Abstract
Aims: The ELUTES Study was a dose-finding trial to determine clinical safety and angiographic efficacy of a polymer-free, paclitaxel-eluting stent. The aim of this paper is to present the longer term follow-up.
Methods and results: 192 patients with de novo lesions were randomised to either control or one of four doses of paclitaxel directly applied to a Cook Incorporated V-Flex Plus™ stent without polymer. The six-month data has been previously published and showed a dose-response effect on diameter stenosis, late loss and binary stenosis, with the highest dose density achieving benefit for late loss and diameter stenosis. Binary restenosis was 3% in this group vs. 21% in controls.
Clinical follow-up data has been obtained in 152 patients (79%) to 24 months and shows sustained effects with no evidence of late stent thrombosis, suggesting drug-eluting non-polymer stents are clinically safe.
Conclusions: Non-polymer, paclitaxel-coated stents effectively inhibited angiographic parameters of restenosis. The stent system appeared safe with no recorded late stent thrombosis.
Introduction
Percutaneous coronary intervention (PCI) with bare metal stents (BMS) has become an accepted method for treating atheromatous coronary disease1,2, reducing the incidence of clinical restenosis compared to angioplasty alone to < 20%. The burden of restenosis has been further reduced in a number of trials by the use of a drug-eluting stent (DES) which report target lesion recurrence rates of ~ 5%3,4.
Studies in animal models demonstrated that paclitaxel, which enhances polymerisation of cellular microtubules and thus prevents mitosis and cell migration, reduced restenosis when delivered locally5. This paper reports the two year clinical data from the European evaLUation of PacliTaxel Eluting Stent (ELUTES) trial, a multicentre, randomised, controlled and triple-blinded clinical study designed to evaluate the safety and efficacy of a polymer-free, paclitaxel-eluting stent (PES) across a range of paclitaxel dose densities.
The angiographic efficacy of such stents in this trial at six months has already been reported, demonstrating a trend towards a reduction in binary restenosis between bare metal and the highest dose of paclitaxel6.
While the stent used as the delivery vehicle for paclitaxel in this trial is no longer clinically available, due to a non-significant reduction in restenosis in the Deliver I trial7, an on-going concern in the use of drug-eluting stents is their long-term clinical safety.
Clinical follow up data from the ELUTES study has now been analysed up to 24 months after the initial stent implantation, with particular attention to the incidence of proven or possible late stent thrombosis.
Methods
Paclitaxel was applied to the abluminal bare-metal surface of V-Flex Plus™ coronary stents (Cook Incorporated, Bloomington, IN, USA) by a proprietary process at four dose densities (0.2 µg/mm2, 0.7 µg/mm2, 1.4 µg/mm2, and 2.7 µg/mm2 stent surface area). These were compared with uncoated stents. The study complied with the Declaration of Helsinki, the locally appointed ethics committee approved the research protocol and informed consent was obtained at ten European investigative sites. Patients with single de novo, type A or B1 lesions, < 15mm length in a native coronary artery were entered into the study. Exclusion criteria were as previously reported6. Stents (16 mm long and 3.0 mm or 3.5 mm diameter) were randomised and implanted as previously described6.
Anti-platelet therapy
Adjunct medications included aspirin and procedural heparin according to operator practice. Antiplatelet therapy with aspirin plus clopidogrel was given for three months only. Glycoprotein IIb/IIIa receptor blocker use was operator dependant (administered in 58 of the 190 patients, with a uniform inter-group distribution).
Statistical analysis
Data on clinical endpoints were analysed on an intention to treat basis with the Fisher exact test for non-parametric data. A two-tailed p-value of <0.05 was taken as significant.
Follow-up
Patients were followed up with repeat angiography at six months. Clinical follow up by telephone interview or review in outpatient clinic was completed at 6, 12 and 24 months. Some centres in the study did not participate in the long term follow-up and some patients refused to continue involvement in the study, so follow-up data are incomplete at the later time points. In total 152/192 (79%) patients did provide two year data.
Results
There were no significant baseline demographic or angiographic differences between groups, except for age (0.2 µg/mm2 group significantly older than 2.7 µg/mm2 group p=0.022). Binary in-stent restenosis (DS > 50% at 6 month follow up) was reduced from 20.6% to 3.2% (p = 0.056).
Clinical endpoints are shown in table 1.
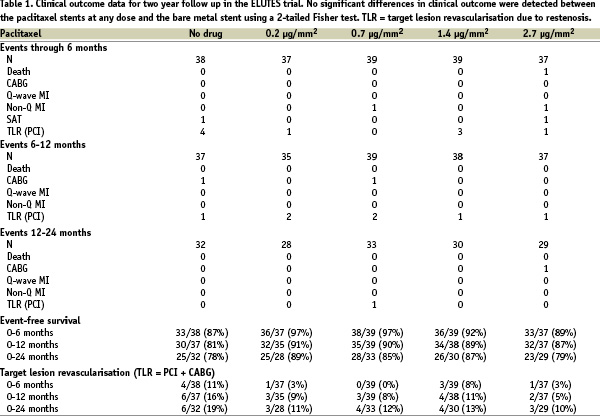
There were no significant differences in clinical outcome compared to the bare metal stents for each of the concentrations used on the drug-eluting stents. The trial was underpowered however to detect significant differences in clinical endpoints.
Two late (12-24 months) events occurred in the study, a TLR and CABG in the 0.7 and 2.7 groups respectively. One case of subacute stent thrombosis occurred in both the control (day of implant) and the highest paclitaxel dose (8 days post implant) arms. The latter event related to stent thrombosis in a BMS that had been placed proximal to the trial stent for dissection (as indicated in the original trial).
One death occurred (with no post mortem) in the PES highest dose group. This patient had a PES implanted in the context of an acute MI, a protocol violation. No reports of suspected late stent thrombosis from 6 to 24 months were reported in any of the arms of the study, despite only three months of clopidogrel given in the study, i.e. no documented stent thrombosis, no MI and no cardiac deaths. Follow-up data are incomplete and therefore some of the patients not followed may have had adverse events that are not recorded.
Discussion
The polymer-free V-Flex Plus stent used in this trial was shown to be an effective method for delivering paclitaxel to the vessel. This led to a significant reduction in angiographically defined restenosis at six months. The stent was safe, with no excess of adverse events over the bare metal stent group. Clinically, a non-significant difference in TLR rates was seen at follow up in favour of the highest dose of paclitaxel. This difference had not changed since the 6-month comparison, suggesting there was no late “catch-up” in the high-dose paclitaxel group. The trial (being predominantly a safety pilot trial) was not powered to determine if these apparent differences were real.
However, late lumen loss angiographically has been shown to correlate with clinical outcome, in particular TLR8. Predicted TLR rates at six months based on late loss in the ELUTES trial using this model created by Mauri et al match closely the TLR rates observed (predicted rate 10.2% vs. 11% observed in the BMS group, 4.4% vs. 3% in the high-dose PES group). Longer term follow up data have not been as closely related to angiographic late loss. Clinical data similar to those reported for ELUTES are available at two year follow up for several other DES trials (Table 2).
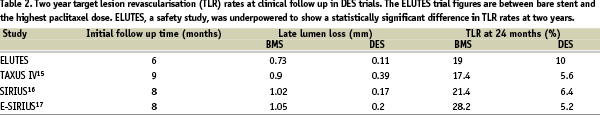
Direct comparison is not possible, considering differing patient populations, but ELUTES clinical results appear in keeping with the benefits seen in other DES trials.
Two early thrombotic events and one death did occur, although it is not evident that these events were due to any adverse effect of the paclitaxel, since endothelialisation would not have been expected to be complete in even the control group at such an early time point. All these adverse events are included in the analyses.
The BASKET-LATE9 trial was designed to look at the incidence of major adverse events in DES patients between 7-18 months post-PCI. It reported a 4.9% rate of cardiac death and non-fatal MI. Late stent thrombosis was not demonstrated to be the cause of these events and the study was underpowered, but such findings (in polymer-coated stents) contribute to the ongoing concern relating DES to LST. In contrast to BASKET-LATE, ELUTES (with polymer-free delivery of paclitaxel) – in a smaller group of patients and with incomplete follow-up – recorded no deaths or MI between six and 24 month follow up in the DES groups.
The Deliver I & II studies used the ACHIEVE polymer-free stent with covalently bound paclitaxel. Deliver I was halted because of a non-significant reduction in binary restenosis. MACE rates at six months were 10.3% vs. 13.3% (p=0.15) in the BMS group. This relative lack of efficacy may be related to the dose of paclitaxel on these stents (3 µg/mm2)10. The use of GIIb/IIIa inhibitors, an independent indicator of angiographic restenosis in this trial (for unknown reasons), also reduced the apparent efficacy of the ACHIEVE stent7. The Deliver II registry reported a composite MACE of 15.7%. Half of these events (7.2%) were due to death or MI, although stent thrombosis was not specifically reported11. The ASPECT trial of a Cook, polymer-free PES reported no late thrombosis, sudden death or MI between one and six months follow-up.
Polymer-free DES trials have been able therefore to report a low incidence of late stent thrombosis.
A contributor to the problem of LST may be adverse responses to the polymers used as a vehicle for drug delivery12. This possibility is supported by the finding of aneurysmal dilatation and inflammatory cells around polymer fragments in an autopsy specimen from a patient with late stent thrombosis after Cypher stent implantation13. Both clinically available PES and SES are polymer-coated. Several studies have assessed the utility of polymer-free, drug-eluting stents in clinical practice. The ISAR TEST trial compared polymer-coated PES or polymer-free (Yukon system) SES. Nine-month data from this study14 show a 0.4% stent thrombosis rate in the polymer-free stent group compared to 0.9% in the polymer group.
In conclusion, the ELUTES trial has shown that this polymer-free, paclitaxel-eluting stent leads to significant reductions in angiographic restenosis. In clinical follow-up, a non-significant reduction in TLR was maintained between 6 and 18 months and the stent appeared to be safe with no reported late stent thrombosis. In an era where real concerns persist about the possibility of polymer-related late stent thrombosis, polymer-free, high-dose drug delivery remains an alternative.
Limitations of the study
The data reported are from a limited number of patients as the trial was powered for angiographic outcomes. The stent used is not available in clinical practice. The clinical benefit or otherwise of the stents cannot be fully known from the data available, although the safety of the polymer-free paclitaxel stent system can be confirmed amongst patients still under active follow-up.
Acknowledgements
The authors gratefully acknowledge the support of Cook Incorporated USA, for the funding and co-ordination of this study.

