Abstract
Since its inception in December 2006, the EuroCTO Club has strived to provide the framework for state-of-the-art chronic total occlusion (CTO) percutaneous coronary intervention (PCI) in Europe and nearby regions. Among its initiatives, the EuroCTO Club has published a set of recommendations regarding the technical aspects of CTO PCI, whose last edition dates to 2012. The EuroCTO Club consensus document discusses CTO PCI clinical indications, techniques and equipment use, as well as the qualifications of operators/centres. Given the considerable amount of progress made by this subspecialty in recent years, there is a need for an updated document that includes data from recent clinical trials and registries, information on novel devices and techniques, and an up-to-date revision on the training requirements to approach CTO PCI. The current updated consensus document of the EuroCTO Club reflects the expertise of European operators to promote the widespread application of state-of-the-art CTO PCI, not only in Europe but also across neighbouring communities.
Introduction
The EuroCTO Club was established in December 2006. The first two consensus documents on the recanalisation of chronic total occlusions (CTOs) were published in 2007 and 20121,2, respectively, to provide an overview of CTO-dedicated material and to try to set standards on clinical indications, techniques and equipment use, and the qualifications of operators/centres. Since 2012, there have been considerable changes in practice, in parallel with the publication of a plethora of novel data, dramatically changing the landscape of CTO percutaneous coronary intervention (PCI).
For this reason, the EuroCTO Club has written this updated consensus document, reflecting the expertise of European operators to promote the widespread application of state-of-the-art CTO PCI in Europe and neighbouring communities.
DEFINITION AND EPIDEMIOLOGY
Coronary CTO is defined as a 100% stenosis with Thrombolysis In Myocardial Infarction (TIMI) grade 0 flow for more than three months1,2. Non-intralesional ipsilateral bridging collaterals may provide antegrade flow to the vessel beyond the occlusion and give a false impression of a functional incomplete occlusion. This should be distinguished from flow within the occluded segment by a careful frame-by-frame assessment in different angiographic projections.
Coronary CTOs are relatively common, observed in approximatively 15-25% of patients with coronary artery disease undergoing coronary angiography3,4,5,6,7. The right coronary artery is the most common CTO vessel, representing about half of the CTO cases5. The CTO prevalence is much higher (~90%) among patients with prior coronary artery bypass grafting (CABG)5, while a CTO is found in only one tenth of patients referred for ST-elevation myocardial infarction (STEMI)8.
COLLATERAL CIRCULATION IN CTOs
The typical feature of a CTO is the presence of collaterals, found in ~90% of cases5. They have the capacity to preserve myocardial function but will not prevent ischaemia during exercise due to a limited capacity to increase blood flow9. The collateral supply provides a perfusion pressure in the range of 30-40 mmHg at the occluded territory, a pressure that leads to the functional reduction of distal vessel size, which then leads to the underestimation of the vessel dimensions during a recanalisation procedure10. The presence of collaterals does not predict viability, as they also develop in patients with prior myocardial infarction and large akinetic territories, i.e., viability still needs to be tested in well collateralised CTOs11. Moreover, the presence of a well-developed network of collaterals is not protective towards ischaemic insults, as even in such a patient population revascularisation might provide a survival benefit compared with medical therapy12.
The angiographic assessment has been refined beyond the classic Rentrop classification by introduction of the grading of the collateral connection size13, which is also helpful to select the appropriate guidewires and techniques for collateral crossing. A more detailed analysis of collateral supply led to the introduction of a collateral scoring system for the suitability for retrograde transcollateral interventions14.
RATIONALE OF CTO REVASCULARISATION
Viable myocardium subtended by a CTO is generally ischaemic, regardless of the degree of collateralisation, as has been shown in fractional flow reserve (FFR) studies15,16. CTO recanalisation aims to improve myocardial perfusion of the corresponding ischaemic territory17. This in turn has beneficial effects at multiple levels (attendant on CTO success). First, successful CTO PCI relieves ischaemia18,19, which has been shown to be associated with a decrease in severity and frequency of angina, as well as improved functional status and better quality of life19,20. Second, an untreated CTO is associated with incomplete revascularisation21,22, which in turn has been associated with persistent left ventricular dysfunction at follow-up23. As such, CTO recanalisation allows complete revascularisation which might lead to improvement in left ventricular function23. Third, CTOs have an arrhythmic potential, as up to 3% of CTO patients present with malignant ventricular arrhythmias5. In a cohort of patients with implantable cardioverter-defibrillator, CTO subjects experienced a higher incidence of appropriate shocks for malignant ventricular arrhythmias, as compared to patients without CTO24. Moreover, ventricular arrhythmias can be observed even in the absence of myocardial scar, thus possibly being related to an ischaemic phenomenon25.
Since there are only observational data12 suggesting a mortality benefit for CTO recanalisation, consideration for intervention should be in order to improve symptoms and quality of life. Appropriate indication is thus important in CTO intervention, not least since this procedure is associated with increased costs, cath lab resource utilisation, and procedural risks, as compared with non-CTO PCI. CTO recanalisation is indicated in cases where a significant benefit is likely in terms of symptom relief, ischaemia reduction, and/or improvement in left ventricular function17. A baseline echocardiogram should be performed in all CTO patients. In patients with normal wall motion or hypokinesia of the CTO territory, myocardial viability is assumed to be present, and CTO recanalisation should be undertaken in patients who remain symptomatic (with angina or dyspnoea on exertion) despite optimal medical therapy (OMT) (e.g., two anti-anginal medications), with the aim of achieving complete revascularisation. In asymptomatic patients, ischaemic burden evaluation is recommended, and CTO recanalisation is indicated if ischaemia is present in ≥10% of the left ventricular mass18,26. In case of akinesia/dyskinesia of the CTO territory, proof of viability with non-invasive imaging should be pursued, and CTO recanalisation is indicated only in patients with viable myocardium in the CTO territory, associated with symptoms or ischaemia26. While these are general consensus recommendations, patients should be judged for intervention on a case-by-case basis (e.g., in case of anti-anginal drug intolerance).
GUIDELINES, CTO PCI REGISTRIES, AND RANDOMISED TRIALS
Current guidelines recommend (class IIa B) that percutaneous revascularisation of CTOs should be considered in patients with angina resistant to medical therapy or with a large area of documented ischaemia in the territory of the occluded vessel27.
One obstacle to a wider adoption of CTO recanalisation is the absence of robust evidence on the benefits of this treatment. Non-randomised comparative studies showed a beneficial effect of CTO recanalisation on symptoms, quality of life, and left ventricular function12,28,29, while its impact on survival remains controversial28,29,30. However, this evidence is derived mostly from studies comparing patients with successful vs. unsuccessful CTO PCI and is therefore prone to important confounders.
Four randomised trials (RCTs) have now been published or presented. Each of these trials was modest in size and open-label in design. Definitive trials remain a scientific gap. The EXPLORE (Evaluating XIENCE and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Elevation Myocardial Infarction) trial is based on the well-established observation of the disadvantage of a patient with a CTO when experiencing a STEMI (“double jeopardy”)31. The trial randomised survivors of a STEMI within seven days to receive PCI or conservative management for a concomitant CTO in order to observe differences in left ventricular function at four months31. The trial did not show a difference, but it was hampered by a low success rate for CTO PCI (73%), a high crossover rate (23%), and the fact that those most likely to profit from CTO PCI were those who died early from shock.
The DECISION-CTO trial was presented at ACC 2017, and has not yet been published as an article. It randomised patients with a CTO to OMT vs. CTO PCI. Critical flaws in trial design include the fact that revascularisation of non-CTO lesions was allowed in both groups and observed in more than 70% of patients (thus diluting the real impact of CTO recanalisation on patient outcomes), extremely low enrolment even from high-volume centres (which suggests a strong selection bias), an 18% crossover rate, and the inclusion of all-cause death and stroke in the primary endpoint (while PCI in all-comers in an elective setting has never shown benefits with regard to such endpoints). Not surprisingly, the trial did not find any difference in the primary endpoint.
In contrast, the EUROCTO trial32, which also randomised CTO patients to OMT vs. CTO PCI, had all non-CTO lesions treated before randomisation, so the sole effect of a remaining CTO on symptoms could be evaluated (primary endpoint). The trial showed a superior effect of PCI on angina frequency and quality of life as compared with OMT, 12 months after randomisation32. Furthermore, physical limitation and functional angina class were improved. Of note, the success rate in that trial was 86.6%, thus in line with state-of-the-art CTO PCI worldwide32.
Recently, the IMPACTOR-CTO (Impact on Inducible Myocardial Ischemia of PercutAneous Coronary InTervention versus Optimal Medical TheRapy in Patients with Right Coronary Artery Chronic Total Occlusion) trial randomly assigned patients with isolated CTO of the right coronary artery (RCA) to either PCI (n=32) or OMT (n=33)33. In the PCI group, Obedinskiy et al33 demonstrated that the decrease in myocardial ischaemia burden at 12 months (primary endpoint) was significantly higher in comparison with the OMT group (13.9±6.1% vs. 0.3±4.2%; p<0.01). Similarly, functional status and quality of life only improved in the PCI group33, confirming the findings of the EUROCTO trial in the setting of single-vessel disease CTO patients.
Figure 1 summarises the major results of the published randomised CTO trials.
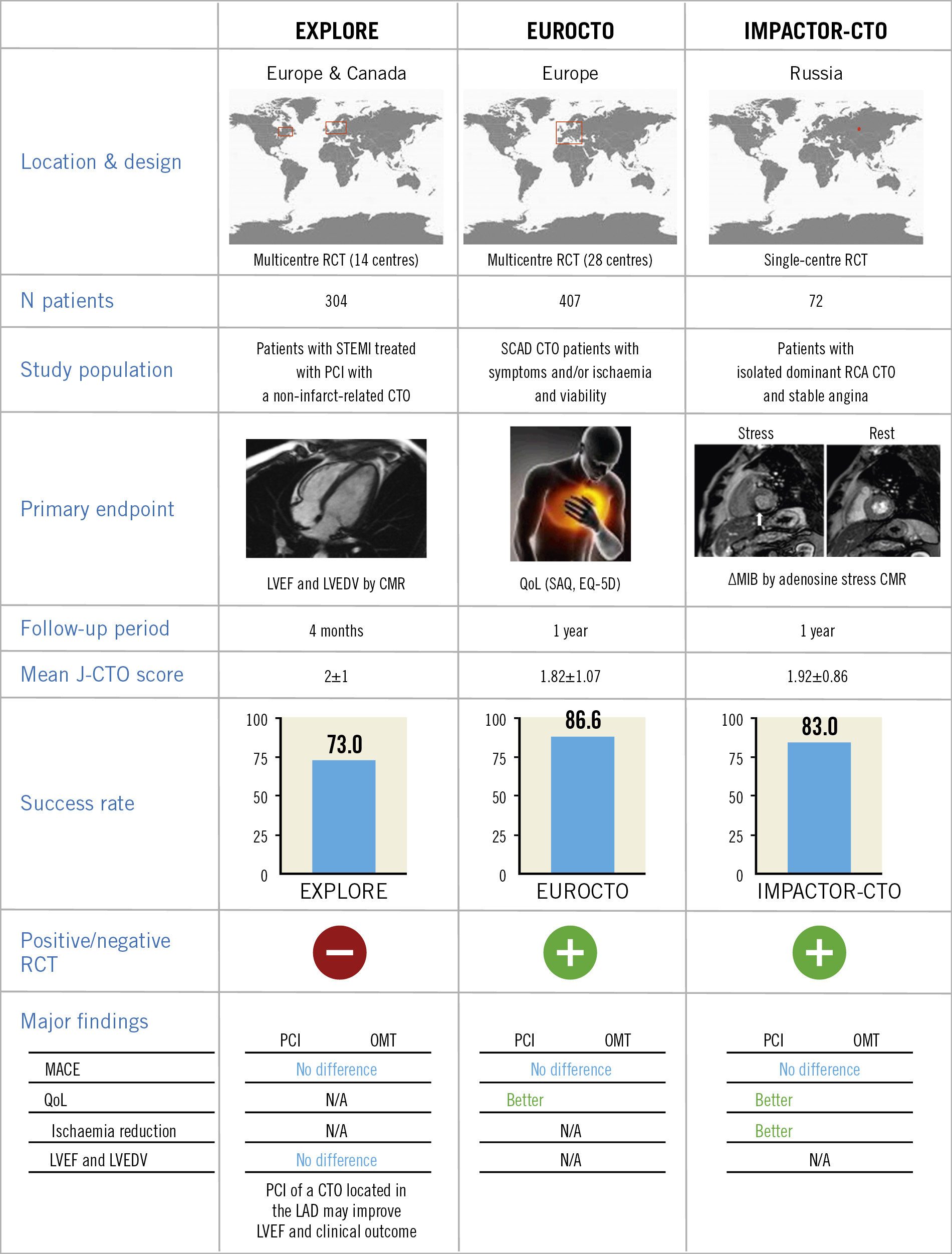
Figure 1. Major findings of the published RCTs comparing PCI vs. OMT in CTO patients. ∆MIB: decrease in myocardial ischaemia burden; CMR: cardiac magnetic resonance; CTO: chronic total occlusion; EQ-5D: EuroQol 5 dimensions questionnaire; J-CTO: Japanese chronic total occlusion; LAD: left anterior descending; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MACCE: major adverse cardiac and cerebrovascular events; MACE: major adverse cardiovascular events; MI: myocardial infarction; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; QoL: quality of life; RCA: right coronary artery; RCT: randomised clinical trial; SAQ: Seattle Angina Questionnaire; STEMI: ST-segment elevation myocardial infarction
Next to randomised trials, which always include a selected patient group, registries including all-comers are important for the assessment of trends in treatment. Currently, the longest-running and largest registry is the ERCTO registry, with more than 17,000 procedures recorded. Since its inception in 2008, this registry has witnessed an increase in lesion complexity, with a parallel increase in the use of the retrograde approach and in success rates34.
Planning the CTO procedures
PREDICTIVE CTO SCORES
A key contributor to achieving success in CTO PCI is meticulous preparation. While the indication for CTO recanalisation should be clinical and not based on any score predicting the probability of technical success of the procedure, scoring models provide a quantitative measure of procedural difficulty and the probability of recanalisation success, which can help with clinical decision making. Furthermore, by providing more objective evaluation of anatomic and clinical complexity, CTO scores enable better case selection according to operators’ experience35. Finally, within the Heart Team, the decision to revascularise and the optimal strategy can be tailored to each CTO patient, taking into account the objective probability of achieving angiographic/clinical success with PCI.
The J-CTO (multicentre CTO registry in Japan) score is currently the most widely used score36. Independent predictors of failure (each given one point) that make up the J-CTO score include prior failed attempt, angiographic evidence of heavy calcification, bending ≥45° within the occluded segment, blunt proximal stump, and occlusion length >20 mm. CTO lesions are then graded as easy, intermediate, difficult, and very difficult (J-CTO scores of 0, 1, 2, and ≥3, respectively)36. While experienced operators can attempt even the toughest of cases with high success rates, operators early in their learning curve can select “simpler” cases (J-CTO score 0 or 1), referring those judged to be more difficult (J-CTO ≥2) to CTO-dedicated centres, or performing them with the guidance of a proctor35.
Newer CTO scores highlight the variety of approaches to CTO PCI. The clinical and lesion-related (CL) score created based on primarily antegrade procedures may perform better for antegrade-only operators37, whereas the ORA (ostial location, Rentrop grade <2, age ≥75 years) score and the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score may be more suitable for predicting recanalisation success in retrograde or hybrid procedures38,39.
MULTIDETECTOR COMPUTED TOMOGRAPHY (MDCT)
The importance of preprocedural multidetector computed tomography (MDCT) remains debatable. MDCT is more accurate than angiography for the definition of the CTO length, the presence of calcium, and the determination of vessel size and remodelling40. Recently, two MDCT-based scores (CT-RECTOR and KKCT) have been introduced41,42. Compared with the angiography-based J-CTO score, these MDCT scoring systems provide a more accurate non-invasive tool for predicting time-efficient guidewire crossing and final procedural success. MDCT is also indicated in case of long ambiguous CTOs, with poor path visualisation via collaterals and previously failed procedures40. In the cath lab, MDCT can also be of benefit for visualisation of the guidewire advancement and optimisation of viewing angles, taking advantage of co-registration. However, its availability is limited. Further studies are required to determine the added value of preprocedural MDCT in CTO PCI better.
FAILING CTO PCI – WHEN TO STOP
Although failure can be highly frustrating and demoralising, being able to accept it and learn and apply the lessons that failure provides is a critical step for the CTO operator. Indeed, the art of “knowing when to stop” is a key issue in CTO PCI to avoid major complications. Operators should consider stopping a CTO PCI attempt if the procedure time is >3 hours, if more than 4x the estimated glomerular filtration rate of contrast has been used or if the radiation dose is >5 Gy air kerma, unless recanalisation is already about to be completed (e.g., antegrade wire in the distal true lumen, or having crossed the retrograde collateral channel). In case of subintimal tracking with failure to re-enter the true lumen, planning a new attempt could be associated with better procedural outcome. Subintimal plaque modification by balloon angioplasty may alter the occlusion anatomy, favouring true-to-true wiring on a subsequent attempt43. In certain cases, the vessel can heal quite favourably and occlusion recanalisation can be observed. It is therefore not surprising that subintimal plaque modification can promote an improvement in quality of life and angina44. In case of no symptomatic improvement, the patient can be brought back and recanalisation re-attempted after six to eight weeks from the first attempt.
Although CTOs re-attempted after initial failure are associated with higher angiographic complexity, longer procedural duration and fluoroscopy time in comparison with first attempt CTO lesions, in experienced hands the success and complication rates were reported to be similar45. The number of times a procedure may be re-attempted is an individual question to be assessed carefully by the treating physician and the patient, and must take into consideration the severity of symptoms, likelihood of success, risk of complications, and the patient’s wishes.
CTO materials and dedicated devices
GUIDEWIRES
Guidewire technology has largely evolved during the last decade and contributed to technique evolution and procedural efficiency. No single wire serves all lesions and all circumstances. Understanding the interaction of different kinds of guidewire (soft <1 gr, intermediate stiff 2-6 gr, stiff >9 gr) with different types of tissue (soft, hard and calcified) is of paramount importance. Soft (<1 gr) tapered polymeric composite core guidewires are suitable for soft tissue tracking (passive wire control). Intermediate stiffness (2-6 gr) tapered composite core guidewires are used for hard tissue tracking (active wire control), while very stiff (>9 gr) tapered guidewires are suitable for calcified tissue penetration46.
In the early era of CTO PCI stiff wires, non-tapered (Miracle family) and tapered (Confianza family; both Asahi Intecc, Aichi, Japan), were predominantly used for lesion crossing. These wires were limited by incapacity to deflect and poor steerability in tortuous anatomy. In 2008, low tip stiffness (<1 gr) in combination with tapering, polymer and hydrophilic coating was introduced (Fielder™ XT; Asahi Intecc) and was proven to be very good for soft tissue tracking, offering a success rate of 30-40%, but it tended to over-deflect and was also not steerable in more complex anatomy. The advent of two technologies addressed the abovementioned limitations. The first one was the composite core technology, that became available in 2010-2011 (Asahi SION family), dramatically enhancing wire steerability and tip shape retention and which was also introduced in the low stiffness wires to improve their performance (Fielder XT-A/Fielder XT-R). The second one was based on the growing experience and understanding of CTOs over the years, that made it evident that penetration power and steerability can only be combined in intermediate stiffness wires. In 2013 a combination of composite core, tapering, polymeric and hydrophilic coating in intermediate stiffness wires introduced the “deflection and rotation” concept that prevailed in modern CTO wiring (Gaia family; Asahi Intecc). The use of these wires will continue to increase in all anatomies except heavily calcified lesions, where very stiff wires are indispensable.
The milestones of CTO guidewire development are presented in Figure 2 and an overview of the currently available CTO guidewires is presented in Supplementary Table 1,2,46.
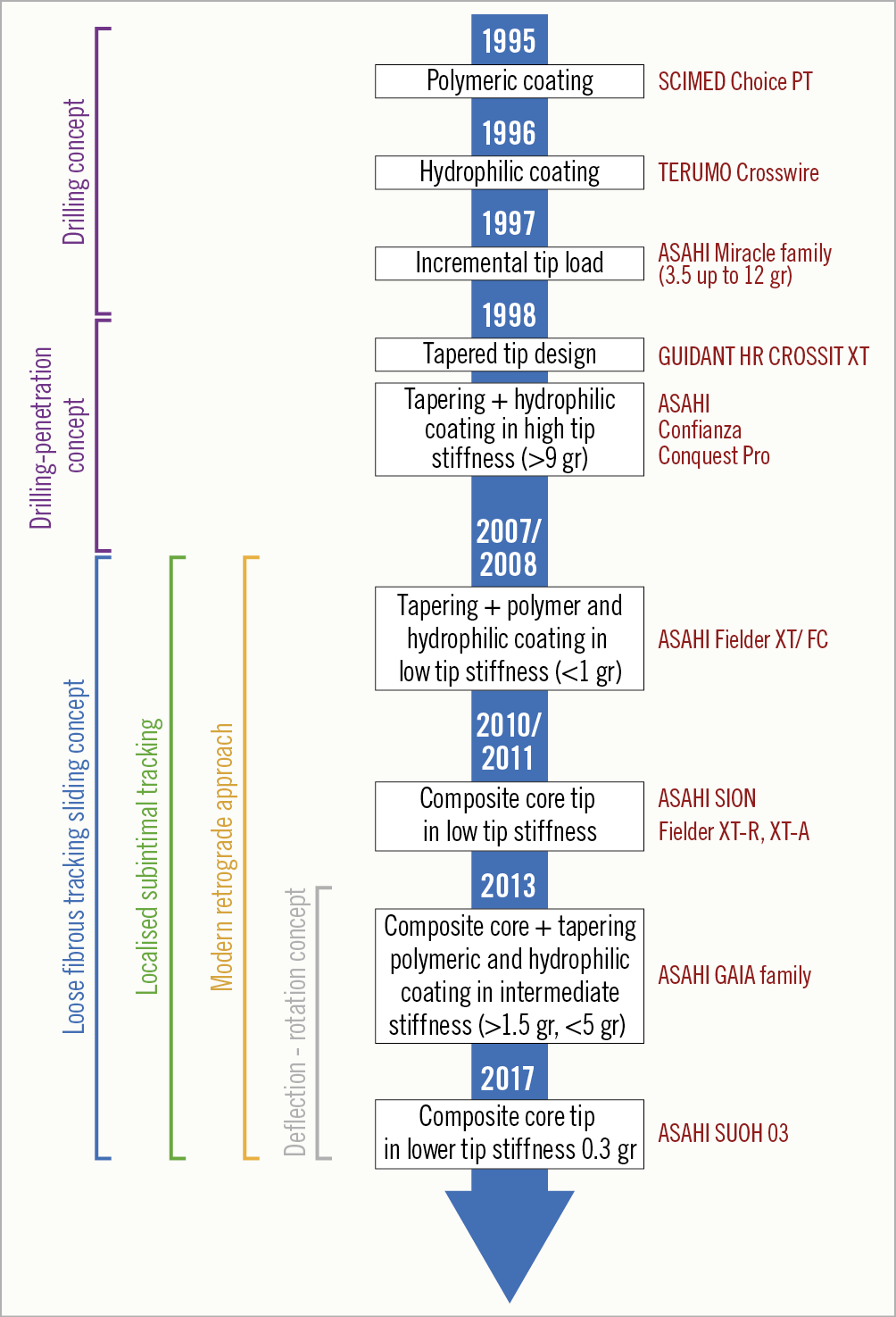
Figure 2. Milestones in CTO guidewire technology. CTO: chronic total occlusion
MICROCATHETERS
In the current era of CTO PCI, guidewires should always be used with an over-the-wire (OTW) device (microcatheter or over-the-wire balloon) in order to ease torque in the tip response, preventing flexion, kinking, prolapse of the guidewire, and improving penetration ability. Microcatheters also allow reshaping or changing the guidewire without losing the distal position. Microcatheters are generally preferred to OTW balloons. Indeed, they are more flexible and track better. Moreover, they allow better understanding of distal tip position and have less tendency to kink than OTW balloons.
Table 1 illustrates the most commonly used microcatheters in CTO PCI.
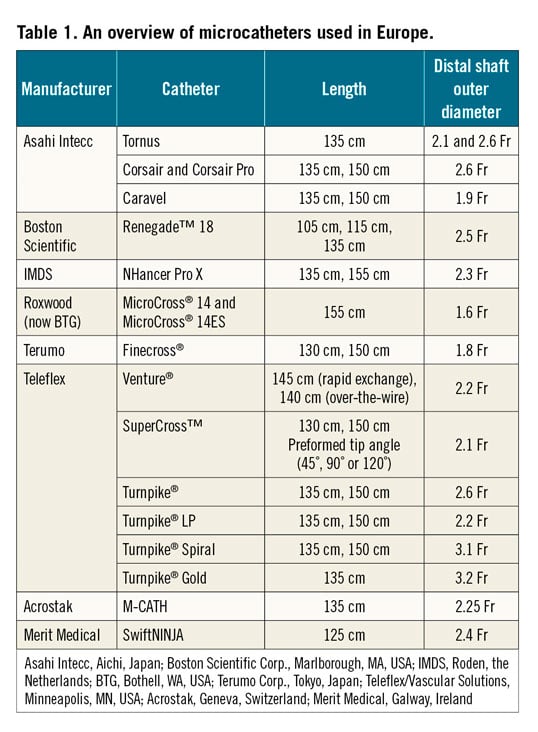
Details concerning the use of several microcatheters are given in Supplementary Appendix 1.
DUAL LUMEN MICROCATHETERS
Dual lumen microcatheters consist of a rapid exchange delivery system in the distal segment associated with an OTW lumen along the catheter. A radiopaque marker band identifies the distal tip of each lumen; the distal band corresponds to the exit point of the rapid exchange segment and the proximal band marks the exit point of the OTW lumen.
The indications for dual lumen microcatheters in CTO PCI can be summarised as follows: a) parallel wire technique; b) wiring a CTO in the presence of a side branch at the proximal cap; c) wiring the distal true lumen without losing access to a side branch near the distal cap; d) antegrade wiring of the distal true lumen if the externalised retrograde guidewire crossed a collateral near the distal cap; e) wiring of difficult-to-access collaterals for the retrograde approach. Hence, such devices are useful to treat bifurcation lesions within the CTO body or located in close proximity to the proximal or distal cap, particularly when a long dissection is present and the second wire has to follow the same course as the first wire for optimal bifurcation treatment using either the one- or two-stent technique.
An overview of the currently available dual lumen microcatheters is presented in Supplementary Table 2.
SUPPORT CATHETERS AND GUIDE EXTENSION CATHETERS
An overview of the utility and use of currently available support catheters and guide extension catheters is presented in Supplementary Appendix 2 and Supplementary Table 3.
Techniques for CTO recanalisation
ACCESS ROUTE – GUIDING CATHETER SELECTION, CONTRALATERAL INJECTION
For CTO recanalisation, it is mandatory to ensure an optimal guide catheter support with a large enough lumen to host devices in parallel, as well as two arterial sheaths for contralateral injections. Good ipsilateral collateralisation may allow one to start unilaterally; however, performing a CTO recanalisation without displaying the distal coronary target is irresponsible. Indeed, dual injection allows better visualisation and understanding of CTO morphology and complexity. In addition, it can improve procedural safety by elucidating guidewire location during crossing attempts. CTO PCI with a single guide can be performed in selected cases with absent collateral circulation or only ipsilateral collateralisation.
Most operators use 7 Fr guides for recanalisation and 5 or 6 Fr guides for contralateral injection47. We recommend the use of guide catheters with side holes to prevent forceful intraplaque injections that may easily cause spiral dissections and also to allow proper monitoring of the arterial pressure. For the left coronary artery, extra backup guides (EBU) are most appropriate; sometimes Amplatz left curves are valid alternatives for the circumflex artery. For the right coronary, Judkins right or Amplatz left (AL1 or AL 2) represent the most employed catheters, while internal mammary artery (IMA) guides, shepherd’s crook (SC) or hockey stick curves are useful for SC origin engagement. The operator should be familiar with deep intubation, anchoring balloon and mother-and-child techniques (guide-in-guide). The various choices for access are: bifemoral artery, biradial (or ulnar) artery48, radial and femoral, and two ipsilateral femoral sheaths49. While the radial approach appears more cumbersome, especially in very complex CTO procedures, none of the approaches was shown to be superior to any other48.
Regarding perprocedural anticoagulation, an initial bolus of intravenous unfractionated heparin (100 IU/kg) is generally administered. The activated clotting time is then monitored every 30 minutes to determine whether an additional bolus of unfractionated heparin is necessary to maintain an activated clotting time >250-300 s. Upstream use of low molecular weight heparin, glycoprotein IIb/IIIa inhibitor therapy or bivalirudin is generally not recommended.
ANTEGRADE APPROACH
The antegrade approach, and particularly antegrade wire escalation (AWE), remains the cornerstone of CTO PCI, being performed in approximately three quarters of cases50,51. All experts agree that AWE is the strategy of choice in case of non-ambiguous proximal cap, good distal landing zone, and lesion length <20 mm. Indeed, a true-to-true lumen approach – when feasible – seems recommendable, as extensive vessel disruption with dissection/re-entry techniques is associated with greater intravascular ultrasound (IVUS)-detected vascular injury, angiographic dye staining/extravasation, branch occlusion, and periprocedural myocardial infarction52. However, the clinical significance of such findings is debated, since no clear evidence of the superiority of a true-to-true approach has been demonstrated so far53,54. The choice of microcatheters and guidewires depends on operator preference and specific angiographic features: in general, highly manoeuvrable guidewires with 1:1 torque response are recommended as initial choices (e.g., Gaia family, Fielder XT-R; FIGHTER™ [Boston Scientific, Marlborough, MA, USA]) to maintain an intra-plaque track. If a subintimal situation at the landing zone is observed, a wide array of techniques for true lumen re-entry is available. These encompass both wire-based and device-based (Stingray™; Boston Scientific) re-entry55. Among wire-based techniques, preference should be given to those providing targeted re-entry56 (e.g., parallel wires, mini-STAR, limited antegrade subintimal tracking, IVUS-guided re-entry …). Alternatively, antegrade dissection/re-entry (ADR) is suggested by some operators as a first-line strategy. This is usually performed in case of ambiguous proximal cap, adequate distal landing zone, and occlusion length >20 mm. In this setting, the CrossBoss™ (Boston Scientific) is recommended by some operators, since this catheter is meant to create a smaller subintimal space disruption compared with knuckle wires. Subsequently, Stingray-based re-entry may be performed.
RETROGRADE APPROACH
The retrograde approach should be considered in occlusions with “interventional” collaterals (i.e., collaterals deemed to be negotiable by the operator depending on his/her experience), diseased landing zone, bifurcation at distal cap, and/or proximal cap ambiguity57. Dedicated microcatheters and guidewires should be used; the utilisation of OTW balloons and guidewires not specifically designed for collateral crossing should be avoided. Preference should be given to septal collaterals (due to lower risk in case of perforation as compared with epicardial channels)50,57,58 and diseased/occluded saphenous vein grafts (which do not present side branches and therefore allow easy navigation)58. Epicardial collaterals should be considered only by very experienced operators and, in any case, as a second-line option59. Fortunately, septal channels are most frequently found in both right coronary artery (72%) and left anterior descending (52%) CTOs60. However, circumflex CTOs most frequently present ipsilateral epicardial collaterals, which should be tackled with great care, utilising dedicated wires (e.g., SUOH 03, SION black; both Asahi Intecc) and techniques (e.g., tip injection to delineate vessel course, rendezvous or tip-in for externalisation, etc.). In any case, specific complication-solving skills are needed when undertaking the retrograde approach, including pericardiocentesis, coiling, covered stent implantation, and establishing haemodynamic support. With regard to the procedural techniques specific to the retrograde approach, although retrograde true-to-true wire crossing is ideally pursued in cases of short, non-calcified occlusions, reverse controlled antegrade and retrograde subintimal tracking (CART) is effectively performed in the majority of cases50,61. The recently introduced concept of “directed reverse CART” seems promising to maximise the effectiveness of this technique while limiting the extent of vessel damage. In directed reverse CART, close proximity between the antegrade and retrograde wires is achieved, before performing balloon dilatation with a small balloon (usually 2.0 mm). Then, a highly manoeuvrable retrograde wire (e.g., Gaia) is carefully manoeuvred towards the (lateral side of the) antegrade balloon, thus creating a connection between the retrograde and antegrade systems. If there is difficulty in making the connection, the use of IVUS should be considered to understand the antegrade and retrograde wire position62. Finally, in long occlusions with ambiguity or unclear vessel course and severe calcifications, a retrograde knuckle wiring (with polymer-jacketed guidewires) facilitates overcoming the occluded segment with a low risk of perforation.
THE CONCEPT OF THE “HYBRID ALGORITHM”
The integration of the aforementioned techniques into a homogeneous set of strategies has become known as the “hybrid algorithm”57. This approach was developed by North American operators in 2012 and is based on the concept of a rapid switch from one approach to another in case of low likelihood of success, in order to optimise procedural efficiency. After dual coronary angiography, four angiographic parameters are assessed: 1) proximal cap location and (non-) ambiguity; 2) occlusion length; 3) quality of the distal vessel; 4) presence of collaterals suitable for retrograde techniques (“interventional collaterals”). Based on these four features, an initial strategy and hierarchy for subsequent approaches are established. The hybrid algorithm has been shown to be effective (success rates of ~90%), safe (low rates of complications: tamponade 1.3%, periprocedural myocardial infarction 1.0%, death 0.4%), and efficient (favourable procedural metrics)50. Additionally, the mantra of this algorithm is its reproducibility and the fact that it can be easily taught/learned, resulting in high success rates obtained by new operators63.
Recently, the Asia Pacific Chronic Total Occlusion Club developed a modified hybrid algorithm for CTO PCI35. Despite similarities with the traditional hybrid algorithm, major changes exist in this new algorithm: i) the role of IVUS-guided entry to overcome proximal cap ambiguity is clearly highlighted; ii) the CTO length alone does not determine the choice of either a wire escalation strategy or a dissection re-entry strategy; iii) the use of the parallel wire technique and IVUS-guided wiring as a bail-out strategy in the antegrade arm; iv) the CrossBoss catheter should be considered as the first-line device for in-stent CTO recanalisation35.
In Figure 3, we propose a modified hybrid algorithm for CTO PCI using the contemporary techniques.
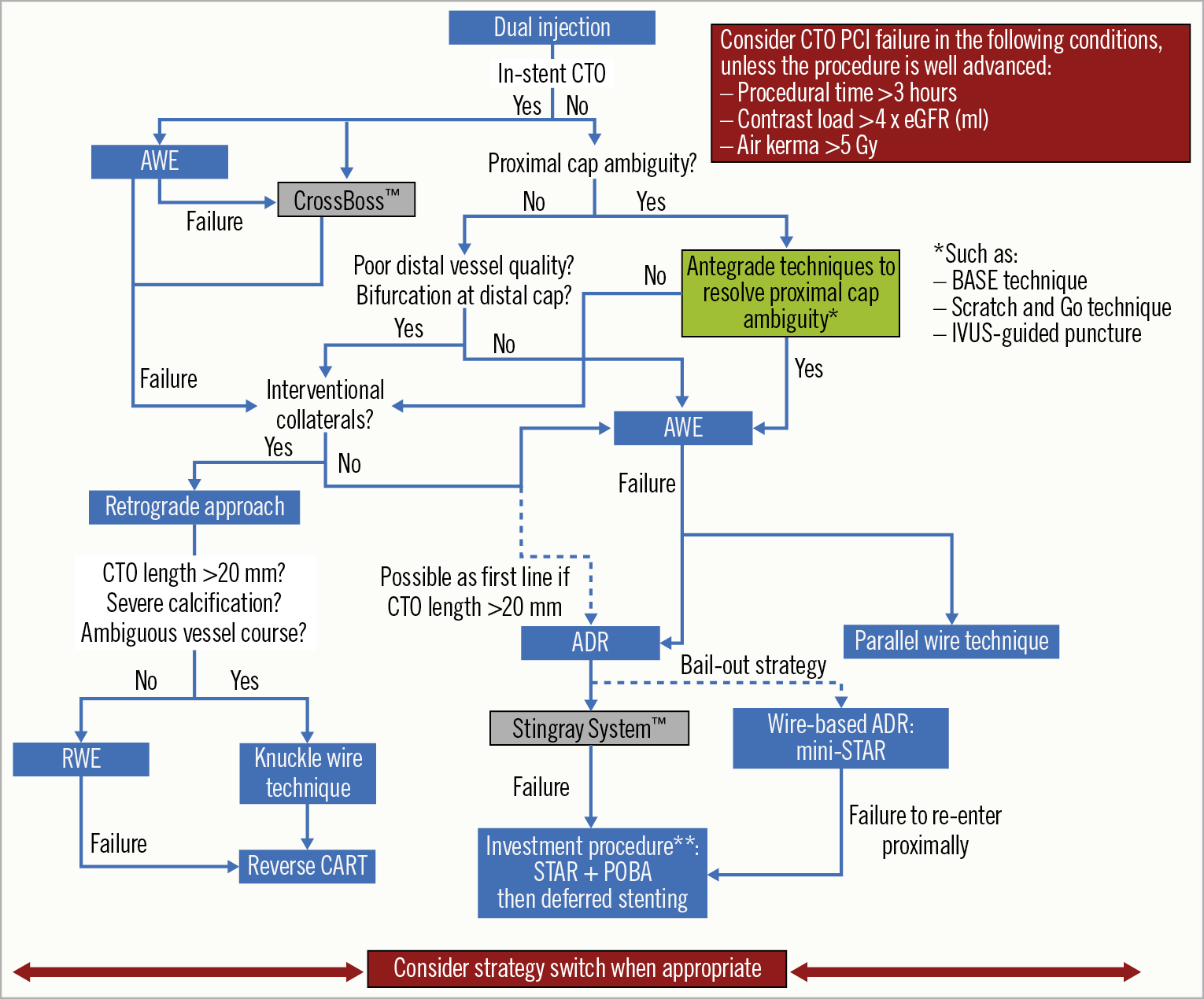
Figure 3. Modified hybrid algorithm for CTO PCI. **Investment procedure refers to deferred stenting (during a second procedure) after subintimal plaque modification via the STAR technique and balloon dilatation43. ADR: antegrade dissection re-entry; AWE: antegrade wire escalation; BASE: balloon-assisted subintimal entry; CART: controlled antegrade retrograde tracking; CTO: chronic total occlusion; eGFR: estimated glomerular filtration rate; IVUS: intravascular ultrasound; PCI: percutaneous coronary intervention; POBA: plain old balloon angioplasty; RWE: retrograde wire escalation; STAR: subintimal tracking and re-entry
Intravascular imaging in CTO PCI
The section on IVUS62,64,65 can be found in Supplementary Appendix 3.
The section on OCT66,67 can be found in Supplementary Appendix 4.
Stents in CTO PCI
After successful recanalisation of CTOs, implantation of drug-eluting stents (DES) reduces the rates of major cardiac events, restenosis and stent re-occlusion as compared to bare metal stents68. Everolimus-eluting (EES) and zotarolimus-eluting stents (second-generation DES) are currently preferred for CTO interventions as they enable better outcomes compared to the first generation of DES69,70,71,72,73 (Table 2). Although the BIOFLOW V study showed superiority of a hybrid ultra-thin sirolimus-eluting stent (SES) compared with EES, this finding was not confirmed in the PRISON IV study (non-inferiority for in-segment late lumen loss not reached, and a higher rate of binary restenosis in the hybrid SES group)74,75. The promising concept of this stent design needs further research in CTO lesions. In the EUROCTO trial, biolimus-eluting stents with abluminal biodegradable polymer were used with satisfactory one-year outcome (ischaemia-driven revascularisation – 2.9%)32.
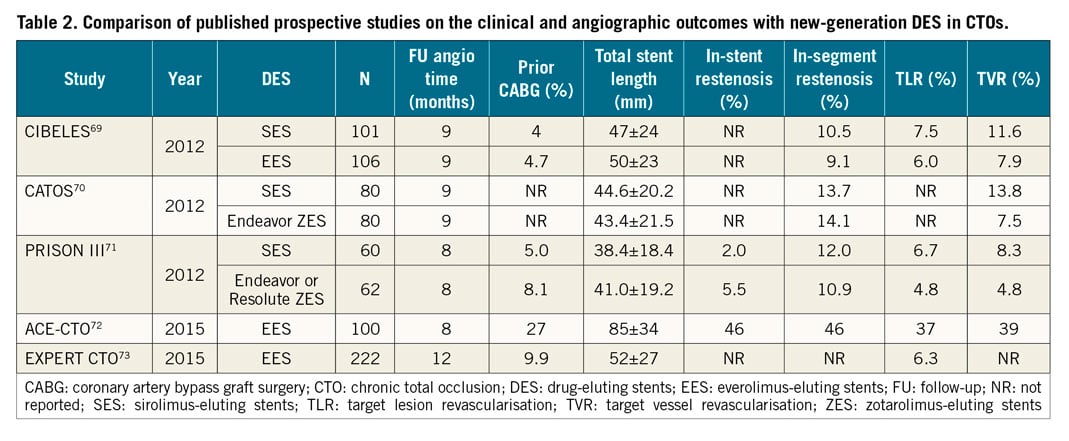
Although the concept of biodegradable scaffold use in CTOs appeared to be attractive, they are actually no longer recommended for clinical use. Some authors have also proposed the concept of using drug-coated balloons without stent implantation in CTO PCI; however, this needs further evaluation.
CTO in particular settings
Supplementary Appendix 5 is dedicated to CTO PCI in the following particular settings: CTO associated with bifurcation lesions76,77,78, in-stent CTO79,80,81,82,83 and CTO patients with low left ventricular ejection fraction (LVEF)27,84,85,86.
Immediate outcome, complications and safety issues
The results of opening a CTO are mainly operator-dependent as well as being driven by a few patient- and morphology-related parameters. Our experience, now with far more than 20,000 procedures in the monitor-reviewed online registry of the EuroCTO club, shows that operators who performed more than 300 CTOs and maintain an annual procedure number of at least 50 cases will have a success rate of more than 85%, which is still lower than that of non-occlusive lesions51. As success rates have increased during recent years, the rate of major complications has decreased to less than 2% and appears close to that of PCI of non-occluded coronary arteries62. Periprocedural myocardial injury discovered by measurement of cardiac biomarkers may occur in 5-10% of the patients, is more common with the retrograde approach and is associated with worse clinical outcome at follow-up87. Aortocoronary dissection may occur in <1% of CTO PCI attempts88; the therapeutic strategy and outcome depend on the rapidity of the entry point sealing and the degree of extension of the dissection89. Donor vessel injury during retrograde CTO PCI requires rapid identification and management, since it is frequently associated with extensive ischaemia and haemodynamic instability90. Small perforations and pericardial effusions are more likely to occur (3-5%)51,89, but are uneventful if addressed rapidly and properly89,90,91,92,93. Figure 4 and Supplementary Appendix 6 focus on the management of coronary perforations during CTO PCI attempts.
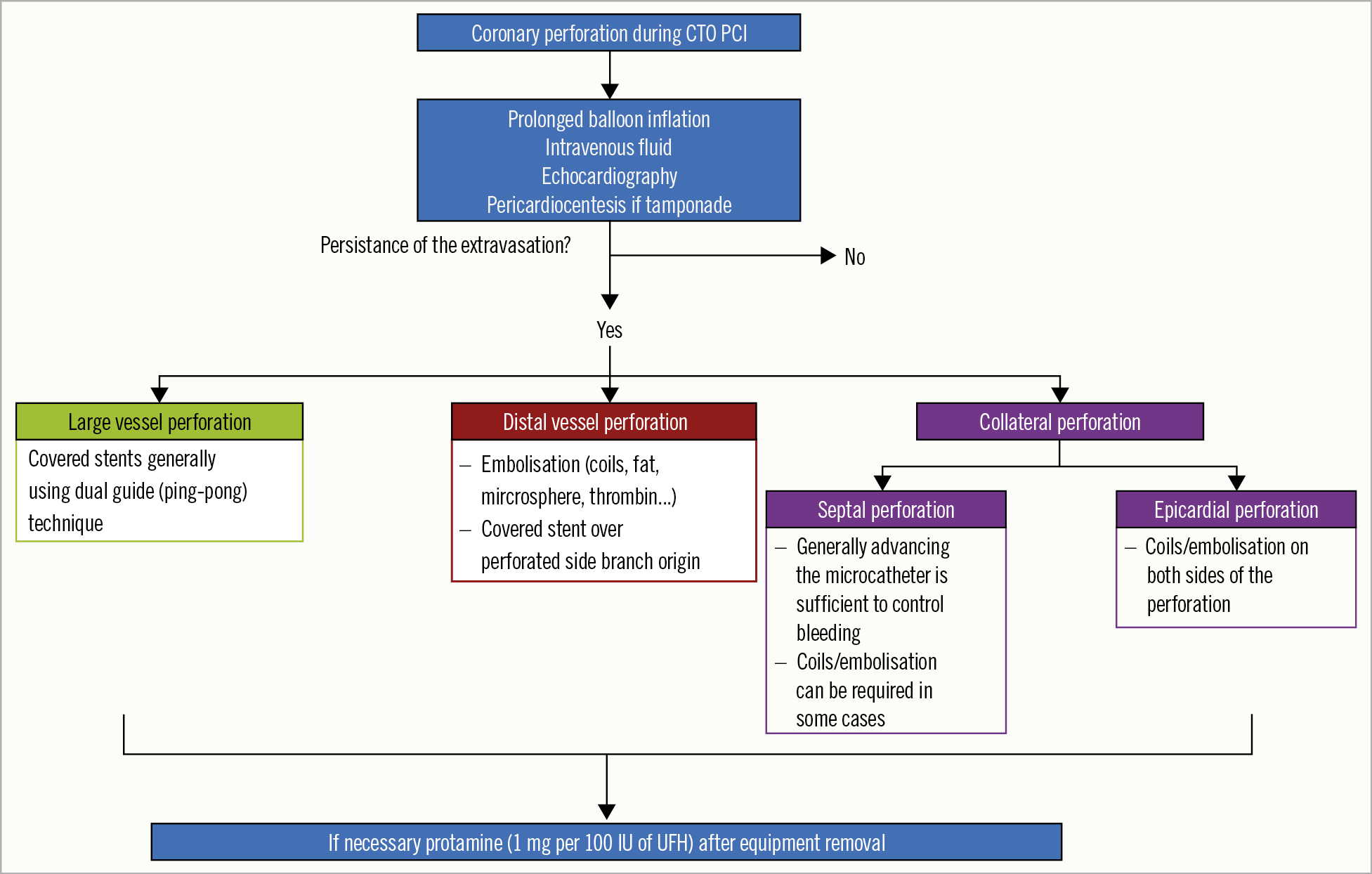
Figure 4. Management of coronary perforation in CTO PCI. CTO: chronic total occlusion; UFH: unfractionated heparin; PCI: percutaneous coronary intervention
The section on radiation and contrast use94,95,96,97 can be found in Supplementary Appendix 7.
Dual antiplatelet therapy (DAPT) after CTO PCI
Dual antiplatelet therapy (DAPT) for at least six months is currently recommended post-stenting in patients with stable ischaemic heart disease (class I recommendation)98. Prolonged (up to 30 months) DAPT duration may be considered (class IIb recommendation) in patients who have undergone complex PCI98. The optimal duration of DAPT in patients who underwent CTO PCI, considered to be at higher ischaemic risk, remains unknown. In a meta-analysis of six RCTs, Giustino et al99 showed that longer DAPT duration after PCI (>12 months) was not associated with improved clinical outcomes in CTO patients, differently from other subsets of complex non-CTO PCI, such as long stent length (>60 mm), two-stent bifurcation technique and ≥3 stents implanted. In another retrospective study, Lee et al100 showed no differences in the incidence of major adverse cardiac and cerebrovascular events or moderate to severe bleeding between CTO patients taking >12-month DAPT and those switched to single antiplatelet therapy after 12 months.
Hence, in the absence of strong clear evidence, DAPT in CTO patients undergoing PCI should be prescribed on a tailored (case-by-case) basis and the duration should be indicated according to the clinical presentation and the assessment of both ischaemic and bleeding risks98.
How to set a CTO programme
Supplementary Appendix 8 focuses on the learning/training process in CTO PCI as well as centre requirements and centre/operator qualifications.
Conclusion
During recent years, major advances have been achieved in the field of CTO PCI. The current updated consensus summarises the contemporary European practice in CTO PCI influenced by the development of dedicated material and the growing expertise among European CTO operators. The aim of the EuroCTO Club is to contribute actively to the training of interventional cardiologists in contemporary CTO techniques in order to achieve high levels of success, low rates of complication and to improve the outcome of CTO patients.
Conflict of interest statement
L. Azzalini reports research grants from Abbott Vascular, ACIST Medical Systems, Guerbet, and Terumo, and honoraria from Guerbet, Terumo and Sahajanand Medical Technologies. A. Avran reports honoraria from Abbott Vascular, Boston Scientific, Biosensors, Terumo and Biotronik for teaching courses and proctoring. A. Gershlick has received travel sponsorship and speaker’s fees from Abbott Vascular and Medtronic. C. Di Mario reports speaker’s fees from Philips Volcano. The other authors have no conflicts of interest to declare.

