
Abstract
Aims: In addition to patients with pure/predominant aortic stenosis (PAS), real-world transcatheter aortic valve implantation (TAVI) referrals include patients with mixed aortic valve disease (MAVD; severe stenosis+moderate-severe regurgitation). We sought to compare TAVI outcomes in patients with MAVD vs. PAS.
Methods and results: Out of 793 consecutive patients undergoing TAVI, 106 (13.4%) had MAVD. Patients with MAVD were younger and had a higher operative risk, a more severe adverse cardiac remodelling, and a worse functional status than patients with PAS. Moderate-severe prosthetic valve regurgitation (PVR) was significantly more frequent in patients with MAVD than in patients with PAS (15.7% vs. 3.6%, p=0.003), even after propensity-score and multivariable adjustments. Moderate-severe PVR was associated with increased one-year mortality in patients with PAS (log-rank p=0.002), but not in patients with MAVD (log-rank p=0.27). Eventually, all-cause and cardiac mortality as well as the functional capacity were similar in the two study groups up to one year.
Conclusions: A significant proportion of patients referred for TAVI in a real-world registry has MAVD. Moderate-severe AR at baseline can influence the rate and modify the clinical sequelae of post-TAVI PVR. Eventually, clinical outcomes in patients with MAVD are comparable to those in patients with PAS in the acute and midterm phases, in spite of a baseline higher risk. MAVD should not be considered a contraindication for TAVI.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
CABG: coronary artery bypass grafting
EuroSCORE: European System for Cardiac Operative Risk Evaluation
LVEF: left ventricular ejection fraction
MAVD: mixed aortic valve disease
NYHA: New York Heart Association
PG: pressure gradient
PVR: prosthetic valve regurgitation
SAVR: surgical aortic valve replacement
STS-PROM: Society of Thoracic Surgeons predicted risk of mortality
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
In patients with severe symptomatic aortic stenosis (AS), transcatheter aortic valve implantation (TAVI) can improve quality of life and significantly reduce mortality1-3. However, the role of TAVI in the management of patients with native aortic valve regurgitation (AR) is less established4. Although mixed aortic valve disease (MAVD) is frequently encountered in clinical practice5, data on its prevalence and natural history are scarce6. MAVD (moderately severe AR co-existing with severe AS) was considered an exclusion criterion in the landmark PARTNER trial1-3 as well as in the SURTAVI trial7. Likewise, TAVI is not recommended in some of the practice guidelines in patients with AS who also have severe AR8.
However, post-approval real-world TAVI practice has expanded to groups of patients who were excluded from the pivotal clinical trials, including patients with MAVD9. As TAVI is suggested to be increasingly performed in younger patients as well as in patients with bicuspid aortic valve disease, more MAVD will be encountered among TAVI referrals.
The aim of this study was 1) to define the frequency and characteristics of patients with MAVD referred for TAVI in a real-world multicentre registry, and 2) to compare the outcomes of TAVI in patients with MAVD vs. pure/predominant AS, using a propensity score-adjusted analysis.
Methods
The study included consecutive patients enrolled in a prospective multicentre TAVI registry from January 2008 to January 2015. The list of participating centres, details of inclusion and exclusion criteria, and TAVI procedure technical aspects have been previously described elsewhere10. The study protocol was approved by the ethics committee at each of the participating centres and all patients provided written informed consent. Patients were considered eligible for inclusion if they had severe symptomatic AS and were considered by the Heart Team to be inoperable or at high surgical risk.
Aortic regurgitation (AR) severity was graded in accordance with the recommendations of the American Society of Echocardiography/European Association of Cardiovascular Imaging11,12. According to the severity of AR at baseline, the study population was divided into two groups: pure/predominant aortic stenosis (PAS, if AR was mild-or-less), and mixed aortic valve disease (MAVD, if AR was moderate or severe). The cover index was calculated as: 100×([prosthesis diameter–computed tomographic annular diameter]/prosthesis diameter).
OUTCOMES
An independent committee (including a neurologist) adjudicated all events, and all endpoints are reported according to the Valve Academic Research Consortium-2 (VARC-2) definitions13.
The primary endpoint of the present study was device success, defined as absence of procedural mortality, correct positioning of a single device into the proper anatomical location, absence of prosthesis–patient mismatch with a transaortic mean pressure gradient (PG) <20 mmHg, and absence of moderate or severe prosthetic valve regurgitation (PVR)13. Secondary endpoints included individual valve performance indices (transvalvular gradient and PVR), early safety endpoints (at 30 days) and clinical efficacy endpoints at one year.
PROPENSITY ANALYSIS
To account for baseline and procedural differences between the two groups, a score for propensity14 to MAVD has been developed using a multivariable logistic regression analysis to represent the probability of a given patient having MAVD (range, 0.003-0.986). The model was inclusive and comprised 19 variables (Table 1). This model yielded a c-statistic of 0.784 (95% confidence interval, 0.733-0.834; p<0.001), denoting a substantial ability to predict MAVD (vs. PAS).
STATISTICAL ANALYSIS
Quantitative variables are summarised as mean±standard deviation (SD) or median (interquartile range [IQR]) and are compared by the Student’s t-test or Mann-Whitney test, as appropriate. Categorical variables are summarised as frequencies and proportions and are compared by the chi-square test.
The association between MAVD and the study endpoints was tested using univariable and multivariable logistic regression analyses, and was expressed as odds ratio (OR) and 95% confidence interval (CI). In multivariable analysis, the propensity score for MAVD was entered into the model (the propensity score-adjusted multivariable regression analysis).
Cumulative survival curves for patients with MAVD vs. PAS were constructed using the Kaplan-Meier method and compared with the log-rank test. All analyses were performed with SPSS, Version 23 (IBM Corp., Armonk, NY, USA). All probability values were two-tailed, and a p-value <0.05 was considered significant.
Results
PATIENT CHARACTERISTICS
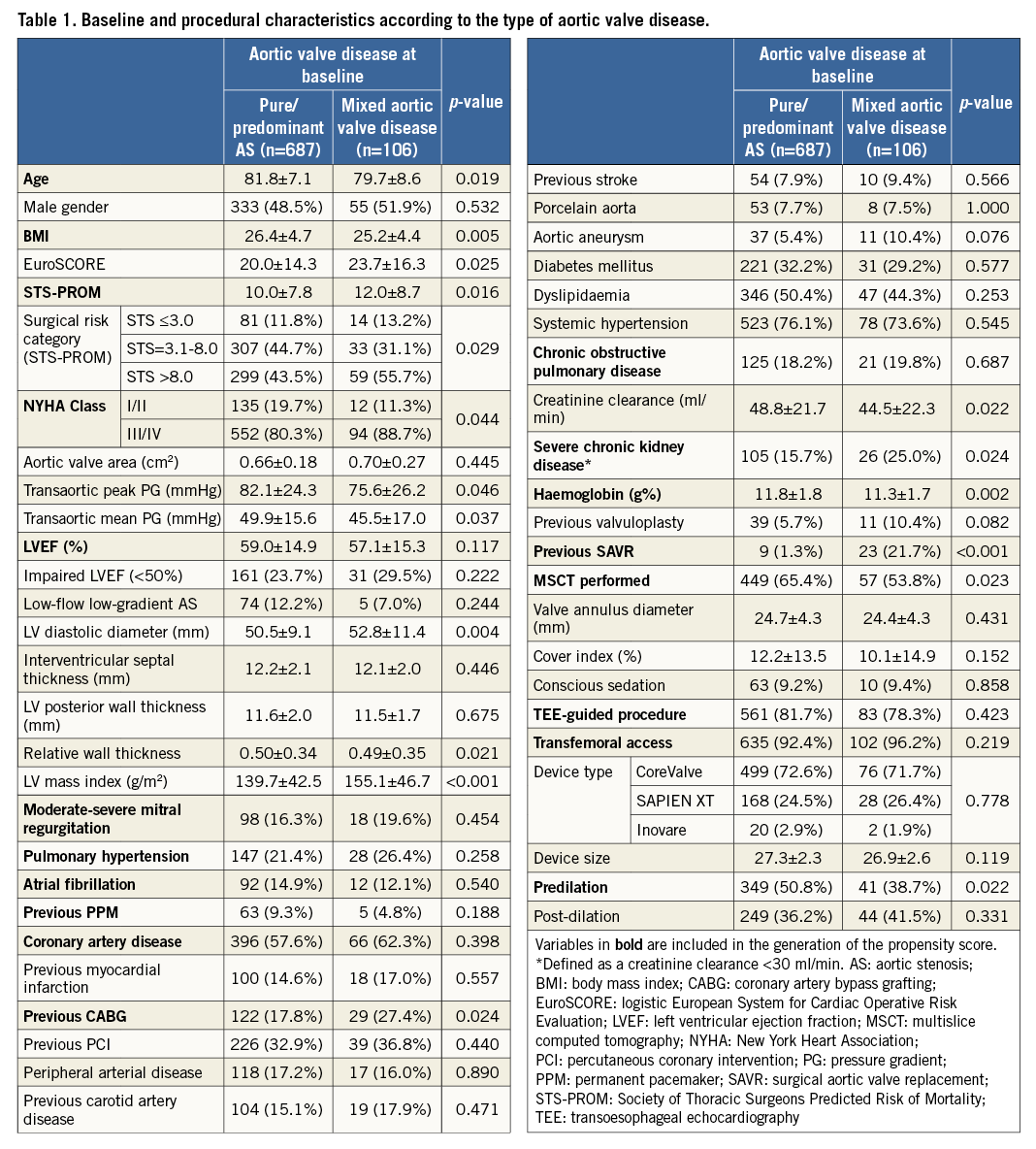
Out of 793 consecutive patients undergoing TAVI, 106 (13.4%) had MAVD. Baseline and procedural characteristics of patients with MAVD vs. PAS are summarised in Table 1. Compared to patients with PAS, those with MAVD, although younger, were at higher surgical risk and had a higher New York Heart Association (NYHA) class. MAVD patients also had a lower transaortic PG, and a larger left ventricular (LV) diastolic diameter and mass. They were also more likely to have a history of coronary artery bypass grafting (CABG) or surgical aortic valve replacement (SAVR) and to have lower creatinine clearance and haemoglobin.
MAVD AND PROCEDURAL OUTCOMES (Table 2)
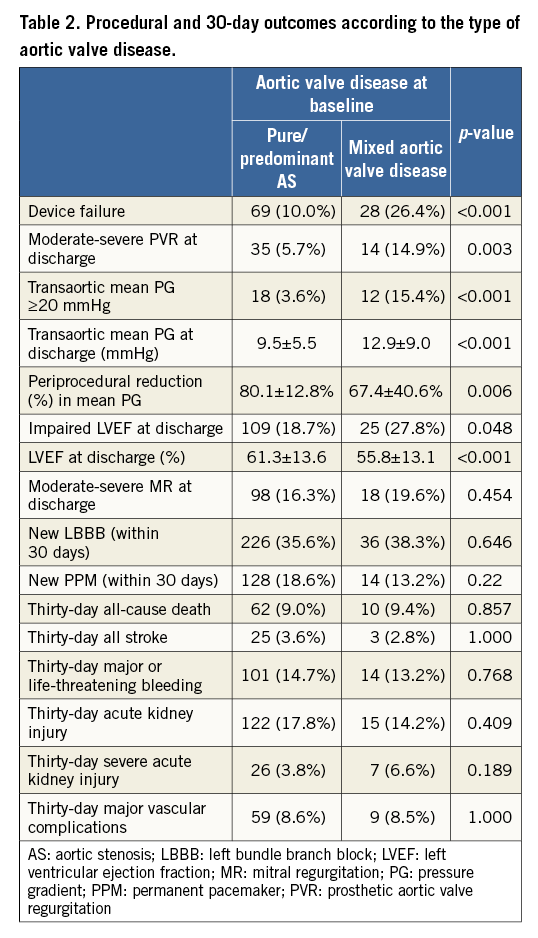
Device failure (VARC-2 definition) was significantly more frequent in patients with MAVD than in patients with PAS in the overall patient population (26.4% vs. 10.0%, p<0.001) as well as after excluding patients with previous SAVR (22.9% vs. 9.7%, p=0.001). After propensity-score adjustment, the risk of device failure remained significantly higher in MAVD patients (OR: 2.14 [1.07-4.27], p=0.032).
In univariable analysis, the two components of prosthetic valve performance were worse in MAVD than in PAS; moderate-severe PVR (15.7% vs. 3.6%, p=0.003; OR: 2.89 [1.49-5.61], p=0.002) and residual transaortic mean PG ≥20 mmHg (15.4% vs. 3.6%, p<0.001; OR: 4.81 [2.22-10.43], p<0.001). After propensity-score adjustment, MAVD was no longer significantly associated with residual PG ≥20 mmHg (OR: 0.48 [0.06-3.97], p=0.49).
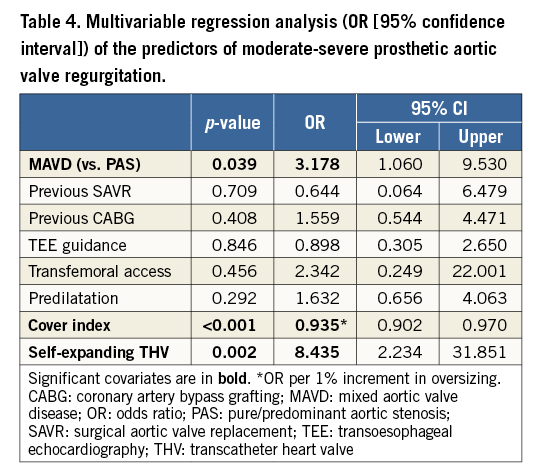
On the other hand, MAVD remained significantly associated with moderate-severe PVR after excluding patients with previous SAVR (15.1% vs. 5.8%, p=0.011) as well as after propensity-score adjustment (OR: 2.824 [1.294-6.163], p=0.009) and multivariable adjustment (OR: 3.178 [1.060-9.530], p=0.039) (Table 3). In addition to MAVD, cover index (OR: 0.935 [0.902-0.970] per 1% increment in oversizing, p<0.001) and the implantation of a self-expanding device (OR: 8.435 [2.234-31.851], p=0.002) were associated with moderate-severe PVR in multivariable regression analysis (Table 4).

The incidence of all other procedural/30-day outcomes was similar between groups, with the exception of LV ejection fraction (LVEF) which was significantly lower at discharge in MAVD patients than in PAS patients (55.8±13.1% vs. 61.3±13.6%, p<0.001). Similarly, impaired LVEF (<50%) at discharge was more common in MAVD patients (28% vs. 19%, p=0.048), with the odds ratio being significant in univariable analysis (OR: 1.68 [1.01-2.78,], p=0.045) but not in propensity-score adjusted analysis (OR: 1.15 [0.58-2.28,], p=0.695).
ONE-YEAR OUTCOMES
At one year, the overall mortality rate was 19.3% and was very much the same in the two study groups (MAVD: 19.8% and PAS: 19.2%, log-rank p=0.99) (Figure 1). Cardiac deaths constituted 70.5% of all mortalities, with their incidence being similar in both groups (MAVD: 15.1% and PAS: 13.4%, log-rank p=0.72). At the latest follow-up (median [IQR], 375 [79-742] days post TAVI), dyspnoea resolved completely (NYHA I) in 60% and 66%, was mild (NYHA II) in 30% and 26%, and was moderate-severe (NYHA III-IV) in 10% and 8% of MAVD and PAS patients, respectively (p=0.49). Accordingly, 76.4% of MAVD patients and 75.8% of PAS patients were alive beyond one year in NYHA functional Class I or II.
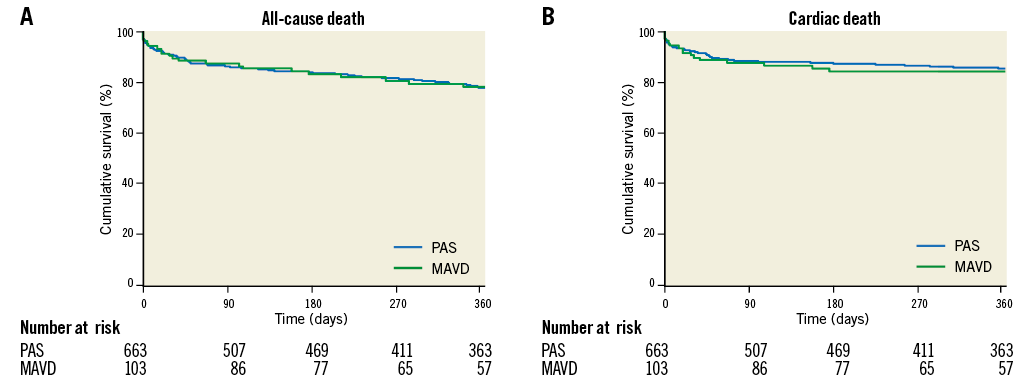
Figure 1. Kaplan-Meier survival curves for one-year mortality after TAVI according to the type of aortic valve disease (pure/predominant aortic stenosis [PAS] vs. mixed aortic valve disease [MAVD]). A) All-cause mortality. B) Cardiac mortality.
IMPACT OF PVR ON CLINICAL OUTCOMES
Overall, moderate-severe PVR developed in 49 (6.9%) patients with available echocardiographic data at discharge (n=707) and was associated with a higher one-year all-cause mortality (28.6%) compared to patients with mild-or-less PVR (13.8%, log-rank p=0.005; HR: 2.20, 95% CI: 1.25-3.86). As a higher mortality was expected to arise from the more severe PVR in the MAVD group, the impact of PVR on outcomes was studied in each of the study groups (MAVD vs. PAS) separately. The increased risk of one-year mortality in patients with moderate-severe PVR vs. mild-or-less PVR was even more pronounced in the PAS group (31.4% vs. 13.8%; log-rank p=0.002; HR: 2.64, 95% CI: 1.40-4.96, p=0.004). On the other hand, in the MAVD group, moderate-severe PVR was not associated with a significant increase in one-year mortality (21.4% vs. 13.8%; log-rank p=0.629) (Figure 2).
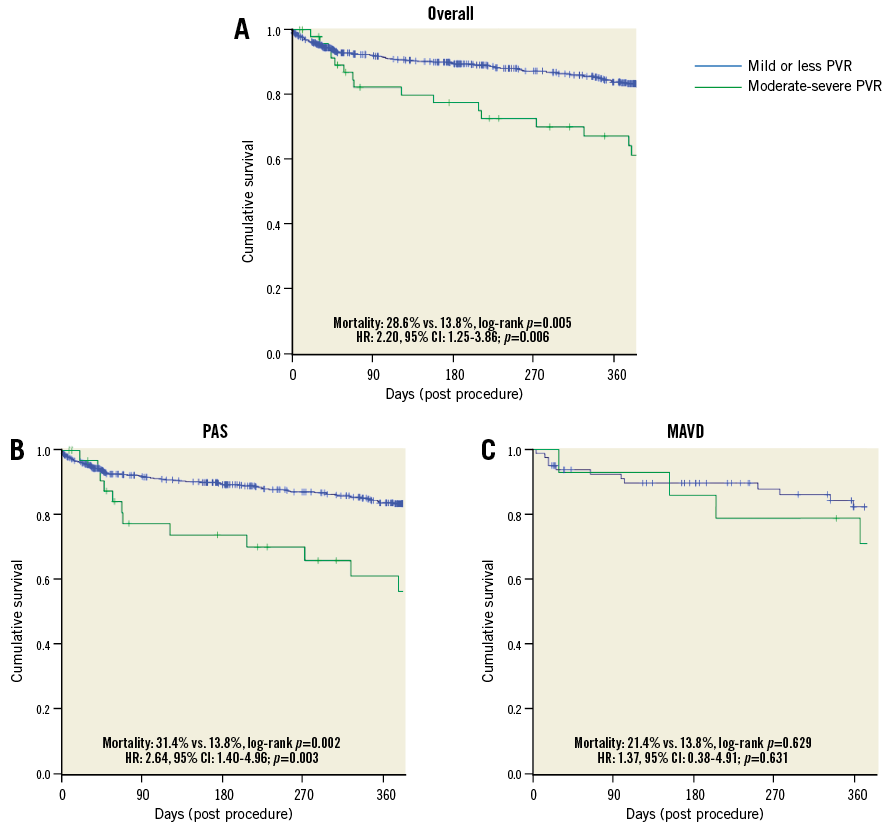
Figure 2. Kaplan-Meier survival curves for all-cause mortality after TAVI according to the severity of prosthetic valve regurgitation (PVR). A) In the entire patient population. B) In patients with pure/predominant aortic stenosis (PAS). C) In patients with mixed aortic valve disease (MAVD).
Discussion
The main findings of the present study are that: 1) MAVD is common among TAVI referrals in real-world practice and is typically associated with more severe symptoms and adverse cardiac remodelling and a higher operative risk, and 2) the incidence of PVR is significantly higher in patients with MAVD but does not impair the long-term outcomes of these patients, possibly due to a protective preconditioning of the LV.
Mixed stenosis and regurgitation is common among patients undergoing isolated SAVR, representing 19.3% of patients in the STS database from 2002 to 2010 (n=141,905)5. Among patients undergoing TAVI, MAVD was reported in 11-17% of patients in all-comers multicentre registries9,15-17. In the present real-world multicentre registry, 13% of TAVI patients had MAVD.
MAVD AS A PECULIAR DISEASE ENTITY
Anatomically, a direct association between AR and aortic valve cusp calcification and bicuspidity has been reported in population-based studies18. Vianello et al19 compared the aortic valve histologic structure in patients with degenerative aortic valve disease presenting with pure AS and patients presenting with combined AS and AR. Overall, pure AS was characterised by real “calcium replacement” of the valvular fibrous tissue, calcification of the lipid component, and bone-endochondral metaplasia, while MAVD was characterised by a higher percentage of tissue fibrosis. The authors suggested the consideration of MAVD as a separate nosological entity within the degenerative aortic valve disease spectrum, rather than considering AR as a comorbidity with AS. Those structural differences might account for a differential interaction between the device and the landing zone, and for the differential rate of PVR seen in the present study.
Haemodynamically, the combination of volume and pressure overload poses a twofold negative impact on LV mechanics and function20,21. Popescu et al22 studied 181 patients with severe AS, 71 (39%) of whom also had significant AR (i.e., MAVD). Patients with MAVD were younger, more symptomatic, and had higher LV mass, pulmonary capillary wedge pressure, LV end-diastolic pressure, and pulmonary artery pressure and a lower LVEF than those with isolated AS. There is evidence that severe AS patients managed conservatively who have concomitant significant AR have a significantly lower event-free survival than patients with pure AS23, and that even those with only moderate AS and AR are exposed to a higher rate of adverse events than those with severe pure AS24. Therefore, the combination of severe AS with moderate-severe AR represents a unique anatomical (on the valvular complex level) and functional entity.
In the present study, not only did MAVD patients present with more severe LV hypertrophy and functional impairment at baseline, but they also had higher overall estimates of operative risk (higher EuroSCORE and STS-PROM). Therefore, and also due to the aforementioned studies linking MAVD to worse outcome, an earlier intervention should be considered and, because of the high surgical risk, TAVI can be the preferred option. In our study, and earlier studies22,24, patients with MAVD were younger than PAS patients at the time when valve implantation was indicated. Accordingly, MAVD represents a disease entity that will be increasingly encountered as TAVI indications are extended to younger patients.
TAVI OUTCOMES IN MAVD
We found that acute TAVI outcomes in MAVD patients were generally favourable, with the exception of an unequivocally higher risk of PVR which remained significant after accounting for patient-, procedure-, and device-related confounders. A similar association with the risk of PVR and the need for balloon post-dilation was reported in AS patients undergoing TAVI who also had >mild AR9,17 or any degree of AR15. In the latter study, however, the group of patients with MAVD included a large number of patients with mild AR at baseline, a degree of regurgitation that typically does not have significant haemodynamic consequences that make it haemodynamically distinct from PAS. Such a relation between baseline AR and the risk of PVR is important to consider in order to understand, at least partially, the marked variability in the incidence of PVR among different TAVI studies, even among those involving the same device25,26. This inconsistency, which can be largely attributed to the limitations of the echocardiographic assessment of PVR27, can also be partially explained by the inter-study variability in the severity of AR considered acceptable for inclusion.
In the present study, MAVD patients did very much the same in terms of mortality and symptomatic status up to one year post TAVI in spite of an increased risk at baseline and an increased rate of PVR after the procedure. It turns out, as has been confirmed in subgroup survival analysis, that the higher risk of PVR is compensated for, possibly by the LV preconditioning9,28. Manoeuvres that can be undertaken to reduce the severity of PVR have their own risks29. Therefore, identification of patient subgroups with poor or good tolerance to PVR is clinically relevant9.
It has been reported in patients with AR (as compared to AS patients) undergoing SAVR, that the postoperative decline of LV end-diastolic volume occurs faster than the normalisation of LV mass, resulting in concentric remodelling, impaired LV relaxation, and to a rise of diastolic filling pressure30. This provides another explanation of PVR being well tolerated in those patients, who seem to “benefit” from some degree of regurgitation that probably prevents this concentric remodelling.
These findings collectively suggest that patients with MAVD gain an equivalent benefit from TAVI to those with PAS. Considering the worse symptomatic status and the poorer survival in patients with MAVD if left untreated, it turns out that this equivalent absolute outcome in fact reflects a higher relative benefit.
Limitations
The assessment of AR severity is challenging in the setting of severe AS. However, the classification of AR into mild-or-less vs. moderate-to-severe is less challenging than more granular classifications.
Propensity score adjustment accounts only for the “observed” covariates included in the propensity score construction. We adopted the following actions to limit such a limitation of the propensity score: 1) the model used for propensity score construction was inclusive not only of covariates which were different between the two groups, but also other covariates relevant to the endpoints of interest; 2) the score was tested for its discriminative accuracy (revealed to be good as evidenced by a substantial c-statistic); and 3) the score was used in conjunction with further model-based adjustment using multivariable regression analysis, after exclusion of significant multicollinearity between the propensity score and its derivative covariates.
Significant AR in conjunction with AS is frequent in bicuspid aortic valve pathology. As data are derived from a real-world registry, the challenges in identifying and confirming a bicuspid pathology on echocardiographic studies existed. Obviously, it cannot be excluded that some of the extensively calcified valves have an underlying masked bicuspid aetiology31.
The present study did not include patients treated with the next-generation transcatheter aortic valves, so extending the findings to those patients should be cautious. However, correlates of MAVD were shown in our analysis to be independent of patient-, procedure-, and device-related characteristics.
Conclusions
A significant proportion of AS patients referred for TAVI in a real-world registry has moderate-severe AR and presents with an overall higher cardiac adverse remodelling and operative risk. The incidence of PVR is significantly higher in patients with MAVD than in patients with PAS, but does not significantly impact on mortality. Overall, the outcome of patients with MAVD is comparable to that of patients with PAS in the acute and midterm phases.
| Impact on daily practice TAVI is probably as effective in patients with mixed aortic valve disease as in patients with pure aortic stenosis. Mixed aortic valve disease is a potential extended indication for TAVI. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Hôpital Bichat, Paris and University Paris VII, Paris, France.
Conflict of interest statement
R. Sarmento-Leite, J. Mangione, and F. de Brito Jr are proctors for Medtronic and Edwards Lifesciences. P. Lemos is a proctor for Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

