Background
In the primary percutaneous intervention (PCI) of an acute ST elevated myocardial infarct (STEMI), the microvascular clogging caused by embolisation of thrombotic or atheromatous debris can affect the myocardial tissue perfusion to increase the infarct size and reduce survival1-5. In the catheterisation laboratory this usually manifests itself by sub-optimal angiographic capillary opacification (myocardial blush)6,7 or delayed ST-segment resolution with an inappropriate enzyme rise8. To circumvent this problem there are a number of thrombectomy and distal protection devices currently available9-12. However, the usefulness of thrombectomy as an adjunct therapy during primary PCI remains contentious13. In some pilot studies, and in small-randomised trials, there have been promising results with good microcirculation blush, improved left ventricular function9,14, and enhanced event-free survival, whilst in others there was no significant benefit15,16. Probable reasons for failure could be have been due to the inefficiency of the device; high thrombotic burden suggesting more appropriate use in a selected patient population and the late clinical presentation meant that the aspirating a thrombus in vessel associated with a transmural infarction offered little benefit15,17. The Thrombuster II (Kaneka Corp. Japan) is a thrombectomy device that offers a low frictional resistance. This together with it’s large circular lumen can provide superior aspiration ability. This technical report details the device and its use in an acute ST-elevated infarction18.
Case detail
A 53-year old male smoker with no other risk factors for coronary artery disease was referred for primary PCI of an acute infero-posterior infarction within two hours of symptoms. In the cathetherisation laboratory he was haemodynamically stable. Angiography revealed a proximally occluded dominant right coronary artery (RCA) with thrombus (Figure 1a), and no significant disease in the left coronary system.
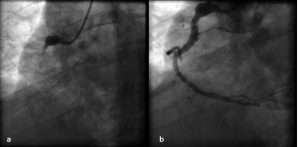
Figure 1. a) Occluded right coronary artery (RCA) with thrombus; b) RCA following clot aspiration with Thrombuster II thrombectomy device and balloon angioplasty.
Successful percutaneous intervention to the RCA (Figure 1b) involved twice manually aspirating the clot (Figure 2) from distal to the proximal segment with a Thrombuster II thrombectomy device advanced over a guidewire through a 6Fr guiding catheter.
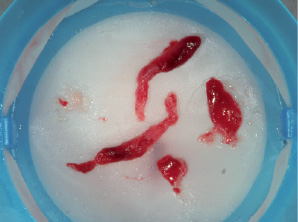
Figure 2. Thrombus extracted from RCA with Thrombuster II thrombectomy device.
This was followed by balloon angioplasty of the distal vessel at 14 atmospheres (Voyager RX balloon 2.5x15mm, Abbott Vascular, Santa Clara CA; USA) together with the administration of boluses (x5) of intracoronary nitroprusside (0.1mg) and the weight adjusted infusion of a GPIIa/IIIb inhibitor (Integrillin) as per protocol. Electrocardiography showed immediate resolution of ST elevation, with TIMI III flow achieved in the target vessel and a TIMI grade II myocardial blush. At discharge, peak enzymes were CK 602U/l, CKMB 63.4ug/l, and troponin T 1.47ug/l. Dual antiplatelet therapy with clopidogrel for 12 months, and aspirin indefinitely was recommended.
Technical data
The Thrombuster II device has CE approval for the removal of thrombus and debris in the coronary or peripheral artery by percutaneous suction. It is a single-user, easy to handle design based on a rapid exchange short monorail system (10mm) using standard guidewire techniques and is available in two sizes for use in 6Fr and 7Fr standard guiding catheters. It has a radiopaque marker at distal guidewire lumen and a proximal luer-lock port. The proximal luer lock connector connects extension tube and the lock type aspiration syringe (30cc) that allows for easy and effective aspiration. The catheter is 140cm long, and its distal portion (30cm) is hydrophilically coated. The inner diameter of aspiration lumen is 1.10mm at proximal part and 1.00mm at distal part as for 6Fr type and 1.32mm at proximal part and 1.20mm at distal part as for 7Fr type (Figure 3).
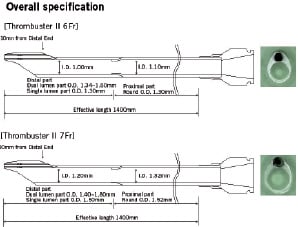
Figure 3. Technical specifications of Thrombuster II 6Fr and 7Fr thrombectomy device.
This feature, together with the better flow kinetics of a circular lumen and the hydrophilic coating, is thought to improve its performance.
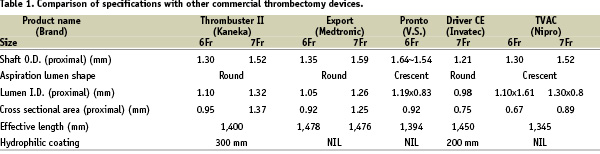
In addition, the proximal cross sectional area of 0.95mm2 and 1.37mm2 (in the 6Fr and 7Fr systems respectively) are the largest currently commercially available thrombectomy devices (Table 1).
The device has a removable core wire that gives better pushability and prevents kinking during insertion. The monorail core extends 2.2mm and 1.8mm beyond the aspirating lumen (in the 6Fr and 7Fr system respectively) resulting in a maximal diameter of 1.47mm and 1.60mm.
Conclusion
The Thrombuster II thrombectomy device was effective in aspirating fresh thrombus in a STEMI patient. This is an expanding field with several devices available with differences in their technical specifications that can influence their performances. Comparative assessment in selected patient population is required to increase the utility of these devices.
Acknowledgement
The authors would like to thank Mr Hiroyasu Higuchi, Kaneka Corp., Osaka, JAPAN for help with the manuscript.

