Abstract
Aims: This follow-up study was performed to assess the long-term effects of paclitaxel-eluting stents (PES) compared with bare-metal stents (BMS) in patients who had undergone a percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI).
Methods and results: The PASSION trial randomly assigned 619 patients with STEMI to receive either a PES or BMS. The composite endpoint for the follow-up study was the occurrence of the combination of cardiac death, recurrent myocardial infarction, target lesion revascularisation (TLR) or stent thrombosis at two years. A trend towards a lower rate of the composite endpoint was observed in the PES compared to the BMS group (hazard ratio [HR] 0.70; 95% C.I. 0.45-1.09). This was driven by a reduced TLR in favour of PES (HR 0.60; 95% C.I. 0.34-1.09). Angiographically proven stent thrombosis at two years did not differ significantly between groups (2.1% in the PES group versus 1.4%; HR 1.48; 95% C.I. 0.42-5.23).
Conclusions: PES implantation for STEMI did not significantly improve clinical outcome at two years after the index event, although there was a trend towards a lower rate of target-lesion revascularisation. The rate of stent thrombosis did not differ significantly between groups.
Introduction
Primary percutaneous coronary intervention (PCI) is now considered the optimal approach in the management of ST-segment elevation myocardial infarction (STEMI) when the procedure is performed expeditiously and at a high-volume centre.1-4 Stent implantation is associated with an improvement in both early and late outcomes, as compared with balloon angioplasty alone, predominantly as a result of a reduction in target-vessel revascularisation.5,6 Drug-eluting coronary artery stents, including paclitaxel-eluting stents (PES), have been shown to improve both early and late outcomes, as compared with bare-metal stents in a variety of clinical settings, predominantly as a result of a reduction in target-vessel revascularisation.7,8 In contrast, the one year follow-up of the Paclitaxel-Eluting Stent versus conventional Stent in Myocardial Infarction with ST-Segment Elevation (PASSION) trial showed no significant superiority of PES compared to bare-metal stents (BMS) in STEMI, although non-significant trends in favour of PES were found.9 However, other studies have indicated a significant benefit of sirolimus-eluting stent use in patients with STEMI.10-11 The current analysis was performed to evaluate whether the differences between the PES and BMS group remained unchanged at two years after stent implantation for STEMI.
In addition, the occurrence of very late stent thrombosis was evaluated. Recently, concern has arisen about the occurrence of serious adverse events caused by stent thrombosis late after drug-eluting stent implantation.12-15 It has been postulated that delayed endothelialisation and malapposition after drug-eluting stent implantation may lead to late stent thrombosis resulting in myocardial infarction (MI) or death.16,17 It was recently stated by the FDA panel (November, 2006) that off-label use of drug-eluting stents (i.e. including STEMI) is associated with increased risks of both early and late stent thrombosis. However, the late rates of serious adverse cardiac events after primary PCI for STEMI with drug-eluting stents are unknown. Therefore, we sought to determine whether paclitaxel-eluting stents are safe compared to bare-metal stents in the setting of primary PCI as measured by the rate of serious adverse cardiac events, including the incidence of stent thrombosis, at two years follow-up.
Methods
Study group
The Paclitaxel-Eluting Stent versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation (PASSION) trial was a prospective, single-blind, randomised study, performed at two centres in the Netherlands (Onze Lieve Vrouwe Gasthuis in Amsterdam and St. Antonius Hospital in Nieuwegein). The study has been registered as an International Randomised Controlled Trial, number ISRCTN65027270. The details of the design and the main results at one year have been published previously.9 Between March, 2003, and December, 2004, 619 patients were enrolled if they were having an acute myocardial infarction with ST-segment elevation (>20 minutes of chest pain and at least 1 mm of ST-segment elevation in at least two contiguous leads or new left bundle-branch block) and reperfusion was expected to be achieved within six hours from the onset of symptoms. The major exclusion criteria were: cardiogenic shock evident before randomisation; uncertain neurological outcome after resuscitation; mechanical ventilation; or an estimated life expectancy less than six months. This study complied with the principles set out in the Declaration of Helsinki. All study participants provided oral informed consent which was documented in the patients’ clinical records. This approach to informed consent was explicitly approved by the authorising ethics committees.
Treatment regimen
Patients were randomly assigned to receive either a paclitaxel-eluting stent (Taxus Express2, Boston Scientific, Natick, MA, USA) or a bare-metal stent (Express2 or Liberté, Boston Scientific, Natick, MA, USA) in a 1:1 ratio using permuted blocks of 50. The assignment to study groups was performed with the use of sealed envelopes. Patients, referring physicians, investigators who were responsible for obtaining follow-up information and the interventionalists performing follow-up procedures were all unaware of treatment assignments. We administered aspirin (100-500 mg) and clopidogrel (300 mg) as soon as patients arrived at the hospital, followed by aspirin 80 to 100 mg daily for life and clopidogrel 75 mg once daily for at least six months.
Angiographic and procedural characteristics
QCA was performed to describe the angiographic profile of the included patient populations. The same QCA system was used in both participating centres and was performed by highly experienced operators familiar with QCA analysis. In the absence of anterograde flow the MLD was estimated as 0 mm and the reference diameter was measured in the angiographically normal-looking segment proximal to the occlusion.
Follow-up and outcome
During each patient’s hospital stay and follow-up visits at 30 days, one and two years, we recorded all serious adverse cardiac events (death from cardiac or non-cardiac causes, recurrent myocardial infarction, revascularisation of the target lesion or target vessel, and coronary-artery bypass grafting [CABG]), as well as interventions to non-target vessels. Patients were contacted by telephone or by mail. In the event of a repeat hospital admission or any reported adverse event, detailed follow-up information was obtained. If the information could not be checked directly with the patient, it was obtained from the patient’s family, family doctor, the insurance company or public records. When patients were lost to follow-up censoring was done at the date of last contact or clinical follow-up.
Drs. Laarman and Suttorp adjudicated all endpoints of the study in a blinded fashion. The primary outcome was the first occurrence of a major adverse cardiac event (MACE; including death from cardiac causes, recurrent myocardial infarction, and ischaemia-driven revascularisation of a target lesion) within 24 months. All deaths were considered to be from cardiac causes unless a noncardiac cause could be identified. Recurrent myocardial infarction was defined by the development of either pathological Q waves lasting at least 0.4 second in at least two contiguous leads or an increase in the creatine kinase level to more than twice the upper limit of normal with an elevation of the creatine kinase MB iso-enzyme in combination with symptoms or the need for intervention. Revascularisation of the target lesion was defined as ischaemia-driven PCI of the target lesion if there had been restenosis or re-occlusion within the stent or in the adjacent distal or proximal 5 mm and included CABG involving the infarct-related artery. As no routine follow-up angiography was performed, recurrent coronary angiography was performed according to the established clinical guidelines. PCI was then performed if the severity of the lesion was >50% on visual analysis.
A secondary outcome of the study was the occurrence of stent thrombosis. Definition of stent thrombosis in the initial protocol was angiographic confirmation of vessel occlusion or thrombus formation within, or adjacent to, the stented segment. Stent thrombosis was categorised as acute (occurring within 24 hours after the procedure), subacute (occurring one to 30 days after the procedure), late (occurring 30 to 365 days after the procedure) or very late (occurring after one year). In addition, the occurrence of stent thrombosis was evaluated according to the Academic Research Consortium (ARC) criteria.18 Definite or confirmed stent thrombosis: Angiographic confirmation of vessel occlusion or thrombus formation within, or adjacent to, the stented segment or proven stent thrombosis at autopsy. Probable stent thrombosis: Unexplained death within 30 days or target vessel recurrent MI without angiographic confirmation. Possible stent thrombosis: Unexplained death after 30 days. Stent thromboses following repeated target lesion revascularisation, with or without repeat stent implantation, were also counted.
Statistical analysis
Baseline data are presented as proportions or mean (±SD) values and were compared with the use of Student’s t-test or the Wilcoxon rank-sum test for continuous variables and with the Fisher’s exact test for categorical variables. A two-sided P value of less than 0.05 was considered to indicate statistical significance. We estimated the cumulative incidence rates of the primary and secondary endpoints at two years with the Kaplan-Meier method.19 Hazards ratios (HR) were calculated with Cox proportional-hazards models with treatment allocation (PES or BMS) as the only covariate. The 95% confidence interval (CI) for the relative risk was calculated with the use of the standard errors from the Kaplan-Meier curve. The significance of differences in rates of the endpoints between treatment groups was assessed by the log-rank test. Statistical analysis was done with the Statistical Package for Social Sciences software (SPSS 13.0 for Windows).
Results
Six-hundred and nineteen patients were enrolled in the study: 310 were randomly assigned to the paclitaxel-stent group and 309 to the bare-metal stent group. The most common reasons for exclusion from the trial were an anticipated delay of more than six hours between the onset of symptoms and reperfusion, coronary anatomy that was not suitable for stent implantation, cardiogenic shock, or mechanical ventilation. The baseline clinical characteristics of both groups were well matched (Table 1).
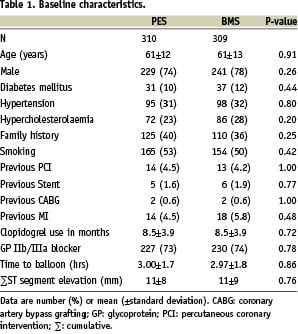
The mean age was 61 years; 76% of the patients were male. Clopidogrel was used for a median of nine months (interquartile range, six to 12).
Angiographic and procedural characteristics
For baseline angiographic and procedural characteristics see Table 2.
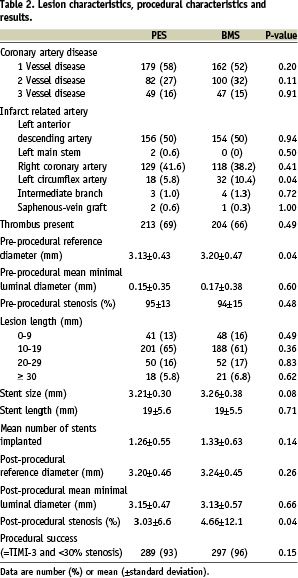
Approximately half of the patients had multivessel disease, and in 50% of the cases the left anterior descending coronary artery was the infarct-related artery. The majority of patients had an estimated lesion length between 10 mm and 19 mm. The mean reference diameter was 3.13±0.43 mm in the paclitaxel-stent group and 3.20±0.47 mm in the bare-metal stent group. The average length of stents was 19 mm in both groups. TIMI grade 3 flow was established in 93% of patients in the paclitaxel-stent group, as compared with 96% of patients in the bare-metal stent group (NS). The sizes of infarcts, reflected by the mean peak value of the creatine kinase MB iso-enzyme, were similar (193±183 in the paclitaxel-stent group and 210±186 in the bare-metal stent group).
Incidence of MACE
A total of 96% of patients in the paclitaxel-stent group and 97% of those in the bare-metal stent group underwent complete two year clinical follow-up. Follow-up for death was complete in 98% in both groups. The cumulative incidence of MACE at two years was 11.1% in the paclitaxel-stent group and 15.4% in the bare-metal stent group (HR 0.70; 95% C.I. 0.45-1.09; P=0.12) (Figure 1 and Table 3).
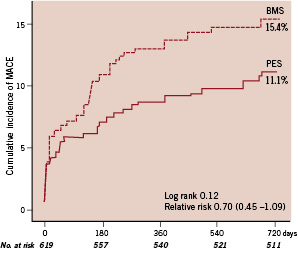
Figure 1. Kaplan-Meier survival curves showing the composite endpoint of death from cardiac causes, recurrent myocardial infarction, or target-lesion revascularisation at two years. The cumulative incidence of the primary endpoint of serious adverse cardiac events was 11.1% in the paclitaxel-stent (PES) group and 15.4% in the bare-metal stent (BMS) group (relative risk, 0.70; 95% CI, 0.45 to 1.09; P: 0.12 by the logrank test).
The cumulative incidence of cardiac death, recurrent MI or stent thrombosis was 8.5% in both groups (HR 0.95; 95% C.I. 0.56-1.63; P=0.86). Figure 2 shows the incidence of target lesion revascularisation up to two years of follow-up.
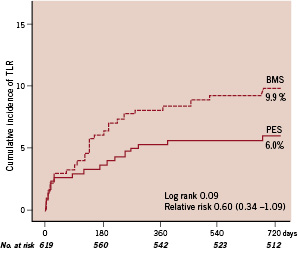
Figure 2. Kaplan-Meier survival curves showing cumulative incidence of target lesion revascularisation (TLR) at two years. The cumulative incidence of TLR was 6.0% in the paclitaxel-stent (PES) group and 9.9% in the bare-metal stent (BMS) group (relative risk, 0.60; 95% CI, 0.34 to 1.09; P: 0.09 by the logrank test).
Stent thrombosis
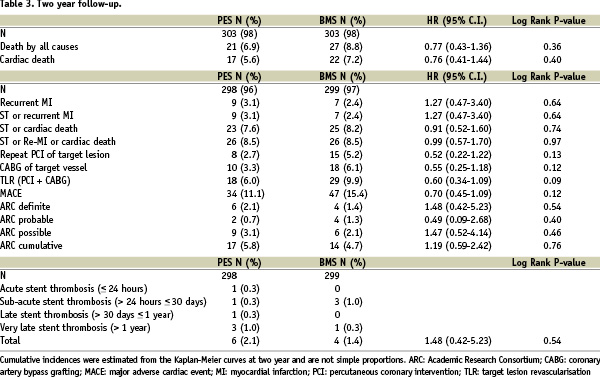
The cumulative incidence of angiographically proven stent thrombosis was similar between the two treatment groups, 2.1% (n=6) in the paclitaxel-stent group versus 1.4% (n=4) in the bare-metal stent group. Acute stent thrombosis (within 24 hours) occurred in one patient (0.3%) in the paclitaxel-stent group. Sub-acute stent thrombosis occurred in one patient (0.3%) in the paclitaxel-stent group and in three patients (1.0%) in the bare-metal stent group. Very-late stent thrombosis occurred in three patients (1.0%) in the paclitaxel-stent group and in one (0.3%) in the bare-metal stent group. These four patients with stent thrombosis beyond one year were all compliant with the use of aspirin at the time of the event and there were no specific confounding factors involved. One of the patients in the PES group had a very late stent thrombosis in the PES that was implanted in a lesion adjacent to the initial stent six months after the index event.
The occurrences of stent thrombosis according to the ARC criteria are shown in Table 3. The cumulative incidences of definite, probable or possible stent thrombosis were 5.8% in the PES group versus 5.1% in the BMS group (HR 1.19; 95% C.I. 0.59-2.42; P=0.62).
Discussion
This is the first randomised study to report clinical outcome with late follow-up two years after PES or BMS implantation for STEMI. The additional information pertaining to two year follow-up is important, as currently the information available on drug-eluting stents post STEMI is almost exclusively limited to one year of follow-up, a duration too short to fully appreciate the risks and/or benefits of drug-eluting stents, particularly as late stent thrombosis beyond one year seems to be of particular concern with drug-eluting stents. In our study, the use of a paclitaxel-eluting stent was not superior to that of a bare-metal stent in patients undergoing a primary PCI for STEMI, apart from a trend towards less target-lesion revascularisation in the PES group. The cumulative incidence of the primary endpoint – a composite of cardiac death, recurrent myocardial infarction, and target-lesion revascularisation at 24 months – was 11.1% in the PES group and 15.4% in the BMS group. The relative risk of 0.70 (95% C.I. 0.45-1.09) was similar to that observed at one year (0.69; 95% C.I. 0.43-1.10) and was not statistically significant.9 The rates of individual adverse events were similar in both groups, except for target lesion revascularisation that was in favour of the PES group, however this did not reach statistical significance. These results are in contrast to other trials with prolonged follow-up comparing the use of a PES with a BMS. Previous trials observed improved outcome with the use of PES in a number of clinical conditions (except for STEMI).7,8 As discussed previously,9 several factors may account for the discrepancies between our results and those reported for PES in other clinical settings. In summary, the event rates in the BMS group were much lower than those anticipated in our power calculations, possibly due to improvements in stent design and/or implantation techniques. This TLR rate was noticeably lower compared to the TLR rates after BMS implantation observed in all other randomised trials with sirolimus- and paclitaxel-eluting stents in STEMI.10,20 Secondly, the study design did not include angiographic follow-up in contrast to the majority of these previous trials. Third, there may be a difference in the response to vascular and myocardial injury after PCI for myocardial infarction, when compared with the response in more elective procedures. Importantly, the relative risks and non-significant trends were similar to those seen at one-year follow-up and we did not see a late ‘catch-up’ phenomenon with the use of PES after longer follow-up.
Whereas restenosis is considered a relatively benign adverse event, stent thrombosis may be fatal in up to 45%.21 Although this study was not powered to detect a difference in stent thrombosis, it is notable that the current study showed an equal safety profile for both stents at two years after implantation in patients with STEMI. There was no significant increase of catastrophic adverse events associated with the use of a PES. The cumulative endpoint of cardiac death, recurrent MI or stent thrombosis was similar in both groups, with a relative risk of 0.95. Although angiographically proven very late stent thrombosis was higher in the PES group (1.0%) compared to the BMS group (0.3%), the cumulative incidences of definite, probable or possible stent thrombosis (according to the ARC criteria) did not show an increase after PES implantation. However, these rates of stent thrombosis must be read with caution given the limited size of the study population evaluated for the occurrence of stent thrombosis. Much larger numbers of patients are needed to prove statistical and clinical significance. In the current analysis we used a conservative definition of stent thrombosis – angiographically proven – and less conservative definitions of stent thrombosis – according to the ARC, including stent thrombosis after repeat revascularisation.
Regardless of the definition, the incidence of stent thrombosis, according to the ARC criteria, was low in both treatment groups, especially given the complex conditions of unstable patients during acute myocardial infarction, with a thrombotic environment at the time of stent placement, augmented platelet activation,22 the potential for suboptimal stent deployment, and decreased blood flow in the infarct-related artery. Therefore, the current analysis of patients two years after PES implantation suggests that the “off-label” use of PES in primary PCI for STEMI shows an equal safety profile compared to BMS. These results are in line with recently published meta-analyses of randomised trials that had long-term follow-up after drug-eluting stent implantation showing no increase in the rates of stent thrombosis with the use of drug-eluting stents.23,24 However, our results differ from previously reported studies that suggest that drug-eluting stent implantation may be associated with an increase of stent thrombosis and death.12-15 It should be noted that none of these studies were performed in a randomised manner in patients with STEMI.
In our study, all patients were expected to have discontinued the use of clopidogrel at one year after the index procedure. Although there was an increase of events in the PES group compared with the BMS group after one year, this was not statistically significant. Currently, all patients are advised to use clopidogrel for one year after drug-eluting stent implantation and the impact of this prolonged strategy on outcome is not yet known. Early discontinuation of anti-platelet therapy may be associated with an increased risk of thrombosis, but the optimal duration of clopidogrel remains undefined. This increase of events should be evaluated with longer follow-up in larger randomised trials.
Taken together, the mandatory use of PES in acute MI remains debatable. Additionally the use of drug-eluting stents may not be necessary for STEMI in view of the predominantly large sizes of culprit vessels that have a low expected risk of restenosis (TLR 9.9% in the BMS group). However, it is necessary to wait for the longer follow-up of larger randomised trials, meta-analyses and substudies on the use of drug-eluting stents for acute MI as the literature shows that angiographic and clinical restenosis after primary PCI remains an important issue.4,5
Study limitations
A limitation of this study lies in the fact that the power calculations were not based on the hypothesis of the current analysis; e.g., the current study was not powered to detect differences in stent thrombosis or death: therefore these data remain observational. Larger studies with longer follow-up periods and larger patient numbers are required to definitively answer the question of whether the use of drug-eluting stents in primary PCI would improve or decrease survival after acute MI. A further limitation is the lack of an independent clinical event committee, as all events were adjudicated in a blinded fashion by two interventional cardiologists.
Conclusions
In conclusion, the PASSION study did not show any significant clinical benefit associated with the use of paclitaxel-eluting stents as compared with the use of bare-metal stents of same design. In addition, there was no significant increase of stent thrombosis after PES implantation in primary PCI at two years after stent implantation and one year after discontinuation of clopidogrel.
Clinical Trial Registration Information
International Randomised Controlled Trial, ISRCTN65027270.
Funding Sources: Amsterdam Department of Interventional Cardiology at Onze Lieve Vrouwe Gasthuis.
Acknowledgements
We thank Femmy Bosman, Marian Viergever, and Mike Bosschaert for providing indispensable administrative support. And we would like to thank all the patients who participated in the trial.

