Case 1
A 58-year-old man was admitted to our coronary care with troponin negative unstable angina. His risk factors included, being an ex-smoker for a year, treated hypertension and dyslipidaemia. Medication on admission was aspirin and a statin. ECG showed T-wave inversion laterally. Nine years previously he had a bare metal stent implanted in his circumflex artery following a lateral infarction the preceding year. Angiography done during his acute presentation revealed an in-stent restenosis in the previously treated circumflex artery and a diffusely diseased marginal branch. The left anterior decending coronary (LAD) was normal and the right coronary artery (RCA) had a non-flow limiting lesion in the mid-vessel with a fractional flow reserve (FFR) of 0.79. Intravascular ultrasound (IVUS) of the circumflex artery was performed using a 20 MHz Eagle eye IVUS catheter (Volcano Therapeutics, Rancho Cordova, CA, USA). The length of the stented segment was 30mm and had a minimal luminal area (MLA) 4.6 mm2. Neo-intimal formation was visible throughout the stent (Figure 1).
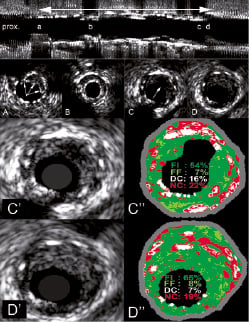
Figure 1. IVUS pullback. The top panel shows the longitudinal reconstruction. The double-headed arrow indicates the location of the stent. The characters “a” through “d” indicate the position of the cross sectional panels “A” through “D”. Panel A: proximal part of the stent, arrows indicatestent malapposition. Panel B : minimal luminal area within the stent (MLA 4.6 mm2). Panel C: eccentric plaque rupture (arrowed). Panel D: directly distal, intact eccentric neo-intimal plaque. Panel C” (magnified frame): IVUS VH of ruptured plaque within the neo-intima. Panel D” (magnified frame): immediately after the rupture. Panels C” and D” show a majority of fibrous tissue. Of note IVUS-VH analysis included the stents’ struts that were seen as spots of calcium and necrotic core. FI, fibrotic tissue; FF, fibrofatty; DC, dense calcium; NC, necrotic core.
This gave a neo-intimal hyperplasia (NIH) volume of 80.3 mm3. The NIH on virtual histology had a tissue composition of necrotic core (NC) 13.6%, (0.08 mm2, 2.4 mm3), calcified tissue 16.8%, (0.10 mm2, 3.0 mm3), fibrofatty 5.8%, (0.03 mm2, 1.04 mm3), fibrotic tissue 63.9%, (0.38 mm2, 11.5 mm3). Distal to the MLA at distance of 18 mm IVUS revealed an eccentric soft neo-intimal ruptured plaque1,2 (Figure 1C) within the stent. The necrotic core was 22% with remnant plaque burden of 54% at the plaque rupture site (Figure 1 C’). Just distal to ruptured plaque the vessel wall was intact and the plaque burden was higher 65% with no necrotic core in contact with the lumen (Figure 1D”).
This ruptured lesion was subsequently managed with a drug eluting stent (DES) that also covered the MLA. The histological findings of a similar plaque rupture are illustrated in Case 2.
Case 2
A 43-year-old obese male underwent percutaneous coronary intervention (PCI) with MINI-CROWN bare metal stent (BMS) implantation in 1999 for a severe lesion in the proximal left anterior descending coronary artery. He was a heavy smoker with a body mass index 26.4 but no diabetes or hyperlipidaemia. His past history includes peripheral vascular disease with intermittent claudication. He was found dead at his friend’s house following a party approximately seven years after stent implantation. At autopsy, the heart showed biventricular hypertrophy with a healed subendocardial infarction in anteroseptal and lateral left ventricle. The coronary system was left dominant and middle left circumflex coronary artery had a 95% luminal narrowing without evidence of plaque rupture. However, there was an occlusive platelet-rich thrombus at the site of the stent in the LAD that was implanted just proximal to origin of the first left diagonal branch. The histological examination revealed a large ruptured atherosclerotic plaque with an underlying occlusive platelet thrombus. This ruptured plaque was composed of a NC rich in cholesterol cleft and an overlying fibrous cap was infiltrated by foamy macrophages (Figure 2).
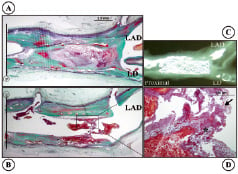
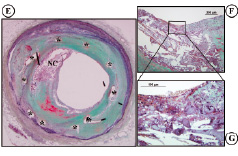
Figure 2. Histology of the case (Movat Pentachrome). A, B: Longitudinal section of the stent. The distal part of the stent shows site of plaque rupture and a superimposed platelet-rich adherent thrombus. C: X-ray of stented artery just above the bifurcation of LAD and left diagonal branch. D: High magnification image of the rupture site (*) and overlying platelet-rich thrombus is identified by the black arrow. E: The cross section of the proximal portion of the LAD stent. (corresponds to the black lines in panel A and B). Note the presence of a thin-cap fibroatheroma (vulnerable plaque) within the stent site (*). F, G: The fibrous cap is extremely thin and infiltrated by foamy macrophages with an underlying necrotic core (NC).
These images are the first reported examples of a plaque rupture within the neo-intima induced by a stent and illustrate progression in the pathological process of atherosclerosis3. Currently there are concerns about late stent thrombosis in DES due to delayed endothelialisation. However, these two cases of very late BMS thrombosis highlight that it can occur despite full stent coverage.
Online data supplement
AVI File 1. IVUS pullback along the stent to reveal the ruptured plaque.
AVI File 2. IVUS-VH superimposed on pullback along the stent to reveal the ruptured plaque.

