subgroup of frail patients (18.2% vs 25.8%; OR 0.72, 95% CI: 0.54-0.94) (Figure 3A). Patients receiving CDT had lower rates of in-hospital mortality compared to patients receiving ST (5.0% vs 22.3%; OR 0.33, 95% CI: 0.28-0.40), as well as in the subgroup of frail patients (6.7% vs 23.9%; OR 0.33, 95% CI: 0.26-0.40) (Supplementary Figure 1).
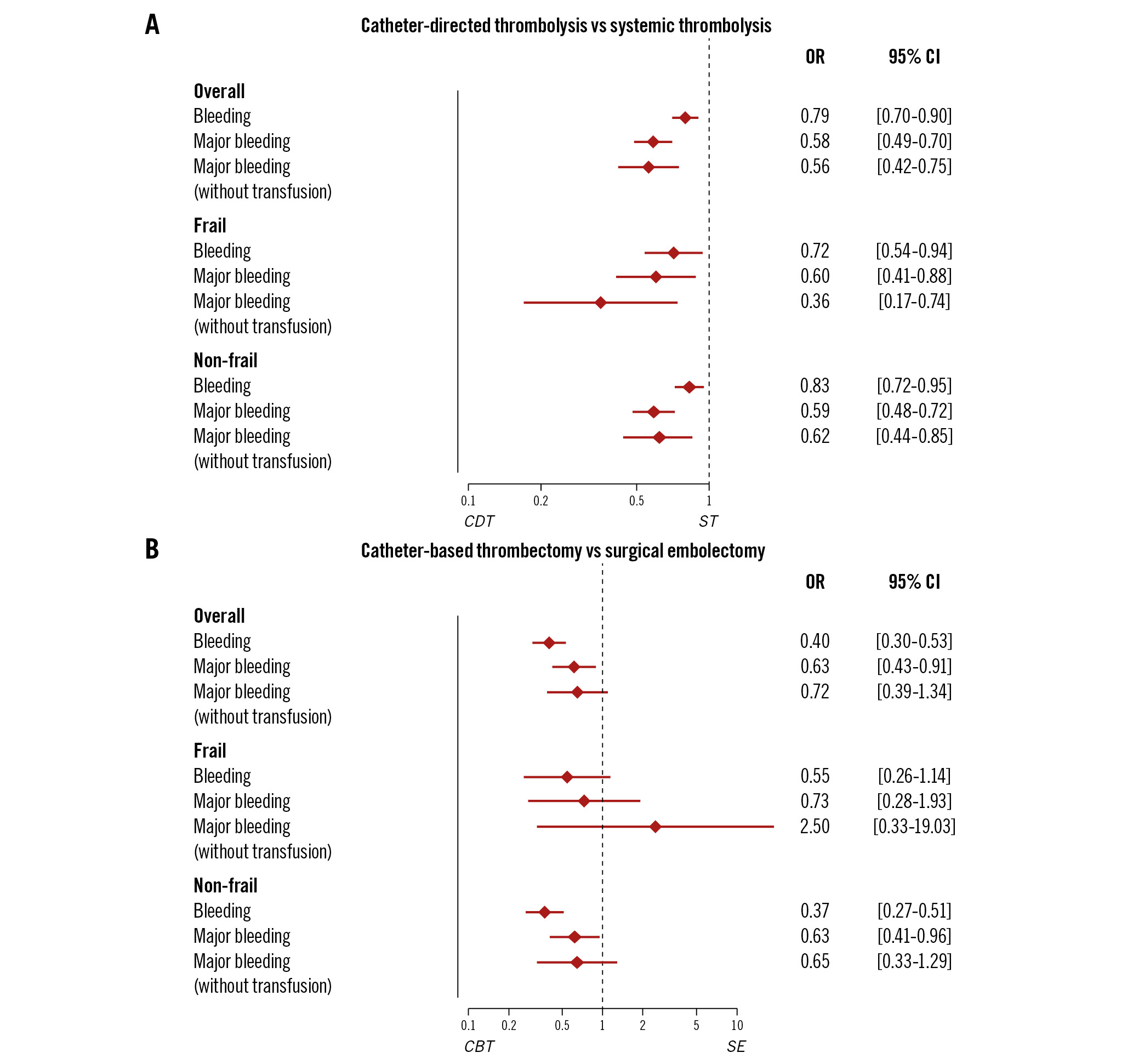
Figure 3. Multivariable logistic regression for overall and major bleeding (with or without transfusion) in elderly patients with PE according to frailty status. A) Catheter-directed thrombolysis vs systemic thrombolysis and (B) catheter-based thrombectomy vs surgical embolectomy. The model was adjusted for age, sex, comorbidities (hypertension, diabetes mellitus, obesity, congestive heart failure, chronic pulmonary disease, and cancer) and high-risk PE. CBT: catheter-based thrombectomy; CDT: catheter-directed thrombolysis; CI: confidence interval; OR: odds ratio; PE: pulmonary embolism; SE: surgical embolectomy; ST: systemic thrombolysis
CATHETER-BASED THROMBECTOMY VERSUS SURGICAL EMBOLECTOMY
Among all hospitalisations of elderly patients who received either SE or CBT, CBT was significantly associated with reduced major bleeding (11.0% vs 22.4%; OR 0.63, 95% CI: 0.43-0.91), without statistical significance among the subgroup of frail patients (14.8% vs 22.5%; OR 0.73, 95% CI: 0.28-1.93) (Figure 3B). Overall bleeding was also significantly less in the CBT group compared to the SE group (23.3% vs 50.4%; OR 0.40, 95% CI: 0.30-0.53); a tendency in the same direction was also observed among frail patients (28.4% vs 45.0%; OR 0.55, 95% CI: 0.26-1.14) (Figure 3B). In regard to in-hospital mortality, patients receiving CBT showed a trend towards lower rates compared to patients receiving SE (11.1% vs 21.1%; OR 0.85, 95% CI: 0.54-1.35), and to frail patients (10.6% vs 20.0%; OR 0.52, 95% CI: 0.20-1.32) (Supplementary Figure 2).
HIGH-RISK PATIENTS
Among the subgroup of high-risk PE, CDT compared to ST was not associated with a reduced incidence of overall or major bleeding, while CBT compared to SE was significantly associated with reduced overall bleeding (OR 0.33, 95% CI: 0.21-0.53) but not major bleeding (Supplementary Figure 3). The additional analyses based on high- or non-high-risk status can also be seen in Supplementary Figure 3.
Discussion
After investigating the NIS from the years 2016 to 2020, the main findings can be summarised as follows (Central illustration): 1) among elderly patients with a diagnosis of PE, the use of reperfusion therapies increased during the investigated time period and reached almost 6% in 2020; 2) use of reperfusion therapies was low among high-risk PE patients − used only in approximately 2 out of 10 patients with high-risk PE − and this decreased even further in the high-risk PE frail patient subgroup, with only 12% undergoing reperfusion therapeutic strategies; 3) CDT and especially CBT use increased continuously throughout the study period; 4) CDT showed lower rates of overall and major bleeding compared to ST, and CBT showed lower rates of overall and major bleeding compared to SE.
Previous analyses have focused on the use of “classical” reperfusion therapies, namely ST and SE. In a German nationwide analysis of the years 2005-2020, the rate of use of such therapies was low (<5% and <0.1%, respectively) in the elderly (>60 years) population4. In addition, based on the US NIS data from the years 1999-2008, 23.3% of patients with unstable (high-risk) primary PE hospitalisation and age >60 years received thrombolytic treatment15. This percentage is comparable to the one based on our analysis of the years 2016-2020 (23.2%) for the use of ST in high-risk primary PE hospitalisations. Similar conclusions apply to the usage rates of SE16.
More recently, CDT has been increasingly used in the treatment of high-risk as well as intermediate-high risk PE17 and is recommended with a class IIa indication in current European guidelines in cases where thrombolysis is contraindicated or has failed56.
Concerning CDT, the ULTIMA and CANARY trials, and other single-arm trials, have shown haemodynamic amelioration in patients receiving these treatments; however, the mean age of included patients was below 65 years, and little efficacy or safety data were mentioned for this particular population1819202122. In a retrospective analysis of 18 elderly patients receiving CDT, only one patient had a bleeding event following the procedure23, while in a systematic review of ultrasound-assisted CDT of 611 patients >60 years, the rate of intracranial haemorrhage was 1.1% and major bleeding was 4.9%24. These results were confirmed in our study, which showed a low rate of intracranial haemorrhage or major bleeding using administrative data of 16,120 elderly patients receiving CDT. In addition, we showed that the rates of overall bleeding, major bleeding, and intracranial haemorrhage were comparable in both the CDT group and the group of elderly patients not receiving any reperfusion therapy, suggesting a potential indication of safety for this population, irrespective of the presence of frailty. However, this safety signal warrants thorough investigation in randomised trials before any concrete recommendation for its use can be given11.
Fewer data exist on the use of CBT in an elderly population. The FLASH registry recently published results on the use of the FlowTriever (Inari Medical) thromboemboli aspiration system and reported a major bleeding rate of 1.4% within 48 hours in a population of PE patients with a mean age of 61 years25. In the EXTRACT-PE trial using the Indigo aspiration system (Penumbra, Inc), the rate of major bleeding within 48 hours was also 1.7% among patients with mean age of 59.8 years26. These results refer to single-arm studies with limited inclusion of elderly, and especially frail, patients, in whom the probability of bleeding outcomes is expected to be higher. In our analysis of elderly patients, rates of major bleeding among CBT procedures were higher, reaching 9% (12% in patients with frailty) and 3% if we exclude the transfusions, which may have been procedure related; however, the rate of major bleeding in patients receiving no reperfusion was also high at 8% and 2%, with and without transfusions, respectively. Compared to SE, CBT may be associated with reduced bleeding and in-hospital mortality, especially among patients without haemodynamic decompensation. Definite answers on the safety of this relatively novel reperfusion approach for PE, which will hopefully apply to elderly patients as well, may be provided by the randomised controlled trials that are currently enrolling (ClinicalTrials.gov: NCT05684796, NCT05612854, NCT05111613).
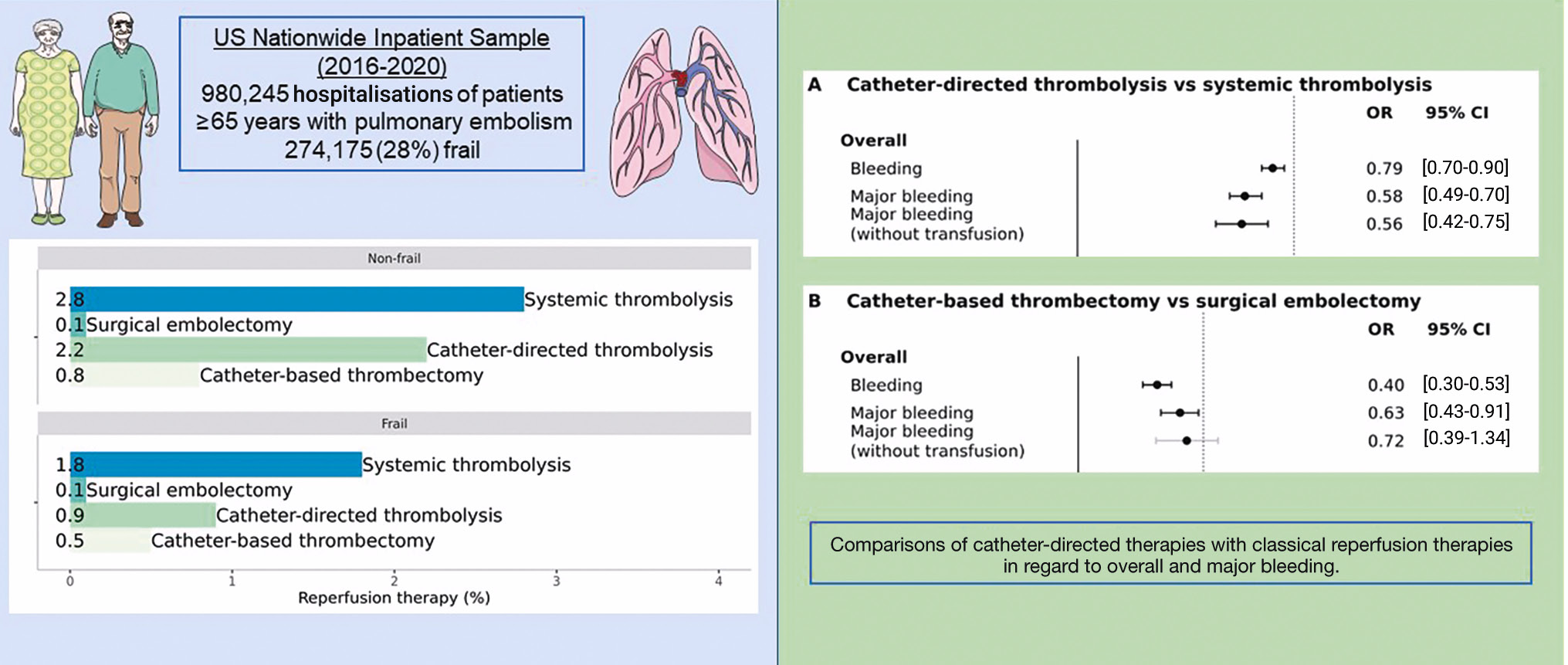
Central illustration. Safety and reperfusion therapies for pulmonary embolism in frail elderly patients. Catheter-directed therapies may be a safer alternative to classical reperfusion therapies for elderly and frail patients with PE requiring reperfusion treatment. CI: confidence interval; OR: odds ratio; PE: pulmonary embolism
Limitations
This study evaluated administrative data and is subject to the limitations of this source of evidence, making the results hypothesis-generating only. The classification of comorbidities, events and the use of advanced therapies was defined according to ICD-10 codes; therefore, misclassification of a few cases cannot be excluded. There is no single definition for major bleeding in the ICD-10 codes. As such, we used ICD-10 codes that topographically define major bleeding (e.g., intracranial, intraperitoneal, etc.), as well as ICD-10 codes for transfusion; therefore, we chose bleeding events that are per se defined as major. Furthermore, it is not certain whether the factors defining high risk were present before or after the PE. Even though adjustment for several clinical factors was performed, confounding by indication could influence the results of the analysis (thus, patients who did not receive reperfusion treatment might have been the sickest, at highest bleeding risk, or perhaps had bleeding already).
Conclusions
In elderly and frail patients with pulmonary embolism, the current use of classical reperfusion treatments (ST or SE) is low, even among high-risk PE hospitalisations, indicating physicians’ increasing fear of bleeding and perioperative complications. The use of CDT has increased and is emerging as a promising and safe alternative to classical reperfusion therapies for this vulnerable patient population.
Impact on daily practice
Reperfusion therapy is challenging in elderly patients with pulmonary embolism. The safety of catheter-directed therapies has not been evaluated in specific vulnerable populations. Catheter-directed therapies may be a safe alternative to classical reperfusion therapies for elderly and frail patients with PE requiring reperfusion treatment. Definite answers on the safety of catheter-directed treatments for elderly and frail patients with PE should be provided by adequately powered randomised controlled trials.
Conflict of interest statement
S. Barco received lecture/consultant fees from Bayer HealthCare, Concept Medical, BTG Pharmaceuticals, INARI, Boston Scientific, and LeoPharma; institutional grants from Boston Scientific, Bentley, Bayer HealthCare, INARI, Medtronic, Concept Medical, Bard, and Sanofi; and financial support for travel/congress costs from Daiichi Sankyo, BTG Pharmaceuticals, and Bayer HealthCare, outside the submitted work. G. Giannakoulas reports speaker or consulting fees from ELPEN Pharmaceuticals, Galenica, GlaxoSmithKline, Janssen Pharmaceutical Companies of Johnson & Johnson, and MSD, outside the submitted work. T. Tichelbäcker reports consultant fees from Bayer, SMT, AstraZeneca, and INARI Medical. I. Ahrens reports lecture/consultant fees from Bayer HealthCare, Boeringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, and Boston Scientific. S.V. Konstantinides reports institutional grants and personal lecture/advisory fees from Bayer AG, Daiichi Sankyo, and Boston Scientific; institutional grants from Penumbra Inc. and Lumira Dx; and personal lecture/advisory fees from MSD and Bristol-Myers Squibb/Pfizer. L. Hobohm received lecture/consultant fees from MSD, Johnson & Johnson, and Boston Scientific, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

