Abstract
Aims: Proximal endovascular clamping (PEC) appears to be a safe and effective cerebral protection technique for carotid artery stenting (CAS). So far the presence of severe stenosis of the ipsilateral external carotid artery (ECA) was considered a contraindication to PEC. This study presents the results of PEC for CAS in patients simultaneously presenting an ipsilateral ECA stenosis.
Methods and results: From September 2004 to December 2006, CAS was performed in 500 unselected cases using PEC. In sixty cases (12%) a severe ipsilateral ECA stenosis (> 80%) was identified by angiography. In all these cases procedural success, defined as the ability to establish cerebral protection as well as stent deployment with a residual stenosis <30%, was achieved. Time of flow blockage was 188±37 sec. Clamping intolerance, a transient neurological deficit lasting < 20 min, occurred in five of 60 patients. In-hospital MACCE included only one minor stroke, but no major strokes, AMI or death.
Conclusions: This experience indicates that the use of PEC, also in the presence of a critical ECA stenosis, is safe and effective. Therefore critical ECA stenosis should no longer be considered a contraindication to PEC.
Introduction
Carotid artery stenting (CAS) is considered a reasonable alternative to carotid endarterectomy (CEA), particularly in patients at high risk for CEA1. Although there are no randomised studies comparing CAS with and without embolic protection devices (EPDs), the use of EPDs appears to be determinant in reducing the risk of stroke during CAS2.
Two types of EPDs are in clinical use2: proximal EPDs (distal balloon occlusion or filter device) having the theoretical advantage of providing embolic protection during all phases of the intervention and, distal EPDs in which the target lesion needs to be crossed first with the delivery system, before the deployment of the EPD distal to the target lesion. In consequence, with the employment of all the types of distal protection embolisation may occur before placement of the protection2.
It should be underlined that when various distal filter systems have been tested, using a bench top vascular model of carotid stenting, a robust amount of distal debris embolisation could be detected in all of them3. Similarly, in a clinical study, when the transcranial Doppler was used to monitor procedural micro-embolic signals (MES) during CAS, it was observed that the use of proximal endovascular clamping, as a proximal EPDs, is associated with a reduced amount of MES when compared to filter devices4.
In the initial reports on the use of proximal endovascular clamping (PEC) during CAS, the investigators considered the presence of a severe stenosis of the ipsilateral ECA as a contraindication for the use of PEC5,6. This was mainly due to the hypothetical difficulty of navigating a stiff guidewire into the ECA. This step is essential: first, to achieve an appropriate device placement; second, to establish a complete antegrade, as well as retrograde, blood flow blockage after inflating the two integrated balloons; and finally, to guarantee an adequate neuroprotection before starting the planned treatment of the lesion.
In this study the immediate and mid-term results are presented of 60 out of 500 patients who underwent CAS using endovascular clamping although an ipsilateral critical stenosis of the external carotid artery had been documented in a pre-interventional selective angiography.
Methods
Study population
Five-hundred patients underwent elective carotid artery stenting between September 2004 and December 2006. Sixty patients out of these 500 showed a concomitant presence of an ipsilateral external carotid artery stenosis of >80%. This subpopulation of 60 patients is the object of this present study.
Symptomatic stenoses of the internal carotid artery (ICA) > 50% were observed in 14/60 cases (23.3%)7,8. Patients were considered symptomatic if they had suffered from a TIA or a stroke in the previous six months2.
Asymptomatic stenosis > 80% based on pre-interventional angiography occurred in 46/60 cases (76.7%)9,10.
Concomitant therapy
Prior to intervention all patients received acetylsalicylate (ASA 75-160 mg/day) and had been simultaneously either on ticlopidine therapy (250 mg twice daily) or clopidogrel (75 mg/day) for at least seven days. Alternatively, patients received 500 mg ASA i.v. before the intervention and a loading dose of clopidogrel (300 mg) 24 hours before the scheduled procedure. At the beginning of the procedure all patients received 70 IU/kg b.w. heparin i.v. Before predilation of the target lesion or stent-delivery 0.5-1.0 mg i.v., atropine were routinely applied. After CAS, thienopyridine was continued for three months and ASA was prescribed on a lifetime basis. Additionally, all drugs used for risk factor reduction, such as oral anti-diabetics, insulin, statins and all other antihypertensive as well as anti-anginal drugs were administered to provide optimal secondary treatment care for the patient.
Technique of the CAS procedure
All CAS procedures were performed percutaneously using the retrograde femoral artery access, with the patient under local anaesthesia. Physicians involved in the procedures fulfilled qualification defined by the ICCS-SPREAD Joint Committee parameters8.
During the intervention an anaesthesiologist was involved to monitor haemodynamic as well as neurological status.
At the beginning of the procedure, an 8 Fr to 10 Fr, 25 cm long (Terumo, Tokyo, Japan) introducer sheath was inserted in the infrarenal aorta via the common femoral artery.
Routinely in our department a complete coronary angiography was performed in all patients prior to CAS due to the high incidence of coronary artery disease in patients with carotid artery disease11,12.
The presence of at least one > 70% angiographic coronary artery stenosis was considered as significant coronary artery disease. An aortic arch angiography was performed to delineate aortic arch type and supra-aortic branch anatomy. Selective carotid artery catheterisation was then performed using a 5 Fr JR4 diagnostic catheter advanced over a .035”, 150 cm long, 3 cm angled, soft hydrophilic guidewire (Standard Glidewire®, Terumo, Tokyo, Japan)
Once the diagnostic angiogram was completed, the tip of the hydrophilic wire was advanced in the distal common carotid artery and then rotated towards the ostium of the ECA. The tip of the soft wire was than gently navigated in the ECA stenotic segment and positioned in one of the ECA distal branches, then a JR4 diagnostic catheter could be advanced in the distal part of the ECA, using a gently clock- and counter- clock rotational movement as well as a push-and-pull technique.
After stabilising the diagnostic catheter in the distal ECA, the hydrophilic wire was exchanged for a 300 cm, 0.035-inch stiff wire (Hi- Torque Supracore, Guidant, St Paul, MN, USA).
The Mo.Ma system 8 Fr to 10 Fr (Invatec, Roncadelle, Italy) was advanced over the stiff wire in the aortic arch using fluoroscopy guidance. At this point – gently – the MO.MA system (5 Fr at the distal end) was navigated through the common carotid until the radiopaque marker for the distal balloon was located in the ECA, around 0.5-1 cm beyond the bifurcation and proximal to, or at, the thyroidal branch4-6. At this point, the ECA balloon is inflated. After angiographic control to insure correct and stable device positioning, the 0.035-inch stiff wire was withdrawn. Thereafter, the proximal balloon in the CCA was inflated, blocking the antegrade and the retrograde flow across the target vessel. A 0.014” guidewire was then navigated through the lesion. A predilation of the lesion was performed at the discretion of the operator.
In all cases, self-expanding carotid stents were deployed using a rapid exchange technique. Post-dilatation was performed with 4.5 – 5.5 x 20 mm balloons.
After post-dilation, at least 60 ml of blood were aspirated and filtered through sieves to collect particulate plaque debris. Blood flow was restored after two consecutive aspirations showing absence of debris; first de-clamping the balloon located in the ECA, and than the balloon located in the CCA.
The final angiography included ipsilateral bi-planar carotid and intracranial views to exclude cerebral thromboembolisms.
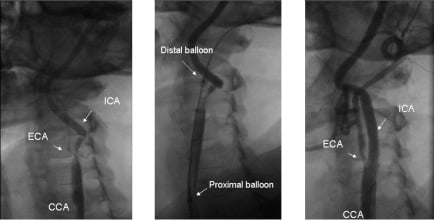
Figure 1. Angiographic images of a CAS procedure performed on a patient presenting with critical stenosis of the internal carotid artery (ICA) and subtotal occlusion of the external carotid artery (ECA) (left). Procedure was performed using the endovascular clamping as a cerebral protection device (middle, arrows indicates the proximal and distal balloons of the system, a stiff wire can be observed in the ECA). The final result (right), after stent deployment and post-dilation, shows procedural success and preserved perfusion of the ECA.
Post-procedural patient management
Femoral sheaths were removed within four hours (162±32 min) after conclusion of the procedure. A complete blood count was obtained before CAS procedure and prior to hospital discharge.
An independent physician evaluated all patients, with emphasis on the neurological status and on local complications at the arterial access site12,13.
Definitions
The primary endpoint of this study was the completion of the CAS procedure in the presence of a relevant ipsilateral stenosis of the external carotid artery using the MoMa endovascular clamping technique.
Procedural success was defined as achieving <30% residual stenosis of the ICA after successful CAS.
Procedure-related complications were defined as adverse events including neurology, cardiopulmonary, and haemorrhagic complications related to the procedure.
Neurology complications were classified as one of the following:
1) Transient ischaemic attack, defined as a new neurological deficit that resolved completely within 24 hours
2) Minor stroke, defined as a new neurological deficit that either resolved completely within 30 days or increased the National Institute of Health Stroke scale (NIHSS) by < 3
3) Major stroke, defines as a new neurological deficit that persisted for >30 days and increased the NIH Stroke Scale by > 4
4) Amaurosis fugax defined as a transient loss of vision14.
Haemorrhagic complications were classified as major or minor as defined by the Thrombolysis In Myocardial Infarction Criteria15.
Clamping time was defined as the time lasting from the inflation to the deflation of the proximal balloon in the CCA (time of flow blockage).
Clamping intolerance was defined as a transient neurological deficit observed during the clamping time, however showing a complete recovery within 20 minutes after restoration of the antegrade flow.
Total procedure time was defined as the time lasting from the completion of the diagnostic angiography to the acquisition of the final intracranial views after stent implantation4-6.
Follow-up
All patients were followed up at 30 days after PTA with a clinical examination assessing overall general conditions, with specific emphasis on neurology symptoms, medication, hospitalisations, or any type of complication after the CAS procedure.
Results
Patients’ demographic characteristics are presented in Table 1.
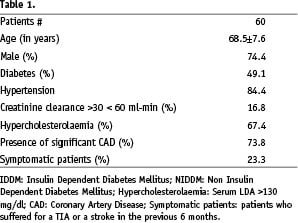
Patients (mean age 74 years) presented an elevated incidence of atherosclerotic risk factors (49.1% diabetic, 84.1% hypertensive and 67.4% hypercholesterolaemic) with a robust incidence of concomitant coronary artery disease (74%).
The presence of a severe ECA stenosis was observed in 60 (12%) patients out of the 500 scheduled for CAS from September 2004 to December 2006.
Procedure related characteristics are presented in Table 2.
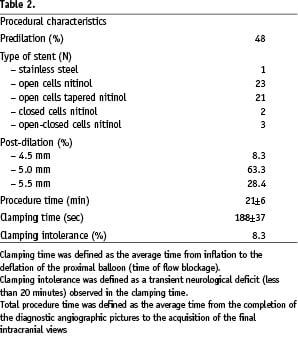
Procedural success, defined as the ability to establish neuroprotection with an adequate placement of the MoMa system, and to deploy the stent, was achieved in 100% of the cases. Total procedure length was 21±6 min. The average time of endovascular clamping was 188±37 sec. Clamping intolerance was observed in five patients (8.3%); in all cases CAS could be concluded under cerebral protection. Symptoms of clamping intolerance usually (3 out of 5 cases), started after stent placement, during blood aspiration collecting particulate debris. In these patients, symptoms resolved within 5-10 minutes after deflation of the Mo.Ma proximal balloon and restoration of the antegrade blood flow. No intraprocedural MACCE was observed.
Balloon predilation was performed in 28 patients. Balloon post-dilations were performed with 4.5 mm (5 patients), 5.0 mm (38 patients) or 5.5 mm (17 patients) balloons.
No ECA damage (i.e. dissection or occlusion) was observed at the time of system retrieval.
During the hospital stay, no patient died or experienced a major stroke. No TIA was observed.
In one patient a minor stroke occurred, manifesting as a hemiparesis four hours after the procedure, symptoms resolved completely within three days. At day four, this patient was discharged to home.
No groin complications or other types of bleeding complications were observed during the in-hospital phase.
During the 30 days follow-up, we did not observe any MACCE.
Despite the presence of coronary artery disease in nearly 74% of the patients, no myocardial infarction was observed intra-procedurally, peri-procedurally, in-hospital and within 30 days after the procedure.
Discussion
Carotid artery stent placement with neuroprotection is generally considered a reasonable alternative to carotid endarterectomy (CEA) in high risk patients1,2.
A major drawback of CAS is cerebral embolisation, and consequently cerebral infarctions during, or immediately after, the stent placement procedure which potentially can result in stroke symptoms. Embolic protection devices, proximal EPDs and distal EPDs, have been developed to reduce embolisation of plaque fragments during carotid stenting.
In comparison to distal filters, systems proximal EPDs provide embolic protection during most phases of the procedure.
Since the initial studies, the presence of a severe stenosis of the common carotid artery is considered a contraindication because it could be responsible for the distal embolisation occurring when the distal tip of the system crosses the common carotid lesion4-6,16.
Consistently, in the data that have been published so far using PEC for CAS, the presence of a severe stenosis of the ipsilateral external carotid artery was not considered an indication for the deployment of PEC4-6,16, probably because of the supposed difficulty in device positioning for stable neuroprotection.
The goal of this study was to demonstrate safety and efficacy of the Mo.Ma proximal endovascular clamping device for CAS in complex anatomic conditions, such as concomitant presence of severe disease of the ipsilateral ECA and independently from aortic arch type.
No data have been before reported addressing this specific topic.
The presence of an atherosclerotic plaque of the carotid bifurcation defining a critical stenosis of both the internal as well as the external carotid arteries seems to be a non-infrequent pathologic condition. The results of this study show the association of critical stenosis of both ICA and ipsilateral ECA in 12% of patients undergoing CAS.
The presented data shows that a critical stenosis of the ipsilateral external carotid artery (>80%) did not preclude either an adequate positioning of the PEC device in the ECA or result in an increased incidence of complications, when compared to published registries treating patients with non-stenotic ECA5,6. In fact, within this group of 60 patients only one minor stroke occurring four hours after the intervention has been observed.
The results of this study indicate that despite the presence of a tight stenosis of the ECA, once the stiff guidewire could pass the stenosis of the ECA, the distal tip of the Mo.Ma system could also safely navigate in the ECA. Moreover, the distal balloon dilation, which can reach a diameter of 6 mm when fully inflated – due to the specific characteristics of compliance of this elastomeric balloon – did not caused any damage to the external carotid artery as demonstrated in the final angiogram performed at the end of the procedure after retrieval of the Mo.Ma system. None of the patients presented any clinical symptoms that could be associated with the selective navigation of the system through the stenotic external carotid artery.
In conclusion, the results of our experience suggests that the use of PEC, in the presence of a critical stenosis of both the internal and the external carotid artery, is both safe and efficient, and complex anatomic situations such as the ones described here should no longer be considered a contraindication to the use of proximal endovascular clamping as neuroprotection during carotid stenting.
Online data supplement
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.
Moving image 4.
Moving image 5.

