Abstract
Background: The use of atherectomy during peripheral endovascular interventions (PVI) has increased dramatically, but data regarding its safety and effectiveness are lacking.
Aims: This study sought to determine the long-term safety of atherectomy in contemporary practice.
Methods: Medicare fee-for-service beneficiaries who underwent femoropopliteal artery PVI from 2015-2018 were identified in a 100% sample of inpatient, outpatient, and carrier file data using procedural claims codes. The primary exposure was the use of atherectomy. Inverse probability of treatment weighting was used to adjust for measured differences in patient populations. Kaplan-Meier methods and multivariable Cox proportional hazards regression were used to compare outcomes.
Results: Among 168,553 patients who underwent PVI, 59,142 (35.1%) underwent atherectomy. The mean patient age was 77.0±7.6 years, 44.9% were female, 81.9% were white, and 46.7% had chronic limb-threatening ischaemia. Over a median follow-up time of 993 days (interquartile range 319-1,377 days), atherectomy use was associated with no difference in the risk of either the composite endpoint of death and amputation (adjusted hazard ratio [aHR] 0.99, 95% confidence interval [CI]: 0.97-1.01; p=0.19) or of major adverse limb events (aHR 1.02, 95% CI: 0.99-1.05; p=0.26). Patients who underwent atherectomy had a modest reduction in the risk of subsequently undergoing amputation or surgical revascularisation (aHR 0.92, 95% CI: 0.90-0.94; p<0.01) but an increase in the risk of undergoing a subsequent PVI (aHR 1.19, 95% CI: 1.16-1.21; p<0.01).
Conclusions: The use of atherectomy during femoropopliteal artery PVI was not associated with an increase in the risk of long-term adverse safety outcomes among patients with peripheral artery disease.
Introduction
Peripheral artery disease (PAD) affects over 200 million people worldwide, and its prevalence is increasing1. Advanced PAD has devastating consequences for patients, including limitations in mobility, foot pain, ulceration, gangrene, and limb amputations. Revascularisation is a critical component of the management of advanced PAD, as it may prevent amputation and improve quality of life. With the development of endovascular revascularisation, there have been dramatic changes in the strategies used for revascularisation of patients with PAD over the last 2 decades, with a significant increase in the use of an endovascular approach23.
As the scope of the percutaneous approach expands to treat more complex lesion subtypes, a need for innovation in endovascular devices, specifically for the management of heavily calcified stenoses, has arisen2. Vascular calcification is associated with worse long-term patency and may inhibit the impact of drug delivery4. As such, numerous atherectomy devices have been developed to modify lesions, to allow for adequate vessel expansion, to address device-uncrossable stenoses, and to facilitate drug transfer. As the number of devices on the market increases, the need to define the short- and long-term safety associated with their use grows.
In the United States (US), atherectomy use during peripheral endovascular intervention (PVI) has increased substantially over the last decade despite a lack of data supporting its long-term safety or clinical advantages over other methods, such as balloon angioplasty alone. For example, in the Vascular Quality Initiative, the proportion of PVI procedures performed with atherectomy increased by 64%: from 11% in 2010 to 18% in 20165. Similarly, in an analysis of Centers for Medicare & Medicaid Services (CMS) data, investigators found an increase in the age- and sex-adjusted procedure rates of atherectomies performed from 2006 to 2011, with a disproportionate increase in atherectomy use in the outpatient setting and a particularly high use among privately owned office-based laboratories67. Atherectomy is also being used more commonly in patients with milder disease, e.g., those with intermittent claudication58. Throughout the world, atherectomy has been adopted into vascular practice to a variable degree.
This increase in atherectomy use in the US has occurred in the context of CMS modifying the reimbursement structures for PVI in 2008. This policy change was designed to shift PVI use from the inpatient to the outpatient setting, with the goal of reducing total expenditures for PVI9. Along with this change, in 2011, the Current Procedural Terminology (CPT) coding system was updated to increase the reimbursement for PVI, particularly those utilising atherectomy10. The combined result of these changes was an increase in the total number of PVI, with a disproportionate increase in PVI involving atherectomy. The total expenditures for PVI have also increased over this time period, with a large portion attributable to the greater use of atherectomy7.
To date, there are insufficient and conflicting data regarding the long-term safety and effectiveness of atherectomy during PVI1112131415. Safety data are critically needed as the use of atherectomy devices has become pervasive. Therefore, this study was designed to evaluate the long-term safety of atherectomy among a contemporary cohort of Medicare patients with PAD undergoing femoropopliteal artery PVI in both inpatient and outpatient settings.
Methods
Study population
Medicare fee-for-service (FFS) beneficiaries ≥66 years of age who underwent PVI of the femoropopliteal arterial segment between 1 April 2015 and 31 December 2018 were included in the study. Inpatient procedures were only included after 1 October 2015 due to the change to the International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) claims codes, which have more specific coding for atherectomy. Patients were excluded if they had less than 1 year of Medicare claims data prior to their index procedure. Patients were also excluded if they were treated at a private non-institutional clinic (i.e., office-based lab or ambulatory surgical centre) due to differences in claims data and reimbursement. Inpatient procedures were identified in the Medicare Provider Analysis and Review files using ICD-10-PCS codes (Supplementary Table 1). Patients who underwent outpatient PVI were identified in a 100% sample of the carrier FFS files using CPT codes. For patients who underwent multiple procedures during the study period, the first procedure was defined as the index procedure and subsequent procedures were considered reinterventions.
Primary exposure
The use of atherectomy was identified among patients who underwent outpatient procedures through specific CPT procedural billing claims codes that include atherectomy use (Supplementary Table 1). Among patients undergoing in-hospital procedures, specific ICD-10-PCS claims codes for atherectomy (04CK3ZZ, 04CL3ZZ, 04CM3ZZ, 04CN3ZZ) were used. Different types of atherectomy devices could not be identified using claims codes.
Baseline characteristics
Baseline sociodemographics were ascertained as of the index procedure date. Patient comorbidities were determined using the Chronic Conditions Data Warehouse. It includes data on 27 comorbidities established using a lookback period of 1 to 3 years and involving claims from multiple clinical sites (i.e., inpatient and outpatient medical claims)16. In addition to these comorbidities, International Classification of Diseases, 9th and 10th Revision, Clinical Modification (ICD-9-CM, ICD-10-CM) claims codes were applied over a 1-year lookback period to identify current or prior tobacco use, chronic limb-threatening ischaemia (CLTI), and prior amputation (Supplementary Table 2). Procedural characteristics included balloon angioplasty (drug-coated and uncoated balloons), stent placement (bare metal and drug-eluting), and procedural setting (inpatient or outpatient). Hospital characteristics were retrieved from the 2016 American Heart Association Annual Survey file which includes hospital teaching status, region, and bed capacity. In addition, the femoropopliteal artery revascularisation procedure volumes of each institution during the study period were computed.
Outcomes
The primary outcomes were major adverse limb events (MALE) and the composite of all-cause death and amputation. MALE included amputation and thrombosis/embolism. Amputations included any amputation of the lower extremity through the forefoot but excluded minor amputations of individual toes. Major amputations were those that occurred at the ankle and more proximally. Minor amputations were distal to the ankle and included toe amputations. Secondary endpoints included all-cause death, all-cause readmission, amputation, major amputation, minor amputation, surgical revascularisation, amputation or surgical revascularisation, and repeat endovascular revascularisation. Both surgical revascularisation and repeat endovascular procedures could involve either leg, as coding was not specific enough to evaluate target vessel revascularisation. Three falsification endpoints were used to evaluate the possibility of residual confounding between treatment groups: hospitalisation for myocardial infarction, congestive heart failure, and pneumonia17.
Statistical methods
All metrics and normally distributed variables were reported as means±standard deviations and compared using the Student’s t-test. Categorical variables were presented as frequencies and percentages and compared using the chi-square test. Standardised mean differences (SMD) were calculated to compare characteristics; values of greater than 0.01 represented meaningful differences between groups18. Kaplan-Meier methods were used to estimate the cumulative incidence of events for each group. Log-rank tests were used for between-group comparisons.
Inverse probability of treatment weighting was used to account for differences in measured characteristics (Supplementary Table 3). In the first step, a propensity score model was fit, connecting treatment exposure (atherectomy versus no atherectomy) with patient, procedure and hospital characteristics. The probability of receiving atherectomy, or the propensity score, p, was then computed. The weight variable, w, was then defined as 1/p for subjects in the atherectomy group and as 1/(1-p) in the non-atherectomy group. Next, a Cox regression model was fit with group membership as the only covariate. Statistical inference was performed using the bootstrap method. For outcomes that did not include death, the Fine-Gray method was used to account for the competing risk of death 19. Prespecified subgroup analyses included female patients, procedural setting (outpatient vs inpatient), advanced age (≤75 vs >75 years), presence of chronic kidney disease, and disease severity (CLTI vs non-CLTI).
A p-value of less than 0.05 was considered significant. SAS statistical software version 9.4 (SAS Institute) was used for all analyses. The Institutional Review Board at the Beth Israel Deaconess Medical Center evaluated this study and waived the need for approval because human subject research was not involved.
Results
Patient characteristics
The study cohort included 168,553 patients who underwent PVI, 35.1% (N=59,142) of whom underwent atherectomy. The mean age of the PVI cohort was 77.0±7.6 years, 44.9% were female, and 81.9% were white (Table 1). CLTI was present in 46.7% of patients, 7.9% had undergone a prior amputation, and 49.0% were current or prior tobacco users.
There were no major differences in comorbidities between patients who were treated with atherectomy and those who were not (Table 1). Specifically, the rates of prior myocardial infarction, chronic kidney disease, diabetes, ischaemic heart disease, stroke/transient ischaemic attack, CLTI, and tobacco use were not significantly different between the 2 groups (SMD <0.1). After adjustment, all SMD were less than 0.01.
Table 1. Baseline characteristics of patients who underwent femoropopliteal endovascular revascularisation stratified by receiving atherectomy.
| Total population (168,553) |
No atherectomy (N=109,411) |
Atherectomy (N=59,142) |
SMD | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 77.02±7.59 | 77.18±7.76 | 76.73±7.45 | 0.06 | |
| Female | 75,744 (44.9) | 49,485 (45.2) | 26,259 (44) | 0.02 | |
| Race | White | 138,026 (81.9) | 90,261 (82.5) | 47,766 (80.8) | 0.04 |
| Black | 21,765 (12.9) | 13,533 (12.4) | 8,232 (13.9) | 0.05 | |
| Asian | 1,500 (0.9) | 986 (0.9) | 514 (0.9) | 0.00 | |
| Hispanic | 4,086 (2.4) | 2,561 (2.3) | 1,525 (2.6) | 0.02 | |
| Native | 1,115 (0.7) | 684 (0.6) | 431 (0.7) | 0.01 | |
| Other | 2,061 (1.2) | 1,387 (1.3) | 673 (1.1) | 0.01 | |
| Comorbidities | |||||
| Alzheimer’s dementia | 28,904 (17.1) | 19,125 (17.5) | 9,779 (16.5) | 0.03 | |
| Alzheimer’s disease | 7,636 (4.5) | 4,961 (4.5) | 2,675 (4.5) | 0.00 | |
| Anaemia | 77,592 (46.0) | 50,678 (46.3) | 26,914 (45.5) | 0.02 | |
| Asthma | 9,989 (5.9) | 6,586 (6.0) | 3,403 (5.8) | 0.01 | |
| Atrial fibrillation | 31,051 (18.4) | 20,407 (18.7) | 10,644 (17.0) | 0.02 | |
| Breast cancer | 4,264 (2.5) | 2,847 (2.6) | 1,417 (2.4) | 0.01 | |
| Cataract | 23,563 (14.0) | 15,011 (13.7) | 8,552 (14.5) | 0.02 | |
| CHF | 63,796 (37.8) | 41,614 (38.0) | 22,182 (37.5) | 0.01 | |
| CKD | 84,519 (50.1) | 55,008 (50.3) | 29,511 (49.9) | 0.01 | |
| CLTI | 78,665 (46.7) | 52,042 (47.6) | 26,623 (45.0) | 0.05 | |
| Colorectal cancer | 3,294 (2.0) | 2,198 (2.0) | 1,096 (1.9) | 0.01 | |
| COPD | 49,949 (29.6) | 32,296 (29.5) | 17,653 (29.8) | 0.01 | |
| Current or prior tobacco use | 82,554 (49.0) | 54,267 (49.6) | 28,287 (47.8) | 0.04 | |
| Depression | 36,501 (21.7) | 24,011 (21.9) | 12,490 (21.1) | 0.02 | |
| Diabetes | 85,880 (51.0) | 54,960 (50.2) | 30,920 (52.3) | 0.04 | |
| Endometrial cancer | 638 (0.4) | 425 (0.4) | 213 (0.4) | 0.00 | |
| Glaucoma | 13,103 (7.8) | 8,293 (7.6) | 4,810 (8.1) | 0.02 | |
| Hip fracture | 2,390 (1.4) | 1,600 (1.5) | 790 (1.3) | 0.01 | |
| Hyperlipidaemia | 118,567 (70.3) | 75,562 (69.1) | 43,005 (72.7) | 0.08 | |
| Hyperparathyroidism | 21,676 (12.9) | 14,112 (12.9) | 7,564 (12.8) | 0.00 | |
| Hyperthyroidism | 135,272 (80.3) | 86,759 (79.3) | 48,513 (82.0) | 0.07 | |
| Hypothyroidism | 29,418 (17.5) | 19,045 (17.4) | 10,373 (17.5) | 0.00 | |
| Ischaemic heart disease | 110,100 (65.3) | 70,357 (64.3) | 39,743 (67.2) | 0.06 | |
| Lung cancer | 3,630 (2.2) | 2,348 (2.1) | 1,282 (2.2) | 0.00 | |
| MI | 7,748 (4.6) | 5,168 (4.7) | 2,580 (4.3) | 0.02 | |
| Osteoporosis | 12,524 (7.4) | 8,301 (7.6) | 4,223 (7.1) | 0.02 | |
| Prior amputation | 13,296 (7.9) | 9,232 (8.4) | 4,064 (6.9) | 0.06 | |
| Prostate cancer | 7,625 (4.5) | 4,868 (4.4) | 2,757 (4.7) | 0.01 | |
| Rheumatoid/osteoarthritis | 66,730 (39.6) | 42,951 (39.3) | 23,779 (40.2) | 0.02 | |
| Stroke/TIA | 15,715 (9.3) | 10,175 (9.3) | 5,540 (9.4) | 0.00 | |
| Geography | |||||
| Northeast | 7,234 (4.3) | 5,535 (5.1) | 1,700 (2.9) | 0.11 | |
| Mid-Atlantic | 21,927 (13.0) | 15,180 (13.9) | 6,749 (11.4) | 0.07 | |
| Central Northeast | 29,325 (17.4) | 18,890 (17.3) | 10,434 (17.6) | 0.01 | |
| Central Southeast | 13,429 (8.0) | 8,032 (7.3) | 5,396 (9.1) | 0.06 | |
| Central Northwest | 12,979 (7.7) | 8,898 (8.1) | 4,082 (6.9) | 0.05 | |
| Central Southwest | 23,140 (13.7) | 13,134 (12.0) | 10,003 (16.9) | 0.14 | |
| South Atlantic | 35,243 (20.9) | 22,716 (20.8) | 12,527 (21.2) | 0.01 | |
| Mountain | 8,483 (5.0) | 5,136 (4.7) | 3,346 (5.7) | 0.04 | |
| Pacific | 16,034 (9.5) | 11,306 (10.3) | 4,730 (8.0) | 0.08 | |
| Other regions | 758 (0.4) | 584 (0.5) | 175 (0.3) | 0.04 | |
| Hospital characteristics | |||||
| 6-24 beds | 147 (0.1) | 93 (0.1) | 55 (0.1) | 0.00 | |
| 25-49 beds | 1,414 (0.8) | 788 (0.7) | 625 (1.1) | 0.04 | |
| 50-99 beds | 7,110 (20.1) | 4,249 (3.9) | 2,860 (4.8) | 0.05 | |
| 100-199 beds | 27,153 (16.1) | 16,467 (15.1) | 10,684 (18.1) | 0.08 | |
| 200-299 beds | 34,342 (20.4) | 22,157 (20.3) | 12,185 (20.6) | 0.01 | |
| 300-399 beds | 31,470 (18.7) | 19,832 (18.1) | 11,638 (19.7) | 0.04 | |
| 400-499 beds | 19,263 (11.4) | 12,775 (11.7) | 6,488 (11.0) | 0.02 | |
| ≥500 beds | 47,655 (28.3) | 33,051 (30.2) | 14,607 (24.7) | 0.12 | |
| Teaching hospital | 122,061 (72.4) | 81,895 (74.9) | 40,170 (67.9) | 0.15 | |
| Procedural characteristics | |||||
| Inpatient procedure | 80,228 (47.6) | 55,640 (50.9) | 24,588 (41.6) | 0.19 | |
| Stent implantation | 69,122 (41.0) | 50,268 (45.9) | 18,854 (31.9) | 0.29 | |
| Bare metal stent | 39,779 (23.6) | 30,197 (27.6) | 9,522 (16.1) | 0.28 | |
| Drug-eluting stent | 29,396 (17.4) | 20,081 (18.4) | 9,315 (15.8) | 0.07 | |
| Drug-coated balloon | 70,584 (41.9) | 40,609 (37.1) | 29,975 (50.7) | 0.28 | |
| Age is expressed as mean±standard deviation. All other values are n (%). *SMD >0.1 is considered significant. CHF: congestive heart failure; CKD: chronic kidney disease; CLTI: chronic limb-threatening ischaemia; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; SMD: standardised mean difference; TIA: transient ischaemic attack | |||||
Practice setting and regional variation
Regions with the greatest proportional use of atherectomy included the Central Southwest (43.2% of PVI), Central Southeast (40.2% of PVI), and Mountain (39.4% of PVI). The Northeast region had the lowest proportional use of atherectomy (23.5% of PVI). Smaller hospitals used more atherectomy than larger hospitals, with atherectomy being used in nearly 40% of all PVI among hospitals with fewer than 200 beds. In addition, non-teaching hospitals used more atherectomy (40.8% of PVI) compared with teaching hospitals (32.9% of PVI) (Table 1).
Outcomes
The median follow-up was 993 days (interquartile range [IQR] 319-1,377). Prior to adjustment, atherectomy was associated with a lower risk of both primary outcomes: MALE (3-year cumulative incidence: 11.38% for atherectomy vs 12.62% for no atherectomy, hazard ratio [HR] 0.89, 95% confidence interval [CI]: 0.86-0.92; p<0.01) and the composite outcome of death and amputation (3-year cumulative incidence: 41.85% for atherectomy vs 45.56% for no atherectomy, HR 0.88, 95% CI: 0.87-0.90; p<0.01) (Table 2, Figure 1). Patients who underwent atherectomy had a lower unadjusted risk of amputation, surgical revascularisation, and either amputation or surgical revascularisation (Table 2, Supplementary Table 4). Conversely, patients treated with atherectomy had a higher unadjusted risk of subsequent endovascular procedures to either leg (Table 2). There were lower unadjusted risks of all-cause readmission and all-cause death (Table 2). In terms of the falsification endpoints, prior to adjustment, there was a lower risk of congestive heart failure among those treated with atherectomy, but there was no difference in the risk of myocardial infarction or pneumonia for patients who underwent PVI with or without atherectomy (Table 2).
After adjustment, there was no difference in the risk of the composite outcome of death and amputation (3-year cumulative incidence: 43.77% for atherectomy vs 44.30% for no atherectomy, adjusted hazard ratio [aHR] 0.99, 95% CI: 0.97-1.01; p=0.19) or MALE (3-year cumulative incidence: 12.15% for atherectomy vs 12.22% for no atherectomy, aHR 1.02, 95% CI: 0.99-1.05; p=0.26) among patients who underwent PVI with atherectomy versus PVI without atherectomy. After adjustment, the reduction in the risk of amputation (3-year cumulative incidence: 13.15% for atherectomy vs 13.99% for no atherectomy, aHR 0.94, 95% CI: 0.91-0.97; p<0.01), surgical revascularisation (3-year cumulative incidence: 8.30% for atherectomy vs 9.20% for no atherectomy, aHR 0.89, 95% CI: 0.86-0.92; p<0.01), and amputation or surgical revascularisation associated with atherectomy remained (3-year cumulative incidence: 37.00% for atherectomy vs 33.60% for no atherectomy; aHR 0.92, 95% CI: 0.90-0.94; p<0.01) (Table 2, Figure 1). After adjustment, atherectomy remained associated with a higher risk of subsequent endovascular procedures (3-year cumulative incidence: 33.28% for atherectomy and 20.06% for no atherectomy, aHR 1.19, 95% CI: 1.16-1.21; p<0.01) (Table 2, Figure 2). There were no differences in the risk of the falsification endpoints of myocardial infarction and pneumonia between treatment groups and there was an attenuation of the association between atherectomy use and congestive heart failure (Table 2).
Table 2. Unadjusted and adjusted analyses for primary and secondary endpoints comparing patients who underwent PVI with atherectomy compared with those who underwent PVI without atherectomy.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Primary endpoints | ||||
| Composite of death and amputation | 0.88 (0.87-0.90) | <0.01 | 0.99 (0.97-1.01) | 0.19 |
| MALE | 0.89 (0.86-0.92) | <0.01 | 1.02 (0.99-1.05) | 0.26 |
| Secondary endpoints | ||||
| All-cause death | 0.92 (0.90-0.93) | <0.01 | 1.00 (0.98-1.01) | 0.85 |
| Amputation | 0.82 (0.80-0.85) | <0.01 | 0.94 (0.91-0.97) | <0.01 |
| Amputation or surgical revascularisation | 0.82 (0.80-0.84) | <0.01 | 0.92 (0.90-0.94) | <0.01 |
| Surgical revascularisation | 0.80 (0.77-0.83) | <0.01 | 0.89 (0.86-0.92) | <0.01 |
| Surgical or endovascular revascularisation | 1.16 (1.14-1.18) | <0.01 | 1.13 (1.11-1.15) | <0.01 |
| Endovascular revascularisation | 1.25 (1.22-1.27) | <0.01 | 1.19 (1.16-1.21) | <0.01 |
| Major amputation | 0.83 (0.80-0.86) | <0.01 | 0.95 (0.91-0.98) | <0.01 |
| Minor amputation | 0.82 (0.79-0.86) | <0.01 | 0.93 (0.89-0.96) | <0.01 |
| All-cause readmission | 0.92 (0.90-0.93) | <0.01 | 0.98 (0.97-1.00) | 0.04 |
| Myocardial infarction | 0.96 (0.93-0.99) | 0.02 | 0.98 (0.95-1.02) | 0.34 |
| Congestive heart failure | 0.93 (0.92-0.95) | <0.01 | 0.98 (0.96-1.00) | 0.02 |
| Pneumonia | 0.99 (0.96-1.01) | 0.32 | 1.01 (0.99-1.04) | 0.37 |
| CI: confidence interval; MALE: major adverse limb events; PVI: peripheral endovascular intervention | ||||
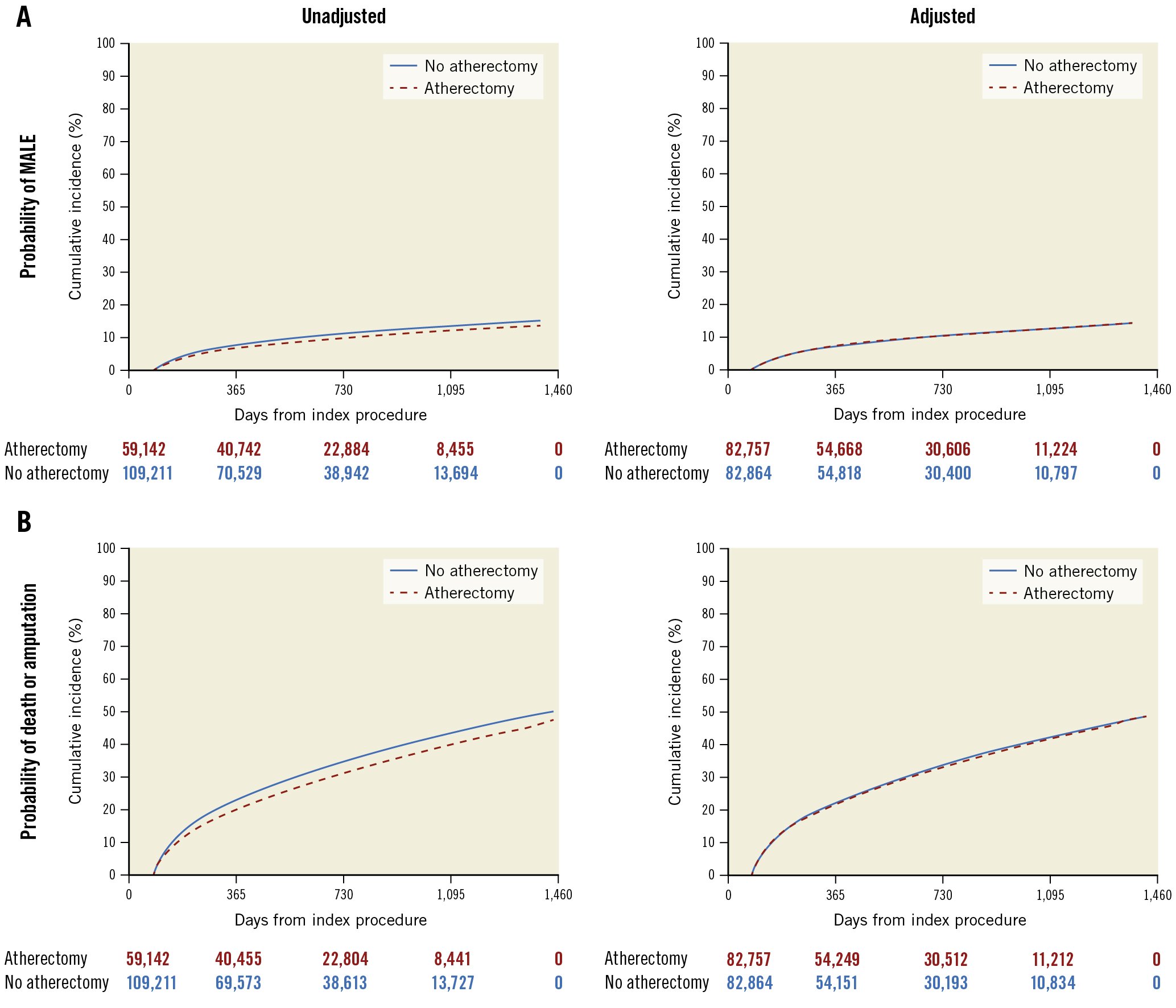
Figure 1. Unadjusted and adjusted risk of MALE and a composite of amputation and death among patients who underwent PVI with or without atherectomy. Kaplan-Meier curves demonstrating that there was a lower unadjusted risk of major adverse limb events (MALE) and no difference in the adjusted risk of MALE over time in patients who underwent atherectomy compared with those who did not undergo atherectomy (A). Prior to adjustment, there was a lower risk of the composite endpoint of death and amputation over time in patients who underwent atherectomy compared with those who did not undergo atherectomy. After adjustment, there was no difference in the composite endpoint of death and amputation over time among patients who underwent atherectomy versus no atherectomy (B). PVI: peripheral endovascular intervention
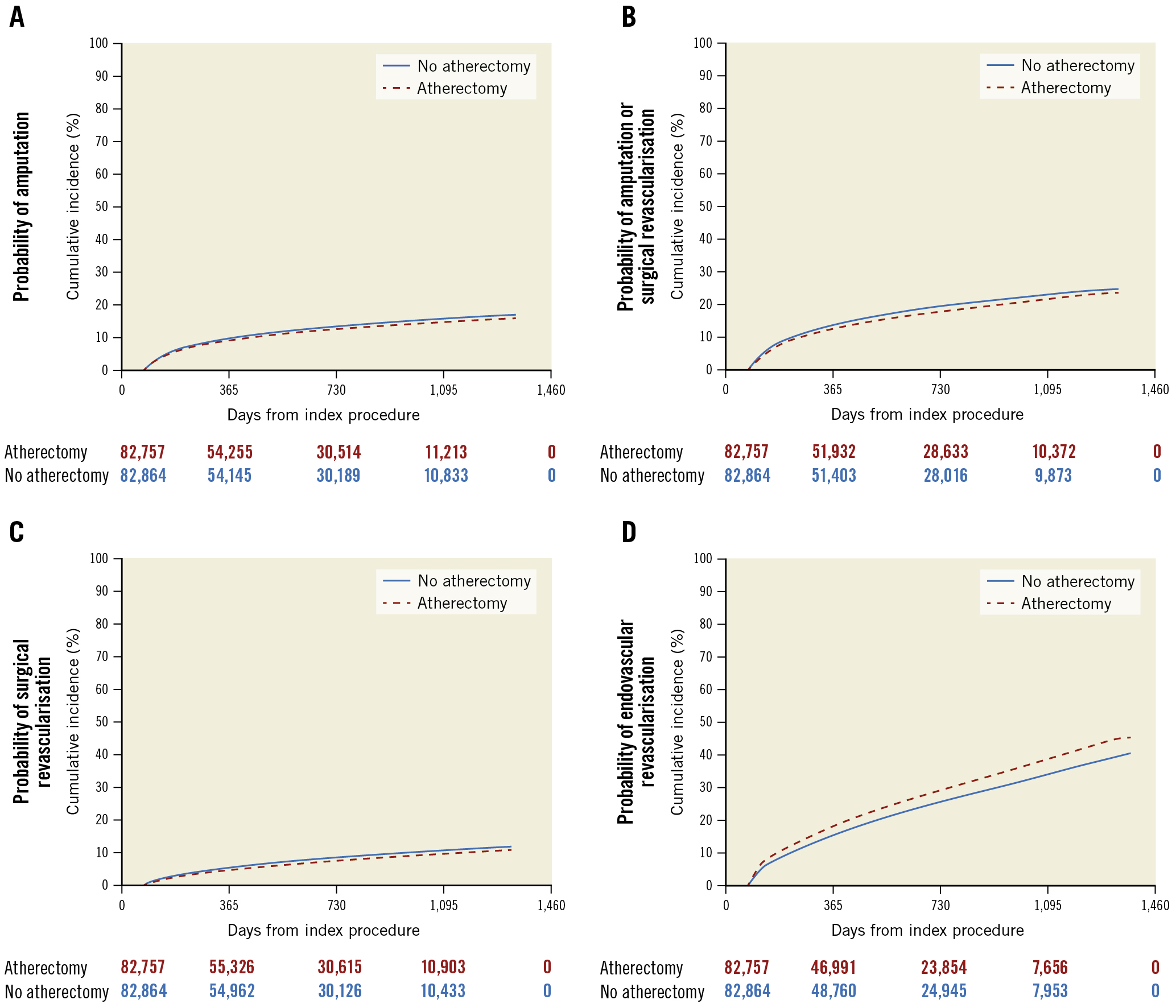
Figure 2. Adjusted risk of subsequent procedures in patients who underwent PVI with or without atherectomy. Kaplan-Meier curves of secondary endpoints including amputation (A), the combined endpoint of amputation and surgical revascularisation (B), surgical revascularisation (C), and endovascular revascularisation (D) among patients who underwent atherectomy versus no atherectomy. Patients who underwent atherectomy had a modestly lower risk of amputation, surgical revascularisation, and the composite endpoint of amputation and surgical revascularisation. Patients who underwent atherectomy had a significant increase in the risk of a subsequent endovascular procedure compared with those who did not undergo atherectomy. PVI: peripheral endovascular intervention
Subgroup analyses
Among the prespecified subgroups, there was no association between the use of atherectomy and death for any subgroup. Outpatients who underwent PVI with atherectomy had a modest reduction in the risk of the composite endpoint of all-cause death and amputation, whereas inpatient PVI with atherectomy was associated with a modestly increased risk (Table 3). Women had a modest increase in the risk of the composite endpoint (aHR 1.03, 95% CI: 1.00-1.05; p=0.04). There was no association between atherectomy use and the composite endpoint of all-cause death and amputation by age (≤75 vs >75), PAD severity (CLTI vs non-CLTI), chronic kidney disease, or diabetes (Table 3).
Table 3. Adjusted risks of outcomes among key subgroups.
| Subgroup analyses | All-cause death | Composite of death and amputation | Amputation | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age >75 | 1.00 (0.98-1.02) | 0.89 | 1.00 (0.98-1.02) | 1.00 | 0.92 (0.88-0.96) | <0.01 |
| Age ≤75 | 1.00 (0.98-1.03) | 0.77 | 1.00 (0.97-1.03) | 1.00 | 0.99 (0.95-1.04) | 0.81 |
| CKD | 1.00 (0.98-1.02) | 0.89 | 1.00 (0.98-1.02) | 0.83 | 0.96 (0.93-1.00) | 0.06 |
| Diabetes | 0.99 (0.97-1.01) | 0.25 | 0.99 (0.97-1.01) | 0.42 | 0.96 (0.93-1.00) | 0.05 |
| Women | 1.01 (0.99-1.03) | 0.34 | 1.03 (1.00-1.05) | 0.04 | 0.97 (0.93-1.02) | 0.27 |
| Inpatients | 1.02 (0.96-1.04) | 0.13 | 1.03 (1.01-1.05) | 0.01 | 0.98 (0.94-1.02) | 0.31 |
| Outpatients | 0.98 (0.95-1.00) | 0.02 | 0.95 (0.93-0.98) | <0.01 | 0.90 (0.86-0.95) | <0.01 |
| Non-CLTI PAD | 1.00 (0.98-1.03) | 0.61 | 1.02 (1.00-1.05) | 0.88 | 0.96 (0.93-0.99) | 0.01 |
| CLTI | 1.00 (0.98-1.02) | 0.67 | 0.98 (0.96-1.00) | 0.07 | 0.93 (0.90-0.96) | <0.01 |
| CI: confidence interval; CKD: chronic kidney disease; CLTI: chronic limb-threatening ischaemia; PAD: peripheral artery disease | ||||||
Discussion
The global impact of PAD is significant and is increasing over time1. Defining safe and effective revascularisation approaches for complex PAD is critical. In this nationwide, longitudinal analysis of Medicare patients undergoing PVI with atherectomy of the femoropopliteal arterial segment, we found that PVI with atherectomy was not associated with a higher risk of death, amputation, MALE, or the need for surgical revascularisation compared with PVI without atherectomy over a median follow-up of approximately 2.7 years (Central illustration). Atherectomy also appeared safe in several critical subgroups, including elderly patients and those with diabetes, chronic kidney disease, and CLTI.
Peripheral atherectomy has been an adjunct during endovascular intervention for several decades, yet its use remains variable. In the US, the frequency of use has been high, but primarily in privately owned clinics secondary to reimbursement incentives5678. However, this pattern is not seen among hospitalised patients, and, globally, practice patterns in atherectomy are highly variable by country and region. Notably, this increase in atherectomy utilisation in the US has occurred without robust evidence supporting the effectiveness of atherectomy or comparative data supporting the clinical advantages of atherectomy over other approaches. For instance, in a meta-analysis of 4 randomised controlled trials comprising 220 patients comparing PVI with and without atherectomy, there was no benefit of atherectomy in terms of primary patency at 6 and 12 months or target lesion revascularisation11.
In addition to effectiveness, the appraisal of the long-term safety of atherectomy versus other revascularisation strategies in broad, real-world patients has, to date, been limited. Many global studies of atherectomy devices have demonstrated overall low event rates, but these have primarily been single-armed studies with relatively short-term endpoints202122. Our study is unique as it demonstrates that peripheral atherectomy appears safe beyond 1 year after the index procedure, with similar rates of amputation-free survival and a lower risk of subsequent surgical procedures. These findings are important as patients with claudication, who have overall low risks of amputation, were included in the study population. As such, any signal of harm would be meaningful. Critically, the falsification endpoints were negligibly different between treatment groups after adjustment, suggesting a low likelihood of residual confounding. These findings persisted in key subgroups, including in the outpatient setting, which has seen the greatest growth in atherectomy use.
These results differ from a prior US study involving patients in the Vascular Quality Initiative registry, which reported higher rates of amputations among patients who received atherectomy compared with those who received percutaneous transluminal angioplasty alone, as well as more limb events with atherectomy relative to stenting12. There are several reasons why the findings differed between that analysis and ours. First, the prior study included patients treated for lesions in multiple arterial segments (iliac, femoropopliteal, and tibial), whereas our study focused on patients treated primarily for lesions in the femoropopliteal segment. In addition, this prior study did not allow for combined procedures such as atherectomy and stenting, which are commonly used in clinical practice. Furthermore, the prior study was performed during an earlier time period when atherectomy was shifting from the inpatient to the outpatient setting. Thus, the population who received atherectomy at that time may have been markedly different from those treated in the present study. Finally, the prior study involved procedures from a predominantly surgical registry, which may have selected a more complex patient population compared with the broad population in the present study. A strength of this analysis was that the database included more granular procedural data. Interestingly, a different analysis that examined outcomes of interventional strategies in patients with CLTI demonstrated lower rates of amputation in patients treated with atherectomy compared with those treated with percutaneous transluminal angioplasty, stenting, or surgical bypass13.
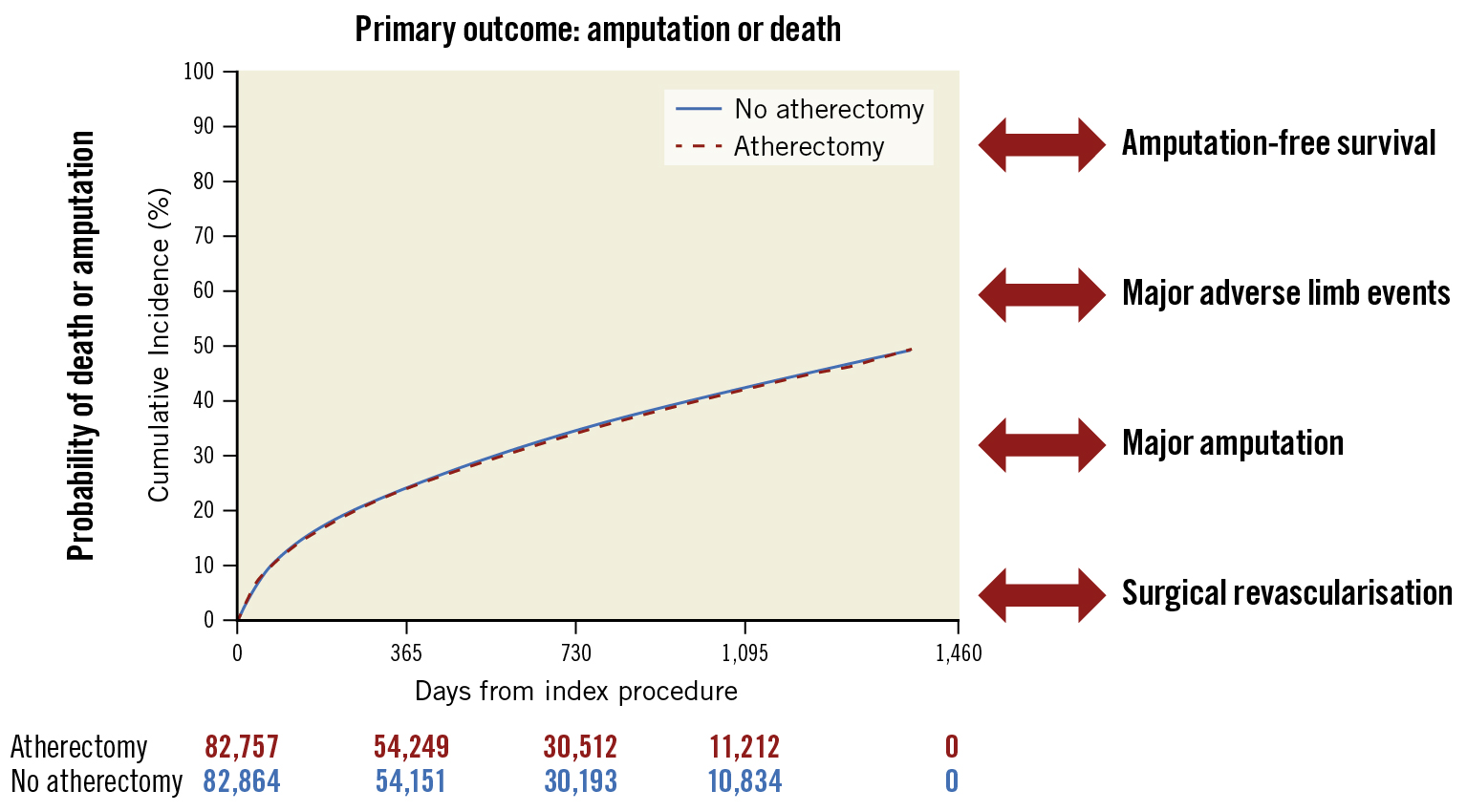
Central illustration. Long-term outcomes following peripheral atherectomy. Kaplan-Meier curve demonstrating that, after adjustment, there is no increase in risk of the composite outcome of amputation or death in patients who underwent PVI with atherectomy compared with those who underwent PVI without atherectomy. Outcomes are equivocal for amputation-free survival, major adverse limb events, major amputation, and surgical revascularisation. PVI: peripheral endovascular intervention
Limitations
There are several limitations of this study. First, this is a retrospective, observational study. As such, patients were not randomised to PVI with or without atherectomy and, thus, the analysis could be subject to unmeasured confounding. However, we used falsification endpoints to evaluate the impact of residual imbalances between groups, and these associations were negligible. Second, the population in our study was primarily white and elderly, and, therefore, the results may not be applicable to the general population. Third, Medicare claims data do not provide information regarding the specific atherectomy device type used or anatomic factors and lesion characteristics that may have influenced the selection of atherectomy. As such, this analysis was unable to assess whether different types of atherectomy yielded different outcomes. In addition, this analysis is unable to further clarify clinical questions such as which lesion characteristics may be best suited to treatment with atherectomy in general or to treatment with specific atherectomy subtypes. Fourth, although insurance claims data lack the granularity to track procedural events like distal embolisation, the major clinical sequelae of these events, including arterial thrombosis/embolism and amputation, are captured and should reflect any major consequence of such events. Last, we excluded atherectomy procedures performed in private office-based labs, and it is possible that this practice setting may have distinct patient outcomes.
Conclusions
In this large analysis of Medicare patients treated with femoropopliteal artery PVI, atherectomy was not associated with an increased risk of adverse outcomes over time, including amputation, the composite of death and amputation, and major adverse limb events. These findings remained consistent in high-risk subgroups, including the elderly and patients with chronic kidney disease, diabetes, or CLTI. In addition, among patients treated in the outpatient setting, there were no additional long-term risks associated with atherectomy use. Further studies are needed to establish the clinical effectiveness of atherectomy during PVI.
Impact on daily practice
This analysis in a large, nationwide cohort demonstrated that the use of atherectomy was not associated with a higher risk of adverse safety outcomes, including amputation and a composite of amputation and death, compared with PVI without atherectomy. Further research is needed to determine whether there is a clinical benefit of atherectomy and to define the patient populations that derive the greatest benefit from atherectomy.
Funding
This work was funded in part by a research grant to the Beth Israel Deaconess Medical Center from Cardiovascular Systems, Inc., (CSI). CSI had no participation in the design of the study, analysis, drafting of the manuscript or decision to publish. Dr. Secemsky is funded by NIH/NHLBI K23HL150290 and Harvard Medical School’s Shore Faculty Development Award.
Conflict of interest statement
W. Schuyler Jones reports research grants from Boehringer Ingelheim, Bristol-Myers Squibb, the Doris Duke Charitable Foundation, Merck, the National Institutes of Health, and the Patient-Centered Outcomes Research Institute; and honoraria for advisory committees from Bayer, Bristol-Myers Squibb, and Janssen Pharmaceuticals. P. Schneider is a member of consulting/scientific advisory boards at Boston Scientific, Medtronic, CSI, Surmodics, Philips, Silk Road, PQ Bypass, Cagent, and LimFlow. C. Shen is an employee at Biogen. M. Schermerhorn is a consultant for Abbott Vascular and Medtronic. E.A. Secemsky is a member of consulting/scientific advisory boards at Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Inari Medical, Janssen, Medtronic, Philips, and VentureMed; and has received research grants from AstraZeneca, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic, and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

