Abstract
There is a growing clinical and scientific interest in catheter-directed therapy (CDT) of acute pulmonary embolism (PE). Currently, CDT should be considered for patients with high-risk PE, in whom thrombolysis is contraindicated or has failed. Also, CDT is a treatment option for initially stable patients in whom anticoagulant treatment fails, i.e., those who experience haemodynamic deterioration despite adequately dosed anticoagulation. However, the definition of treatment failure (primary reperfusion therapy or anticoagulation alone) remains an important area of uncertainty. Moreover, several techniques for CDT are available without evidence supporting one over the other, and variation in practice with regard to periprocedural anticoagulation is considerable. The aim of this position paper is to describe the currently available CDT approaches in PE patients and to standardise patient selection, the timing and technique of the procedure itself as well as anticoagulation regimens during CDT. We discuss several clinical scenarios of the clinical evaluation of the “efficacy” of thrombolysis and anticoagulation, including treatment failure with haemodynamic deterioration and treatment failure based on a lack of improvement. This clinical consensus statement serves as a practical guide for CDT, complementary to the formal guidelines.
Introduction
Clinical manifestations of acute pulmonary embolism (PE) vary, ranging from incidentally diagnosed PE without apparent symptoms to obstructive shock with multisystem organ failure, or even sudden cardiac death1. According to the 2019 European Society of Cardiology (ESC) guidelines on acute PE, reperfusion therapy, preferably systemic thrombolysis, is recommended as the first-line treatment in haemodynamically unstable patients. Reperfusion therapy should also be considered when initially normotensive PE subjects progress to a state of haemodynamic deterioration despite adequate anticoagulation1. However, it has been estimated that more than half of high-risk PE patients do not receive systemic thrombolysis due to a perceived increased risk of bleeding23. In such cases, a surgical embolectomy or catheter-directed therapy (CDT) should be considered1. The increasing use of CDT for the treatment of acute PE over the past decade has been paralleled by a growing scientific and clinical interest in this method, both in Europe and the USA45. Expert multidisciplinary Pulmonary Embolism Response Teams (PERT) are being established widely to standardise and improve the care for PE patients6, and a wide range of mechanical systems for CDT has become available7.
The aim of this clinical consensus statement is to describe the currently available CDT approaches in PE patients and to standardise patient selection, the timing and technique of the procedure itself as well as anticoagulation regimens during CDT.
Indications for catheter-directed therapies – a practical approach to patient selection and the timing of the procedure
According to the current European Society of Cardiology guidelines, the optimal management of acute PE depends on an adequate risk assessment of PE-related early death, which should be assessed in all PE patients (Table 1)1. Early PE-related death in high-risk PE exceeded 15% in some cohort studies, with most deaths occurring during the first hours after admission89. Therefore, urgent systemic thrombolysis is recommended as the treatment of choice1. Additionally, patients with high-risk PE may require haemodynamic or respiratory support, e.g., with intravenous vasopressors, mechanical ventilation, or venoarterial extracorporeal membrane oxygenation (ECMO)10111213.
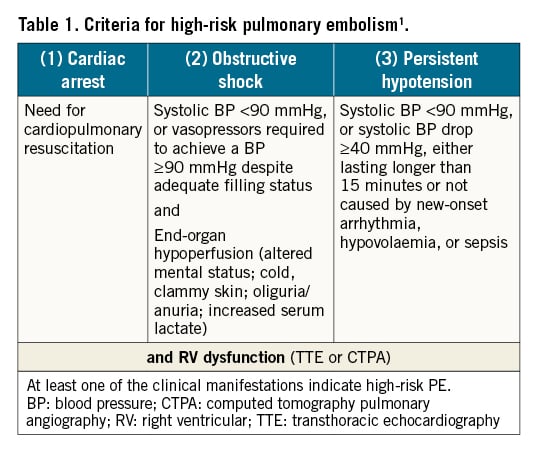
Intermediate-high risk PE patients are characterised by signs of right ventricular dysfunction (RVD) on echocardiography or computed tomography pulmonary angiography (CTPA), in combination with elevated troponin levels demonstrating myocardial injury. Therapeutic anticoagulation alone, without reperfusion treatment, is sufficient in most such cases. However, some patients may deteriorate as a result of progressive right ventricular (RV) failure. The randomised controlled Pulmonary Embolism International THrOmbolysis (PEITHO) trial showed that 5.0% of initially anticoagulated patients suffered haemodynamic decompensation and/or died, mostly within the first 72 hours after their admission, and required rescue reperfusion treatment14. Notably, this number was lower, at 1.4%, in the single-arm PEITHO-2 study15. Cohort data suggest that early mortality may reach 5-10% in initially normotensive patients with RVD and myocardial injury16. This notable range in the incidence of adverse events is likely caused by differences in the study design and patient selection and underlines the need for randomised controlled trials to establish the optimal treatment of patients with intermediate-high risk PE17. Table 2 summarises the most relevant indicators of early PE-related death and haemodynamic deterioration in patients with non-high-risk PE. Initially normotensive patients at an elevated risk for haemodynamic deterioration require close monitoring during the early stage of hospitalisation1.
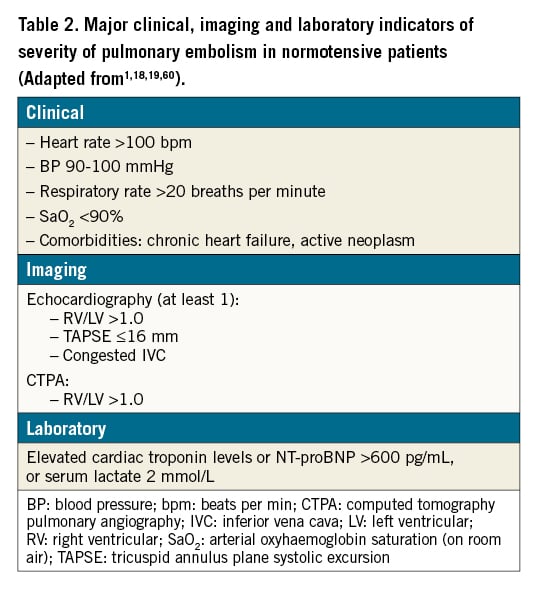
Post hoc data analysis of the PEITHO trial identified clinical parameters that may correlate with all-cause mortality, haemodynamic collapse and recurrent PE among anticoagulated patients with intermediate-high risk PE18. These included a systolic blood pressure ≤110 mmHg, a respiratory rate >20 breaths·min−1, cancer and chronic heart failure. The rate of complications in patients with these risk factors appeared to be much higher in patients receiving anticoagulation only, whereas it was less marked in those initially treated with systemic thrombolysis, suggesting an interaction effect. More specifically, in patients with at least 2 of the above criteria, the aforementioned adverse events occurred in 20% of those randomised to receive anticoagulation only, while this was the case in only 7.0% of the patients randomised to systemic thrombolysis. Lastly, in a post hoc analysis of 2 PE registries, a heart rate ≥100 bpm was suggested as a relevant determinant of PE-related mortality as well as an early adverse outcome19. Although these patient characteristics and presenting symptoms appear to be correlated with adverse outcomes, there are no outcome studies confirming that patients with any combination of risk factors for an early adverse outcome may clinically benefit from upfront reperfusion therapy.
Indications for catheter-directed therapy
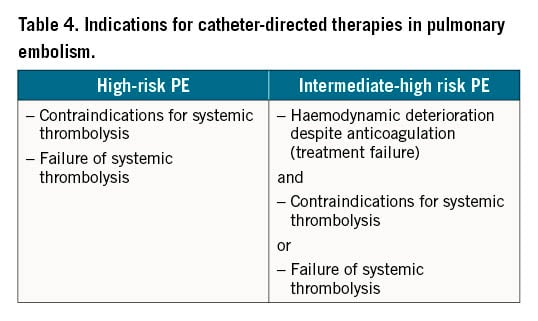
Due to the lack of high-quality evidence from randomised controlled trials with clinical outcomes, primary reperfusion using CDT is not currently the first-line treatment for patients with high-risk acute PE, nor for any of the other PE risk categories. Instead, according to current guidelines, CDT should be considered for patients with high-risk PE in whom thrombolysis is contraindicated or has failed (Table 3)2021. CDT should also be considered a rescue treatment for initially stable patients in whom anticoagulant treatment fails, i.e., those who experience haemodynamic deterioration despite adequate-dose initial anticoagulation (Table 4)1.
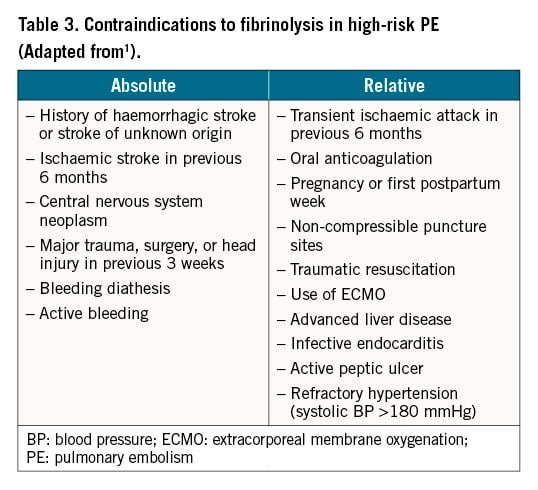
Contraindications to systemic thrombolysis
Recent major surgery, trauma, recent stroke, active bleeding and other comorbidities are absolute contraindications to systemic thrombolysis (Table 3)1. Puncture of central vessels may also be regarded as a relative contraindication to systemic thrombolysis. In PE patients with an absolute contraindication to even small doses of local lytics, such as active bleeding, a mechanical percutaneous embolectomy is probably the optimal reperfusion technique.
Failure of thrombolysis and anticoagulation
Persistent clinical instability and unalleviated RV dysfunction on echocardiography 36 hours after systemic thrombolysis was reported in 8% of high-risk PE patients treated with full-dose systemic thrombolysis22. Apart from this publication, there are no data on high-risk PE indicating failure of thrombolysis. Currently, it is not possible to provide a precise, evidence-based definition of thrombolysis failure for patients with high-risk PE.
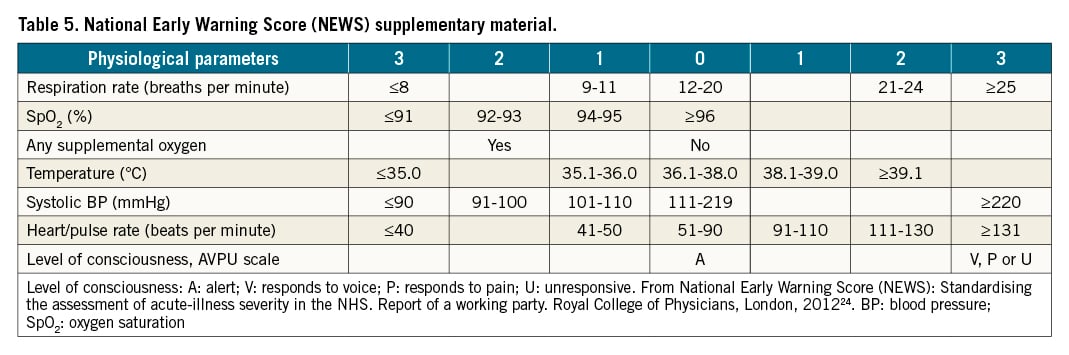
When an initially normotensive PE patient meets the criteria of high-risk PE in the course of the initial anticoagulant treatment, this is clearly considered a treatment failure, and urgent rescue reperfusion should be started. However, ideally, the goal of the initial therapy should be the prevention of shock, rather than “rescue” treatment once it has appeared. The detection of early signs of haemodynamic deterioration and the proper timing for rescue reperfusion therapy in intermediate-risk PE is challenging. Current guidelines recommend that patients with signs of RV dysfunction on echocardiography or CTPA, accompanied by a positive troponin test, should be monitored in the first 2-3 days due to the risk of early haemodynamic decompensation and circulatory collapse. However, no specific advice was provided on which parameters to monitor and how persistent or worsening tachycardia, a decrease in systolic blood pressure, an increase in respiratory rate, and signs of organ hypoperfusion (e.g., decreasing urine output, increasing lactate levels) usually precede haemodynamic collapse (Figure 1)1. Additionally, haemodynamic breakdown can be sudden without any former signs. Several early warning scores have been developed to monitor patients during their hospital stay and identify the likelihood of deterioration of their vital status23. For instance, the National Early Warning Score 2 (NEWS2) has been recommended to monitor acute illness, especially for the detection of clinical deterioration, across all hospitals in the UK (Table 5)24. NEWS2 includes 6 physiological parameters: i) respiratory rate, ii) oxygen saturation, iii) temperature, iv) systolic blood pressure, v) heart rate and vi) level of consciousness. Higher scores indicate higher disease severity and an increased risk of death. A NEWS2 score of 5 or more is a key trigger threshold for urgent clinical re-evaluation and action, and a score of 7 or higher for an emergency response24. Some PE expert centres have started to monitor PE patients using the NEWS2 score; however, at present, no formal recommendation can be given for the use of any scoring system for PE patients. An even more difficult decision is when to consider that the treatment has failed, if patients at intermediate-high risk do not show signs of improvement despite adequate anticoagulation therapy over the first hours and days.
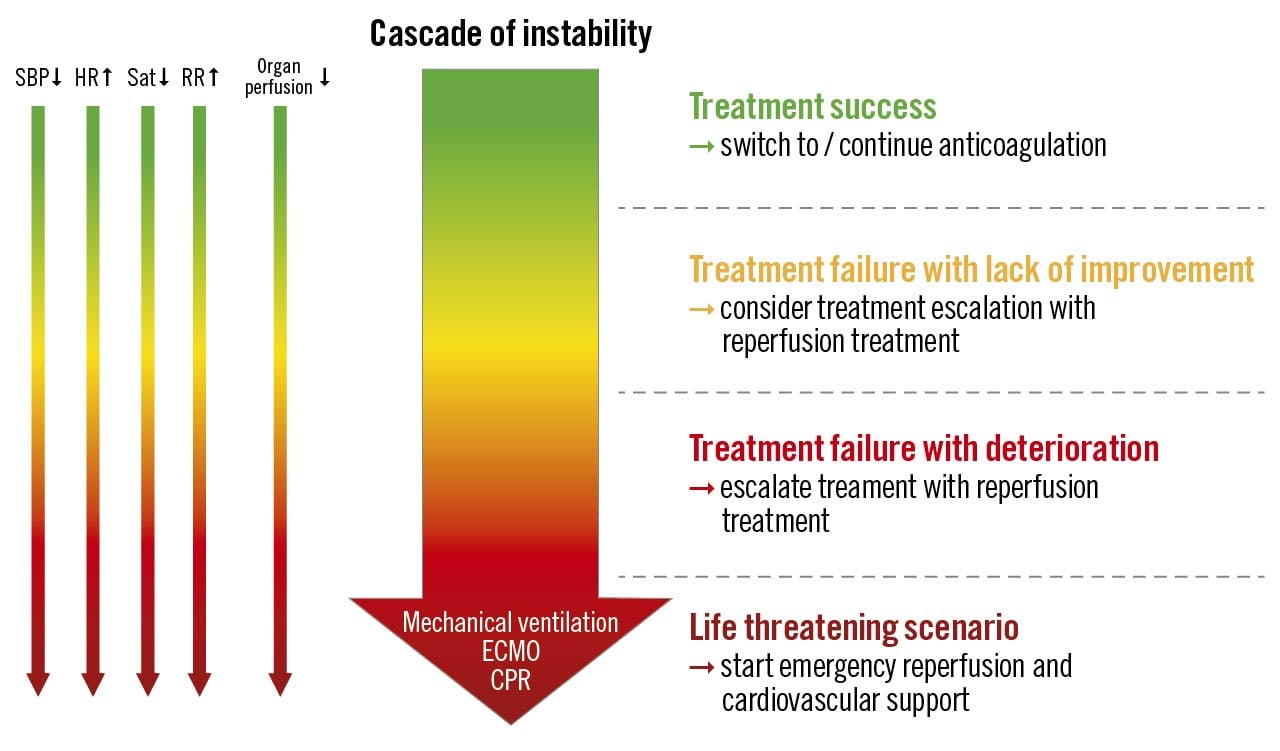
Figure 1. Treatment efficacy of acute pulmonary embolism. CPR: cardiopulmonary resuscitation; ECMO: extracorporeal membrane oxygenation; HR: heart rate; RR: respiratory rate; Sat: saturation; SBP: systolic blood pressure
The panellists agreed that due to a gap in knowledge the definition of “treatment failure” (referring either to primary reperfusion therapy or anticoagulation therapy alone) remains an important area of uncertainty. The assessment of treatment failure combines monitoring physiological signs (level of consciousness, systemic blood pressure, heart rate, respiratory rate, arterial oxygen saturation), signs of organ hypoperfusion (e.g., decreasing urine output, increasing lactate levels) and emergency rescue treatment (cardiopulmonary resuscitation [CPR], mechanical ventilation, ECMO, catecholamines) (Figure 1). Repeated echocardiography may be helpful to detect worsening RV function. Importantly, the decision to perform CDT in a patient with treatment failure depends on many factors including the availability of CDT, expertise, and logistical challenges. Moreover, the overall bleeding risk and comorbidities should be included in the decision-making. A multidisciplinary approach could help determine the optimal treatment of an individual patient.
We propose 3 different clinical scenarios to serve as examples for the evaluation of treatment success (Figure 1).
1. Treatment success. Initial treatment results in the improvement of initially compromised haemodynamic status, i.e., decrease in heart rate and respiratory rate, increase in systemic blood pressure, oxygen saturation, and improvement of peripheral perfusion. In this scenario, no escalation of therapy is required.
2. Treatment failure indicated by a lack of improvement. The panel agreed that in high-risk PE treatment, failure can be recognised when no haemodynamic improvement is achieved 2-4 hours after the completion of full-dose thrombolysis or immediately after the completion of the local thrombolysis infusion. In intermediate-high risk PE patients, a lack of improvement can be diagnosed when compromised vital signs are not alleviated after 24-48 hours of therapeutic-dose anticoagulation. In such patients, we propose considering rescue reperfusion therapy, preferably after a discussion with the PERT, which should be composed of specialists with practical experience in the management of acute PE.
3. Treatment failure indicated by haemodynamic deterioration. If, following the initiation of (fibrinolytic and/or anticoagulant) treatment, the patient develops overt cardiorespiratory instability necessitating CPR, mechanical ventilation, catecholamines, or ECMO, there is a clear indication for emergency treatment escalation. When, on the other hand, worsening in initially haemodynamically stable PE patients is indicated by a progressive increase in heart rate or respiratory rate, a progressive decrease in systemic blood pressure or oxygen saturation, or by worsening signs of organ hypoperfusion (decrease in urinary output, increase in lactate levels) over at least 15 minutes, even without meeting the criteria of an overt shock, this panel agrees that the PERT should also convene as fast as possible to discuss rescue reperfusion therapy.
Description and analysis of contemporary techniques of catheter-directed therapies
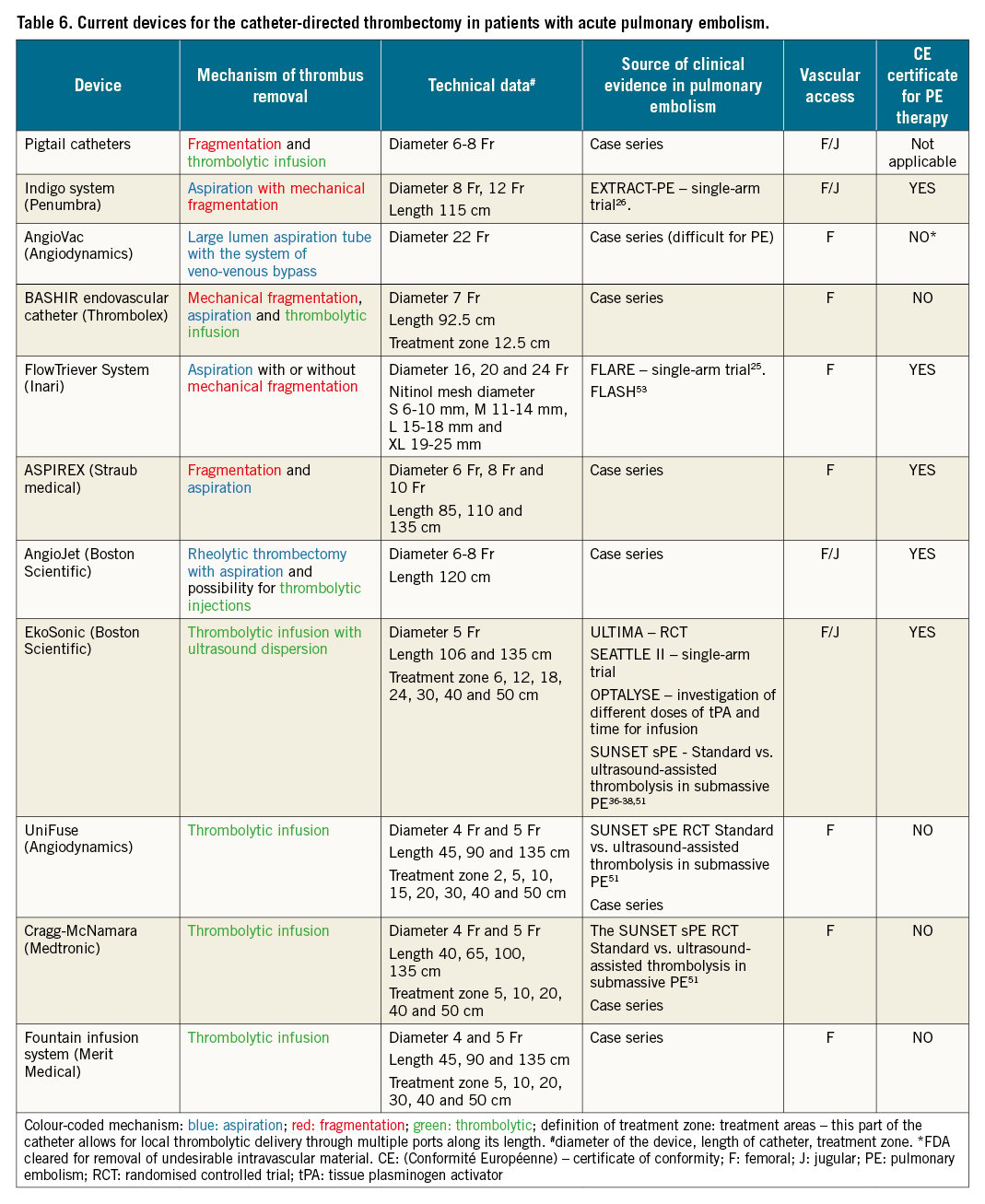
Catheter-directed reperfusion techniques, including catheter-directed clot fragmentation, mechanical embolectomy, local thrombolysis and combined pharmaco-mechanical approaches, act primarily by relieving the thromboembolic obstruction of the proximal pulmonary arteries, restoring pulmonary blood flow and improving RV function. Local thrombolysis can be applied with the drug delivered via pigtail or dedicated sidehole catheters. Catheter-directed ultrasound-assisted thrombolysis is a combination of local thrombolysis facilitated by applying ultrasound energy locally. The most relevant available CDT techniques are summarised in the paragraph below and in Table 6. It should be emphasised that complete removal of thrombus from the pulmonary bed is usually not required for haemodynamic stabilisation. An increase in systemic pressure, improvement of blood gas parameters and/or a decrease in heart rate indicate a reduction in pulmonary obstruction and an improvement in RV size and function, and may be achieved with partial recanalisation.
Thrombus fragmentation
Rotational pigtail thrombus fragmentation is technically non-demanding and widely available. Recently, we performed a survey at 157 interventional cardiology centres across Europe and collected information on current practices in catheter-directed treatment for acute PE. Over 40% of centres reported using any form of CDT. Interestingly, mechanical fragmentation with pigtail catheters is currently still the most commonly used catheter-based therapy for acute PE (Roik M et al. Current use of catheter directed treatment of acute PE in Europe: results of survey of EAPCI and ESC Working Group on Pulmonary Circulation and Right Ventricular Function. Presented at the ESC Congress 2021).
The CLEANER Rotational Thrombectomy System (Rex Medical) allows for clot fragmentation with a motor-driven, rotating, flexible sinusoid tip that is placed directly into the thrombus. Clot fragmentation with the CLEANER carries a risk of distal embolisation and vascular wall injury.
Aspiration embolectomy
The main goal of aspiration embolectomy is to remove embolic material and prevent distal embolisation. Thrombus aspiration is achieved by applying suction (manually or using a dedicated system) through large bore catheters (8 Fr or greater).
The FlowTriever system (Inari Medical) is an aspiration system for interventional treatment of PE. The system has also been used in patients undergoing cardiopulmonary resuscitation or during ECMO for refractory obstructive shock. There is a choice of 3 aspiration catheters (16, 20 and 24 Fr), each with a 60 ml aspiration syringe, and 4 catheters with self-expanding nitinol discs. The small-disc catheter is intended for vessels of 6–10 mm diameter, the medium-disc catheter for 11-14 mm, the large-disc catheter for 15-18 mm and the extra-large catheter for 19-25 mm.
The disc catheters are only required in up to 25% of cases, when conventional aspiration techniques are not successful25. The procedure requires the use of a 24 Fr DrySeal Flex Introducer Sheath (GORE) placed into the common femoral vein using ultrasound-guided puncture; alternatively, the internal jugular vein can be used. Placement of the sheath and the FlowTriever aspiration catheter requires the use of a stiff Amplatz wire (Boston Scientific), with a short flexible tip of 1 cm, placed in a distal pulmonary artery. The aspiration catheter is inserted into a pulmonary artery and advanced proximally to the embolus in the main pulmonary or lobar pulmonary arteries. The aspiration procedure enables 3 different techniques for mechanical clot removal: direct aspiration, “lollipop” technique, and disc aspiration technique.
The Indigo mechanical thrombectomy system (Penumbra) includes an 8 Fr to 12 Fr aspiration catheter, a pump providing suction and a separator wire. When the aspiration catheter is located at the thrombus, the pump creates a negative pressure allowing aspiration of the embolic material. A separator wire with a soft dedicated tip inserted into the aspiration catheter helps to disrupt the thrombus. The catheter can be advanced several times through the thrombus, allowing for removal. The efficacy of the Indigo aspiration catheter was documented in the EXTRACT-PE study26. The majority of patients treated with the Indigo system had an estimated overall blood loss not exceeding 400 ml. Only 2.5% of patients required blood transfusions that were deemed related to the Indigo26. In order to prevent blood loss during CDT (FlowTriever, Indigo), the use of a dedicated valve with proven haemostasis is advised (e.g., DrySeal Flex Introducer Sheath). Recent introduction of the FlowSaver filter (Inari Medical) allows for blood return to the patient. Suction thrombectomy can be used in combination with local thrombolysis. A prospective analysis of local low-dose catheter-directed thrombolysis followed by mechanical thrombectomy with the Indigo system in 54 patients with systemic hypotension and contraindications for systemic thrombolysis was shown to be effective with 11% in-hospital mortality and a low risk of major bleeding27.
The ASPIREX catheter (Straub Medical) is comprised of a sheathed, high-speed spiral that produces negative pressure through an aspiration port at the end of the catheter; this allows for the maceration and aspiration of the thrombus. This technique can be combined with other interventions such as thrombus fragmentation. Catheters with an 8 Fr or 10 Fr diameter are typically used28. The potential drawback of the ASPIREX is the potential for large-volume blood loss, depending on thrombectomy time and damage of the pulmonary arteries with pulmonary haemorrhage.
The AngioVac (AngioDynamics), consisting of an extra-large diameter 24 Fr catheter, has been constructed to remove venous thrombi during extracorporeal veno-venous bypass, allowing the filtration of aspirated blood from thrombi before reintroduction to the systemic circulation. AngioVac is approved for the removal of vascular material from the superior and inferior vena cava and from the right atrium. This system has been occasionally used off-label for PE as well as for thrombus aspiration from the RV29.
Rheolytic thrombectomy
The high-pressure saline jet generated by an AngioJet PE catheter (Boston Scientific) creates a pressure gradient, according to Bernoulli’s law, allowing the disruption and removal of thrombus fragments. This device also allows for the local administration of thrombolytic drugs (Power Pulse option) that directly penetrate the thrombus30. Adverse effects include bradycardia, possibly due to transient increases in bradykinin, adenosine, or potassium secondary to haemolysis, increased dyspnoea, and alveolar bleeding. Bradyarrhythmia (e.g., asystole, atrioventricular blocks) usually occurs following a prolonged single application or an excessively lengthy thrombectomy procedure. Haemolysis may also result in haemoglobinuria; however, this is usually reversible without significant clinical consequences. The US Food and Drug Administration (FDA) has issued a “black box” warning due to reports of asystole and haemodynamic decompensation during rheolytic thrombectomy of pulmonary artery thrombus. Technical improvements of the AngioJet system and shortening of its activation time help to prevent these complications3132. Moreover, a recently published single-centre experience with the AngioJet in 56 high- and intermediate-risk PE patients with absolute or relative contraindications for thrombolytic therapy showed that CDT resulted in a significant and clinically relevant haemodynamic improvement. During hospital stay, major bleeding occurred in 7.1% of patients with a mortality rate of 8.9%. Although post-procedural renal deterioration was observed in 39.3% of patients, 1-session dialysis was required in only 1 (1.8%) patient. These current data suggest that safety issues of rheolytic thrombectomy may be re-evaluated33.
Local thrombolysis
Conventional local thrombolysis via a catheter allows local infusion delivery of a low-dose thrombolytic agent into the pulmonary artery or directly into the thrombus. In addition, the thrombus can be fragmented before drug injection. Thrombolytic drugs can be delivered via pigtail catheter or dedicated catheters (Uni-Fuse [AngioDynamics]; Cragg-McNamara [Medtronic]; or Pulse*Spray infusion system [AngioDynamics]) which have special side holes for intrathrombus thrombolytic injection. Virtually all thrombolytic drugs can be applied in this manner. Dosing regimens vary between centres and may involve the infusion of 0.5-1.0 mg/h alteplase in 1 or both main pulmonary arteries for up to 24 hours. The total alteplase dosage is typically below 30 mg, and the duration of the thrombolysis infusion does not usually exceed 24 hours. In addition, the BASHIR endovascular catheter (Thrombolex), an infusion catheter (7 Fr) with a basket that expands within the thrombus, can be used to deliver 12–14 mg of rtPA (recombinant tissue plasminogen activators)34.
Ultrasound-accelerated catheter-directed thrombolysis
During ultrasound-accelerated catheter-directed thrombolysis, the drug is infused directly into the pulmonary artery thrombus. The catheter is equipped with miniaturised ultrasound transducers, which emit pulsed high-frequency (2-MHz) ultrasound waves35. Ultrasound waves may enhance fibrinolysis by causing disaggregation of unbridged fibrin fibres, thereby increasing permeability of the thrombus and fibrinolytic drug penetration. EkoSonic 5.2 Fr infusion catheters (Boston Scientific) are usually inserted bilaterally into thromboembolic material. The safety and efficacy of the device were documented in the ULTIMA (ULTrasound Accelerated ThrombolysIs of PulMonAry Embolism)36, SEATTLE II37, and OPTALYSE PE trials, using an rtPA dose of 4-24 mg38.
It is always important to monitor the patient during thrombolytic drug infusion (conventional or ultrasound-assisted). Before the initiation of thrombolysis, haemoglobin levels and platelet count should be measured. Several observational studies have suggested that severe fibrinogen depletion during thrombolysis indicates thrombolysis efficacy but also predicts bleeding, including intracranial haemorrhage394041. Although formal guidelines do not make specific recommendations on fibrinogen level monitoring, some centres have adopted the practice of monitoring haemoglobin, platelets, anti-Xa levels (activated clotting time [ACT] or activated partial thromboplastin time [aPTT]), and fibrinogen every 6 hours during the thrombolysis infusion. In patients with >50% decrease in fibrinogen levels, or a drop of fibrinogen to less than 1 g/L, the thrombolytic treatment is adjusted (either temporary discontinuation of the infusion or dose reduction). There is currently no evidence that thrombolytic dose adjustments according to fibrinogen levels improve the safety of catheter-directed thrombolysis for PE.
Due to the lack of randomised trials, it remains debatable whether the anticoagulation intensity and, specifically, the dose of heparin should be reduced during thrombolysis infusion. In some centres, the infusion of unfractionated heparin is reduced to 500 IU per hour, while in other centres the heparin dosage targets an anti-Xa level of 0.5-0.7 IU/mL. Caution is warranted for patients who have been pretreated with low-molecular-weight heparin (LMWH) or with direct oral anticoagulants. In patients who have received these drugs within 8-12 hours prior to thrombolysis infusion, the heparin bolus is usually omitted, and the heparin infusion is initiated with a lower anti-Xa target of 0.3-0.5 IU/mL for the first hours of thrombolysis.
Summary of the current knowledge of the safety and efficacy of CDT
Evidence on the efficacy and safety of CDT is limited to observational studies, a few small randomised trials and small single-arm cohort studies with surrogate outcomes (Table 7). The safety and efficacy depend on the chosen technique, patient characteristics and, most of all, local expertise. Robust data from adequately sized controlled trials designed to prove the efficacy of these procedures for improving the patients’ clinical outcome are still lacking. The estimated overall efficacy of catheter-directed treatment, defined as stabilisation of haemodynamic and blood gas parameters and survival to hospital discharge, has been reported to approach 90%742434445. On the other hand, in a meta-analysis of 16 studies which included 860 patients, in-hospital mortality was 12.9% in patients with high-risk PE, and 0.74% in the intermediate-risk group, while the rate of intracranial haemorrhage was 0.35% and the major complication rate was 4.65%46. Complications during percutaneous embolectomy include haemodynamic decompensation, respiratory failure, alveolar haemorrhage, pulmonary artery perforation, acute kidney injury associated with contrast agent administration and haemolysis, and haematomas at the vascular access site, all dependent on the technique and system that were utilised44344454647.
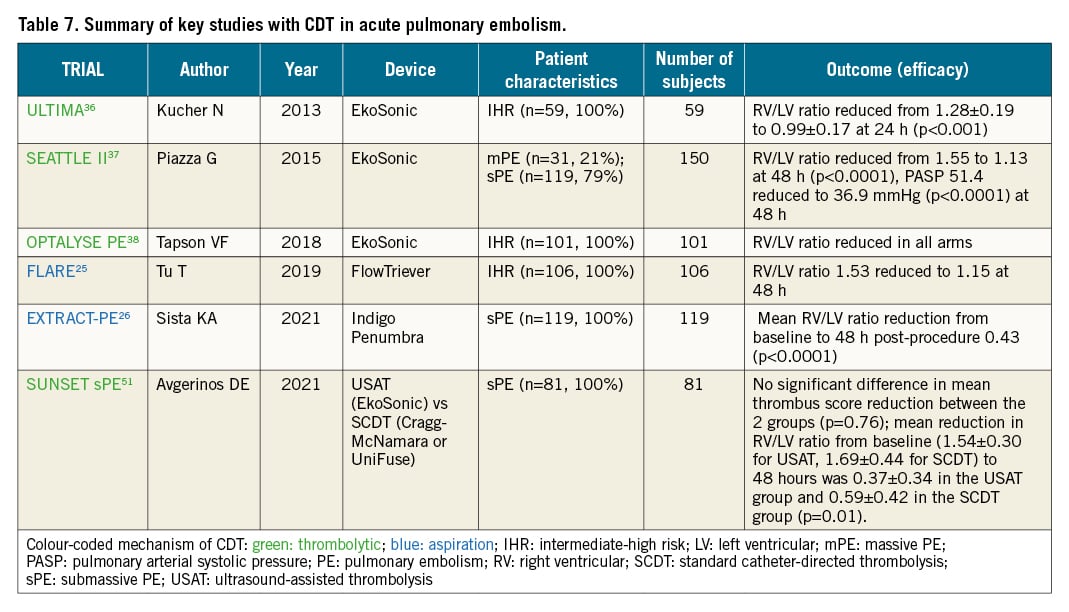
The randomised ULTIMA trial compared the delivery of low-dose (10 to 20 mg) rtPA by ultrasound-assisted catheter-directed thrombolysis (EkoSonic) over 15 hours with standard anticoagulant treatment in 59 intermediate-risk patients; it showed that catheter-directed thrombolysis was superior in improving the 24-hour right-to-left ventricular diameter ratio36. These results on surrogate echocardiographic outcomes were subsequently supported by interventional studies using the same device, which tested different dosing strategies and durations of administration, namely the Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism (SEATTLE II) study and the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism (OPTALYSE PE) randomised trial3738.
Interestingly, a real-life, single-centre, retrospective study of 141 patients with intermediate- or high-risk PE showed that ultrasound-assisted thrombolysis (USAT) was associated with a stabilisation of haemodynamics along with relatively low rates of complications in patients with PE, regardless of the initial risk status48. A meta-analysis of 11 USAT series revealed that USAT resulted in improvements in pulmonary artery mean pressures, RV/left ventricular (LV) ratio, and thrombotic obstruction49. Another meta-analysis, which included 28 studies, reported haemodynamic improvement in patients who underwent USAT, with 2.9% in-hospital mortality and total long-term mortality of 4.1%. Major and minor bleeding complications were seen in 5.4% of patients, suggesting that USAT is a fairly safe and effective procedure associated with significant haemodynamic and clinical improvement in patients with massive and submassive pulmonary embolism50. Of note, a recent analysis of 3-month outcomes of 489 patients from a prospective arm of KNOCOUT-PE study assessing the use of low-dose, short-infusion tPA strategies with USAT in acute PE reported no mortality and a postprocedural 22.6% reduction in the RV/LV ratio. Major bleeding was observed in 2.5% of patients, with no intracranial bleeding. Importantly, significant improvement was found in PE-specific quality of life at 3 months. (Goldhaber et al. KNOCOUT-PE study: International EkoSonic Registry of the Treatment and Clinical Outcomes of Patients with Pulmonary Embolism Prospective Cohort. Presented at the Vascular InterVentional Advances Meeting [VIVA] 2021).
The randomised SUNSET sPE (Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism) trial51, reported similar pulmonary arterial thrombus reduction in patients undergoing ultrasound-assisted thrombolysis compared with those undergoing standard catheter-directed thrombolysis, using comparable mean lytic doses and durations of lysis. However, the lack of standardised dosing regimen across the 2 groups and the small sample size prevented any definitive conclusions. Finally, the recent Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism (FLARE) study reported improvements in RV function in 106 patients with intermediate-high risk PE with 1% risk of major bleeding complications using the FlowTriever system25. A recently published, retrospective, single-centre study in 46 patients provided further evidence of improvement in mean pulmonary artery pressure after a FlowTriever thrombectomy52. Lastly, the recent interim analysis of the prospective FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), an evaluation of 250, mostly intermediate-risk, PE patients (93%) reported 0% 48-hour mortality with a 1.2% rate of major bleeding without sequelae53.
The safety profile of catheter-directed techniques depends on the clinical setting. Compared to the 7-day rates of major bleeding of systemic thrombolysis in PEITHO (8.3% according to Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries [GUSTO] criteria and 11.5% by the International Society on Thrombosis and Haemostasis [ISTH] criteria), the major bleeding rates reported in the ULTIMA, SEATTLE II, and OPTALYSE trials ranged from 0 to 10%, with the above-mentioned meta-analysis of retrospective studies estimating a rate of 6.7% for high-risk and 1.4% for intermediate-risk PE44. None of these were intracranial in the ULTIMA and SEATTLE II studies, whereas in OPTALYSE, 1 intracranial bleeding was reported (1.0%)363738. Notably, the safety of these techniques depends largely on technical expertise. Ultrasound-guided venous puncture for catheter placement likely improves the safety of the CDT procedure, especially if catheter-thrombolytic protocols are used54.
Phase II and III studies provide only limited evidence on acute complications beyond major bleeding and death. In the FLARE study using the FlowTriever device, 4/106 (3.8%) patients experienced 6 major adverse events, including 1 major bleeding, 2 pulmonary vascular injuries, 2 respiratory deteriorations, and 1 ventricular fibrillation; an additional 14 patients experienced other, not otherwise specified, serious adverse events with a broad definition, as well as 2 malfunctions25. A single-centre, retrospective study reported 2/46 (4.3%) complications52. Of the studies using the EkoSonic system, SEATTLE II reported no procedural complications37, ULTIMA reported no serious adverse events related to the study treatment36, and OPTALYSE PE did not report on non-death or non-bleeding safety outcomes38.
We searched the online Manufacturer and User Facility Device Experience (MAUDE) database, which contains medical device reports of suspected device-associated deaths, serious injuries and malfunctions received by the FDA5556. Relevant information is shown in Table 8. While these reports do not provide a reliable quantitative estimate of the absolute risks associated with each catheter-directed technique and the database should not be used to directly compare different devices, they provide an overview of the characteristics of adverse events occurring in everyday practice. Table 8 reports all adverse events included in MAUDE up to September 2019, after removal of duplicates and reports not evaluable by the manufacturer. For EkoSonic and FlowTriever, only reports in PE cases or cases with no indication are included; for AngioJet devices, all reports for all indications are included, as their use in PE is limited by a black box warning label. As each report of malfunction or technical complication may include more than 1 malfunction type, only the most severe malfunction per report is shown. Reports for ULTIMA included complications at 90 days36, reports for the other studies or MAUDE included complications at 30 days and/or in-hospital complications. However, it should be taken into consideration that MAUDE data are limited to US practice and may not reflect the technical or clinical problems related to interventional PE therapies reported from the rest of the world.
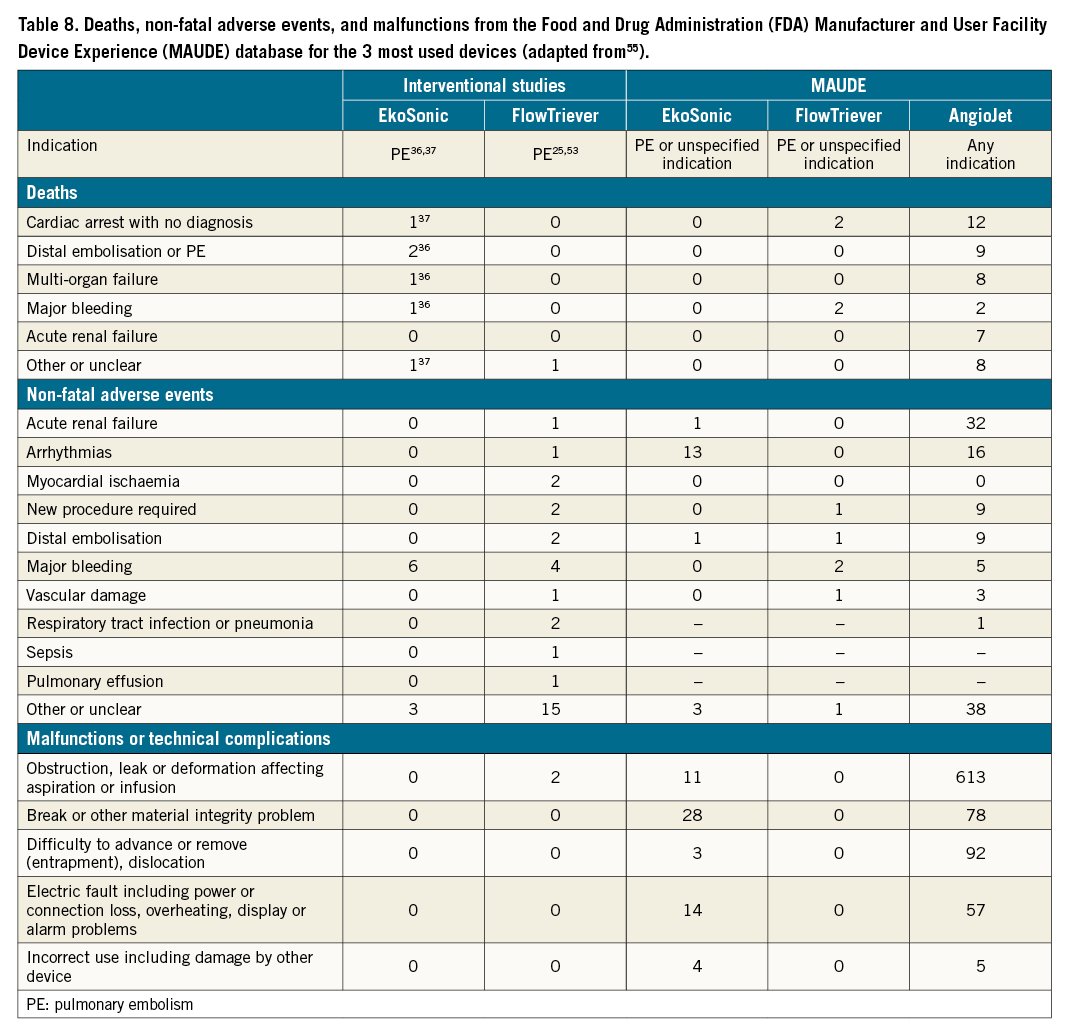
Catheter-directed therapy and EXTRACORPOREAL membrane oxygenation (ECMO)
Mechanical cardiopulmonary support with veno-arterial ECMO is helpful in high-risk PE to maintain the circulation and oxygenation of vital organs during acute RV failure and cardiogenic shock10. Haemodynamic stabilisation with mechanical support offers the potential to provide the additional time necessary to better plan further management. The use of ECMO requires full-dose anticoagulation and is associated with bleeding complications. In patients on ECMO, systemic thrombolysis is contraindicated. Local low-dose lysis should probably also be avoided since it is unclear which minimal pulmonary flow is necessary to allow for an effective local lysis, and the lysis will intrinsically increase the bleeding risk. ECMO support with therapeutic anticoagulation may serve as a bridge to surgical or percutaneous pulmonary embolectomy or, as reported in some cases, to recovery. This panel agrees that the decision on optimal therapy selection in PE patients supported with ECMO should be individualised with a multidisciplinary approach.
Proposed protocol for catheter-directed therapy
Timing of the catheter-directed therapy
We underline that as an important part of determining indications for the CDT procedure it is crucial to establish an unambiguous diagnosis of PE with its haemodynamic consequences, including the assessment of RV dysfunction, preferably with echocardiography, to evaluate bleeding risk, and to assess the efficacy of the already applied therapy. When indications for CDT are established, the panel considers that, in line with ST-elevation myocardial infarction (STEMI) guidelines57, it is reasonable to expect CDT to start without a delay after its indication has been confirmed by the PERT. Although no evidence-based data are available, the panel agreed that for patients already admitted to a centre with CDT capability, CDT should be started within a maximum of 60 minutes from the moment the CDT indication was established (not from PE diagnosis); and for patients that need to be transferred to a CDT centre, preferably within a maximum of 90 minutes (Central illustration). We underline that the timing should only include the indication for CDT, not the moment that PE is diagnosed. Haemodynamic collapse applied therapy is an indication for urgent CDT. However, in intermediate-high risk PE patients, CDT should be considered when no improvement is achieved after 24-48 hours of initial anticoagulation. Thus, in the latter group, time limits could be less stringent. We suggest that this 24-48 hour period of initial anticoagulation should be used by PERT not only to assess indications for CDT but also to prepare the logistics for potential invasive therapy.
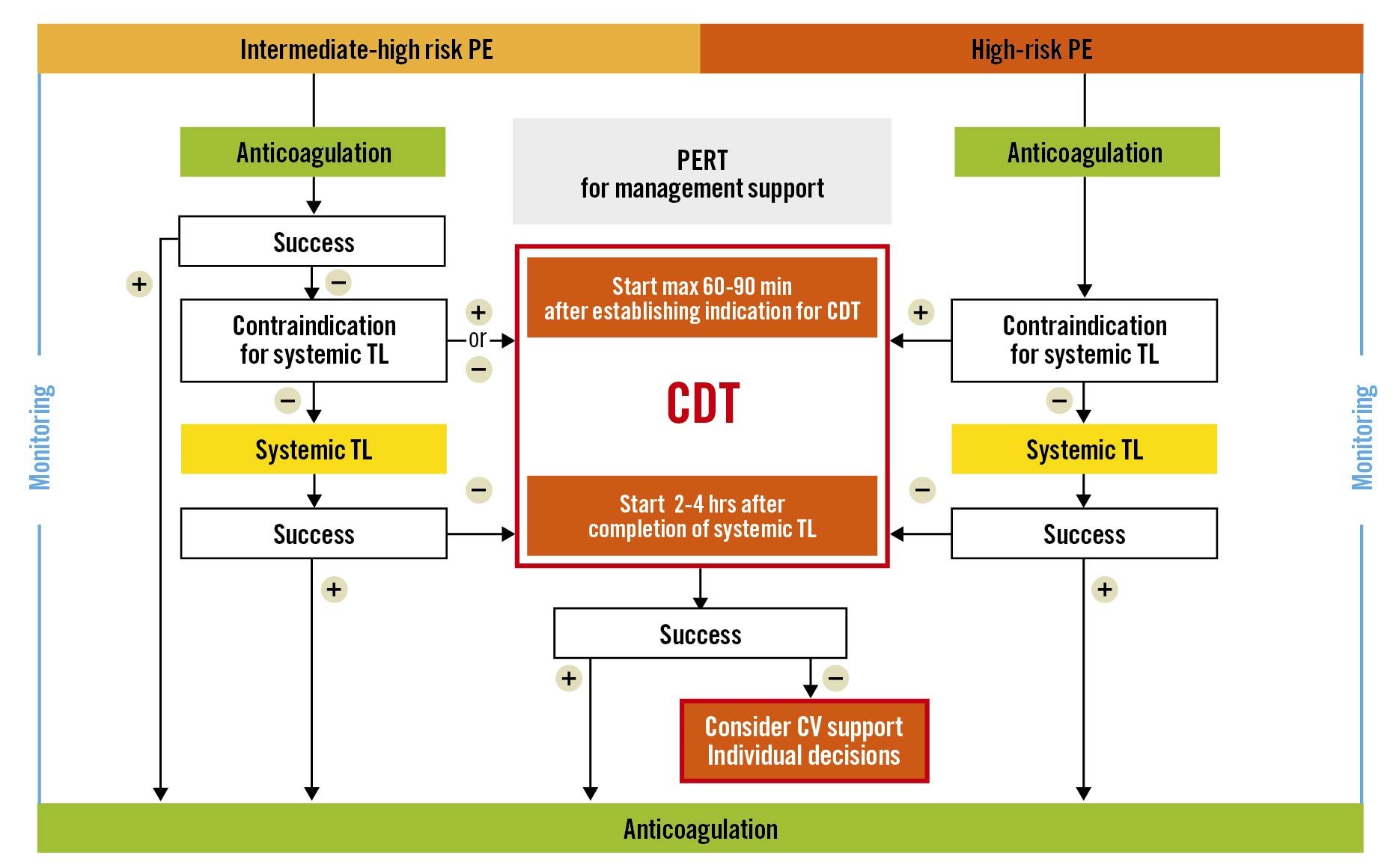
Central illustration. Proposed algorithm and timelines of catheter-directed therapies (CDT) in high-risk and intermediate-high risk pulmonary embolism (PE). CV: cardiovascular; PERT: Pulmonary Embolism Response Team; TL: thrombolysis
PE imaging before intervention
It is advised, when possible, to review CTPA images before deciding on invasive therapy. Assessment of the embolic load and the localisation of clots in the pulmonary arteries will help for proper CDT planning. The majority of patients with PE and RV dysfunction have thromboemboli lodged in lobar or more proximal pulmonary arteries; such thrombi are easily accessible for percutaneous therapies. In contrast, patients with isolated clots at the segmental or more distal level are less suitable for CDT and alternative causes of RVD, such as acute decompensation of pulmonary arterial hypertension, should be considered. Moreover, targeting such distal acute thrombi is more challenging, especially in less experienced centres. In such cases, local thrombolysis might be preferred. Furthermore, some haemodynamically unstable patients with a high clinical probability of PE (for example, after major orthopaedic or oncological surgery) with RVD detected at bedside echocardiography and a high risk of bleeding, in whom CTPA cannot be immediately performed, may be directly transported to a cath lab for CDT preceded by standard pulmonary angiography.
Monitoring and procedural objectives
We underline that the aim of CDT is to stabilise the patient haemodynamically (decreased heart rate, increase in systemic blood pressure, reduction of vasopressor doses), and, in our opinion, haemodynamic stabilisation should be regarded as clinical success. For this purpose, only a partial reduction in thrombotic burden of pulmonary arteries is required. Attempts to remove all clots during the CDT procedure are not necessary and can be harmful, due to increased use of contrast media, potential risk of arterial wall damage, and in the case of AngioJet bradycardia and haemolysis.
During invasive pulmonary artery angiography and percutaneous therapy of acute PE, patients require continuous monitoring of systemic blood pressure, heart rate and electrocardiography. Ultrasonography of proximal leg veins is also indicated to rule out coexisting deep vein thrombosis at the vascular access site. It is important to carefully evaluate haemodynamic parameters (pressure in the right heart chambers and pulmonary artery) before and after the CDT procedure to assess the effects of the therapy. Moreover, the amount of contrast agent used should be minimised. Non-selective angiography with a large amount of contrast administered through an automated syringe should be avoided due to the risk of exacerbating RV failure.
Proposed steps of CDT:
1. Management and medical therapy of PE, including anticoagulation and thrombolysis, should follow current guidelines.
2. Consider indications and potential contraindications for CDT and for local low-dose thrombolysis, preferably in collaboration with the local or regionally active PERT. Assess technical CDT feasibility and technique/device. This requires assessment of the haemodynamic condition of the patients and risk of bleeding, as well as localisation of pulmonary emboli at the CTPA if available (see above).
3. Prepare the patient in the same way as for standard interventional procedures (informed consent is required).
4. Monitor systemic arterial pressure, heart rate, oxygen saturation, and respiratory rate before and after the procedure, at least until 2-4 hours after haemodynamic stabilisation has been achieved.
5. Obtain vascular access through the internal jugular or common femoral vein using ultrasound guidance.
6. Obtain invasive pressure and oxygen saturation from the pulmonary artery.
7. Perform selective conventional angiography by administering 10 ml of contrast at 5 ml/s preferably in the 20° left anterior oblique (LAO) view to visualise the left pulmonary artery and preferably the 20° right anterior oblique (RAO) view to visualise the right pulmonary artery. The RAO cranial view may also be useful.
8. Insert the selected device and perform CDT following recommendations specific for each CDT system.
9. Administer parenteral anticoagulation during the CDT procedure (unless strictly contraindicated), and consider monitoring with anti-Xa, ACT or aPTT. Due to the lack of scientific evidence, it remains debatable whether the anticoagulation intensity and, specifically, the dose of unfractionated heparin should be reduced during local thrombolysis infusion. Caution is warranted for patients who have been pretreated with LMWH or with direct oral anticoagulants.
10. Repeat the invasive pulmonary artery (PA) pressure measurement and mixed venous oxygen saturation before removing the catheters to assess the effect of treatment.
11. Post-CDT management and monitoring. The panellists agree that patients treated with percutaneous techniques should be monitored at least until the catheter is removed, and monitoring should be continued until haemodynamic stabilisation is achieved.
12. Continue full-dose parenteral anticoagulation after completion of the procedure, unless strictly contraindicated. The panel agrees that unfractionated heparin is to be used in patients who remain haemodynamically unstable at the time of catheter removal. Most patients can be directly switched to LMWH or direct oral anticoagulants when the heparin infusion is complete. Further management should follow current ESC guidelines, including the duration of anticoagulation.
Interdisciplinary cooperation in patient management, operating models and logistics of CDT centres
Selection of the optimal management strategy in patients with severe forms or with an atypical course of PE is challenging and usually individualised; it is mostly based on the expert knowledge of a multidisciplinary team of specialists, rather than on evidence alone. Current guidelines recommend setting up a multidisciplinary PERT to discuss the management of high- and intermediate-high risk PE1. This concept for the management of “severe” PE emerged in 20135859. PERT should be composed of specialists with practical experience in the management of acute PE: including emergency medicine specialists; radiologists; physicians experienced in echocardiography and cardiology, interventional cardiology, vascular medicine, haematology, cardiac surgery, and anaesthesiology; intensive care specialists; and/or pulmonologists, depending on local circumstances and resources. Therefore, the final composition of PERT varies among centres. It is key to elaborate a clear PERT operating protocol for each centre. Such a protocol should accommodate the PERT structure, activation pathways and operating mode. It is crucial that a PERT coordinator is available around the clock to quickly organise ad hoc real-time face-to-face or web conferences to discuss the case and make management decisions. A very important task for PERT is to support clinical decisions in the areas of uncertainty.
Future of CDT: ongoing trials
The current evidence regarding the optimal indication and application of CDT techniques is limited. In particular, randomised trials comparing CDT to conventional standard of care, i.e., systemic thrombolysis for high-risk PE and anticoagulation for intermediate-risk PE, and focusing on clinical outcomes are urgently needed. These outcomes involve short-term adverse events including mortality, recurrent PE, haemodynamic collapse, need for organ support and major bleeding, as well as long-term outcomes such as quality of life, functional outcomes, chronic thromboembolic pulmonary hypertension and chronic thromboembolic pulmonary disease. Without such landmark trials, strong guideline recommendations will not be possible.
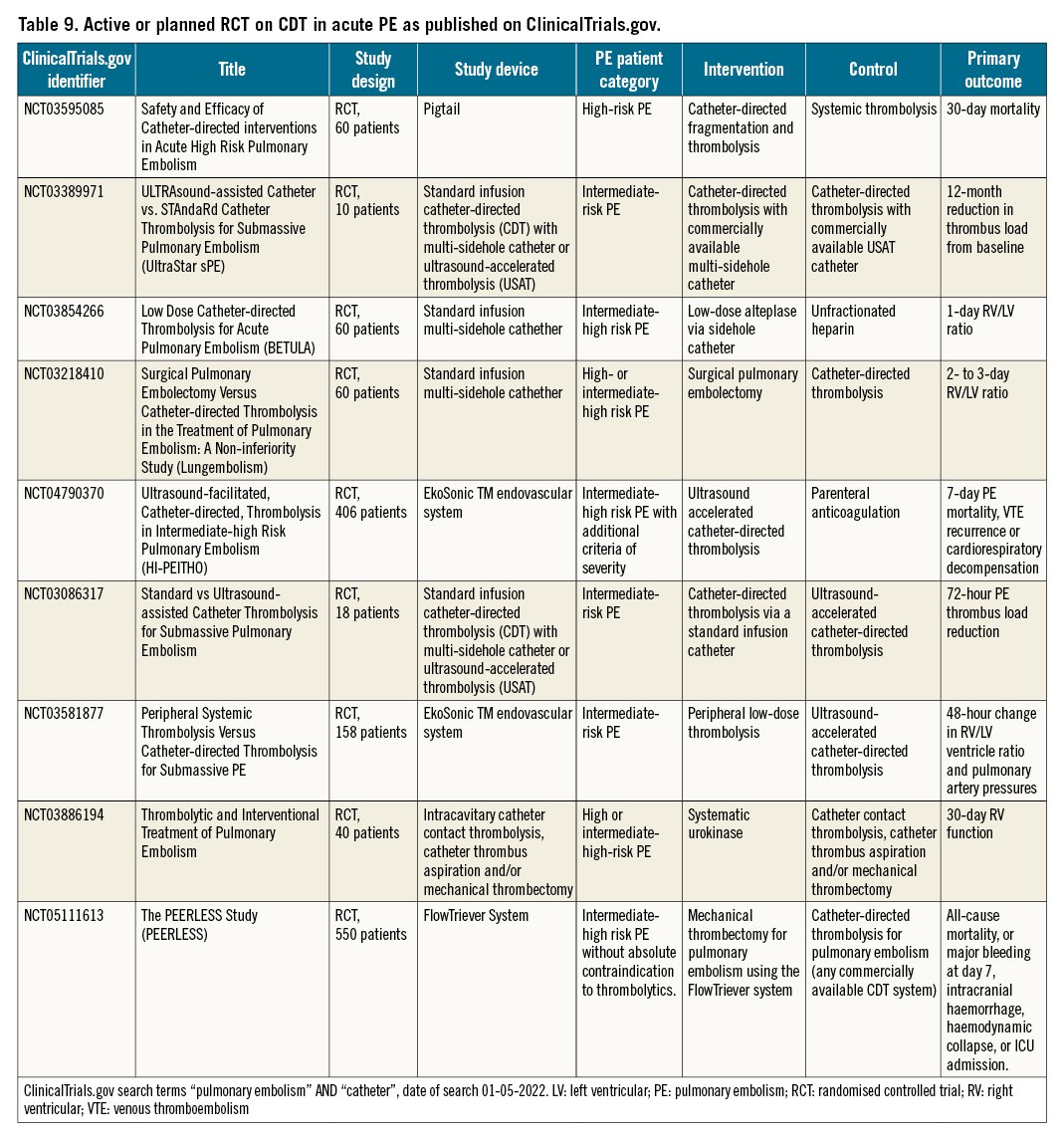
Currently, the ClinicalTrials.gov database indicates 9 planned or ongoing randomised trials focusing on CDT in the management of acute PE (Table 9). Three of these involve patients with high-risk or intermediate-high risk PE, and 6 focus on patients with intermediate-high risk PE. Three compare different types of CDT, 3 compare CDT with systemic full-dose or reduced-dose thrombolysis, 1 compares CDT with surgical embolectomy, and 2 compare CDT to anticoagulation alone. All but 2 have thrombus resolution or RV recovery as the primary outcome. Of the latter, the Safety and Efficacy of Catheter-directed Interventions in Acute High Risk Pulmonary Embolism trial will compare catheter-directed fragmentation and thrombolysis with systemic thrombolysis in 60 patients with high-risk PE, with 30-day mortality as the primary outcome. Secondary outcomes involve changes in blood pressure, oxygen saturation and RV function. The recruitment status of this study is unknown. The Ultrasound-facilitated, Catheter-directed, Thrombolysis in Intermediate-high Risk Pulmonary Embolism (HI-PEITHO) study is comparing CDT with parenteral anticoagulation in 406 patients with intermediate-high risk PE with additional haemodynamic or respiratory criteria of severity. The primary outcome of the HI-PEITHO study is 7-day PE mortality, venous thromboembolism (VTE) recurrence or cardiorespiratory decompensation. Other outcomes involve RV recovery, major bleeding, functional status, quality of life and incidence of chronic thromboembolic pulmonary hypertension (CTEPH) up to 12 months after randomisation, as well as healthcare utilisation. The HI-PEITHO study is currently recruiting. The estimated study completion date is July 2024. The PEERLESS is an ongoing trial aiming to compare FlowTriever System versus catheter-directed thrombolysis in acute intermediate high-risk PE patients. Composite primary outcome will include all-cause mortality, or major bleeding, intracranial hemorrhage, hemodynamic collapse, or ICU admission. Estimated completion date of PEERLESS study is March 2024.
Conclusions
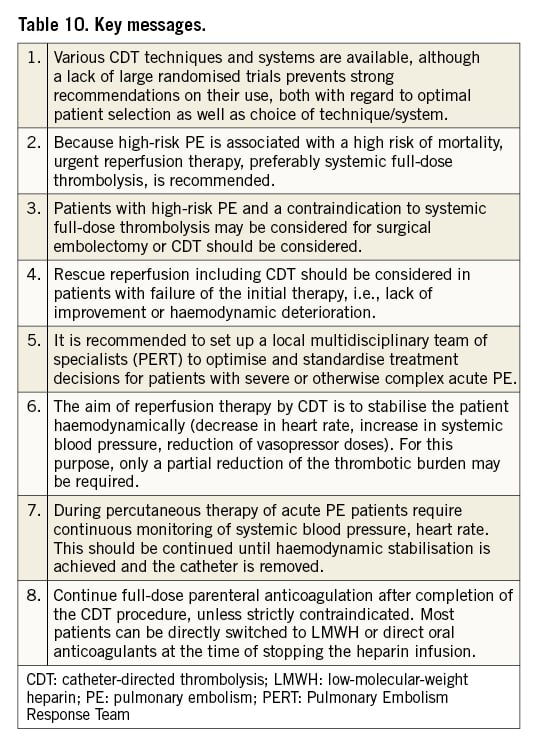
While evidence for the use of CDT is still growing, more randomised trials are needed to determine the specific patient groups that benefit from the various CDT techniques. In this document, we have summarised the current knowledge on CDT for acute PE and provide guidance on the standardisation of patient selection, timing and technique of the procedure, and anticoagulation regimens during CDT (Table 10). Broad implementation of multidisciplinary PERT likely supports the optimal management of patients with PE and appropriate application of CDT.
Conflict of interest statement
P. Pruszczyk received consultancy and speaker fees from Boston Scientific, Bayer Healthcare, BMS-Pfizer, Boehringer and travel grants from BMS-Pfizer and Boehringer Ingelheim. F.A. Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, the Netherlands Organisation for Health Research and Development, Actelion, the Dutch Heart foundation, and the Dutch Thrombosis Association, all outside the submitted work. N. Kucher received research funding from the Swiss National Science foundation, the Swiss Heart foundation, and Concept Medical, as well as honoraria from Boston Scientific, Inari, Bard, OptiMed, Bayer and Pfizer. M. Roik received consultancy and speaker fees from Boston Scientific. N. Meneveau received consultancy and speaker fees from Abbott Vascular, Edwards Lifesciences, Terumo, Boston Scientific, Bayer Healthcare, BMS-Pfizer, Boehringer and AstraZeneca; and personal fees from BTG. A.S.P. Sharp received consultancy or speaker fees from Boston Scientific, Medtronic, Recor Medical, Penumbra Inc, and Philips. J.E. Nielsen-Kudsk is a consultant and proctor for Abbott and Boston Scientific. S. Obradović received speaker fees from BMS-Pfizer, Bayer Healthcare, Boehringer, Sanofi, AstraZeneca, Merck Sharp & Dohme and Novartis. S. Barco received consultancy and speaker fees from Bayer, Concept Medical, Boston Scientific, and Inari; and research grants from Bayer, Bard, Boston Scientific, and Bentley. F. Giannini received consultancy and lectures fees from Abbott Vascular, Edwards Lifesciences, Terumo, Boston Scientific, GADA, Abiomed and AstraZeneca. G. Stefanini received a research grant (to the Institution) from Boston Scientific, and speaker and personal fees from Abbott Vascular, Boston Scientific and BMS-Pfizer. G. Tarantini received consultancy and lecture fees from Abbott Vascular, Edwards Lifesciences, Terumo, Boston Scientific, GADA, Abiomed, and AstraZeneca. S. Konstantinides reports institutional research grants from Bayer, Daiichi Sankyo, Boston Scientific, and Inari; and personal consultancy/speaker fees from Bayer, Daiichi Sankyo, Boston Scientific, BMS-Pfizer, and Merck Sharp & Dohme. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

