Abstract
Aims: The aim of this study was to evaluate the effectiveness of titanium-nitride-oxide (TITANOX) -coated stent and paclitaxel-eluting stent (PES) in patients presenting with acute myocardial infarction (MI).
Methods and results: A total of 425 patients presenting with acute non-ST-elevation MI or ST-elevation MI were randomly assigned to receive TITANOX-coated stent or PES. The primary end point was a composite of MI, target lesion revascularisation (TLR) or death from cardiac causes. At 12 months, there was no significant difference between patients receiving TITANOX-coated stent or PES in the rates of primary end point (10.3% vs. 12.8%, P=0.5), MI (4.2% vs. 8.1%, P=0.1), or TLR (9.3% vs. 7.1%, P=0.5), respectively. The incidence of stent thrombosis, defined according to Academic Research Consortium classification, was significantly lower in the TITANOX group compared to the PES group (0.9% vs. 4.3%, P=0.03).
Conclusions: TITANOX-coated stent and PES resulted in comparable clinical outcomes during 12 months follow-up among patients treated for acute MI.
Introduction
Drug-eluting stents (DES) have been shown to reduce in-stent restenosis after percutaneous coronary intervention (PCI) compared to bare metal stents (BMS).1,2 However, most randomised DES trials have excluded patients with acute myocardial infarction (MI), though invasive approach is currently the preferred method for treatment of acute MI.3-9
Five randomised trials and four meta-analyses of the clinical trials on the use of DES for treatment of acute ST-elevation MI (STEMI) demonstrated that the use of DES in patients with acute STEMI is safe and improves clinical outcomes mainly by decreasing the risk of reintervention compared with BMS.10-18 However, in acute MI, the use of DES is still considered to be an off-label indication. Non-ST-elevation MI (NSTEMI)and STEMI are usually considered to be different entities, but recent reports suggested that the prognosis of either subgroup of MI is similar despite different management strategies.19,20
Recently, there have been concerns about the safety of DES e.g. most notably stent thrombosis (ST). For on-label use, identical rates of ST were observed in both selected DES and BMS patients up to four years according to pooled analyses of randomised DES trials.21,22 On the other hand, stent coating with compounds like titanium-nitride-oxide seem to decrease acute surface thrombogenicity,23-26 and reduce in-stent restenosis when compared with conventional stainless steel stents.23 Moreover, titanium-nitride-oxide-coated stent (TITANOX) are associated with comparable clinical outcomes compared to paclitaxel-eluting stent (PES) in the real world clinical practice of high-risk patients.24
We designed a prospective, randomised, multicentre trial with the main purpose of evaluating the effectiveness of TITANOX-coated stents and PES in patients presenting with acute MI. We report 12 months follow-up results of the trial.
Methods
Study design and patient population
The TITAX AMI (titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction) trial is a prospective, randomised, multicentre trial conducted from December 2005 to November 2006 in six Finnish hospitals. The study was initiated by the investigators and conducted according
to the declaration of Helsinki and written informed consent was obtained from all patients. Study protocol was approved by the Ethics Committees of the coordinating centre Satakunta Central Hospital and the participating hospitals.
Patients >18 years of age presenting with acute MI were eligible for this trial. Diagnostic criteria for NSTEMI included symptoms and signs of myocardial ischaemia, dynamic ECG changes and detection of rise and/or fall of cardiac biomarkers (troponin) with at least one value above the 99th percentile of upper reference limit. STEMI was diagnosed if patient had chest pain at rest >20 minutes and persistent > 1 mm of ST-segment elevation in at least two contiguous limb leads or > 2 mm in two contiguous precordial leads. All patients receiving thrombolysis therapy were eligible for the trial, and it was recommended to perform intra-coronary stenting <24 hours after the admission. Exclusion criteria included unprotected left main disease, ostial or restenotic lesions, contraindication to aspirin, clopidogrel or heparins, life expectancy of less than 12 months and need for a stent longer than 28 mm. According to the trial protocol, randomisation was performed after visualisation of the culprit lesion or a totally occluded infarct-related vessel during coronary angiography. Patients were randomly assigned to the study groups in a 1:1 fashion.
Procedures and clinical follow-up
Culprit lesions were treated according to current interventional techniques, with the final strategy (thrombectomy devices, direct stenting, postdilatation, intravascular ultrasound) left entirely up to the operator’s discretion. Angiographic success was defined as a residual stenosis <30% by visual analysis in the presence of thrombolysis in myocardial infarction (TIMI) flow grade 3. If more than one stent was needed, stents of the same type as the assigned stent were recommended. The study protocol recommended premedication with aspirin (dose 100-500 mg) or a loading dose of intravenous aspirin (250-500 mg), and clopidogrel before the procedure. If clopidogrel was not utilised before the procedure, a loading dose of 300-600mg of clopidogrel was administered immediately after the index procedure. Administration of intravenous heparin, low-molecular-weight-heparin, bivalirudin and glycoprotein IIb/IIIa-receptor inhibitors were left to the investigator’s discretion.
Titan® (Hexacath, Paris, France) stent is a thin strut balloon expandable stent made of stainless steel and coated with TITANOX by plasma enhanced vapour deposition of titanium in a prespecified gas mixture of nitrogen and oxygen in a vacuum chamber.24-26 TITANOX-coated stents were available in lengths of 7, 10, 13, 16, 19, 22 and 28 mm, and in diameters of 2, 2.25, 2.50, 2.75, 3.0, 3.5, 4.0 and 4.5 mm. Taxus-Liberte® stent (Boston Scientific, Natick, MA, USA) is comprised of a stainless steel stent platform, a polyolefin polymer derivative, and a microtubular stabilising agent paclitaxel, with two-phase 30-day polymeric release kinetics that provides antiproliferative effect.2 Paclitaxel release is completed within 30 days of implantation, although a substantial portion (>90%) of the paclitaxel remains within the polymer indefinitely. PES’s were available in lengths of 8, 12, 16, 20, 24, and 28 mm, and in diameters of 2.25, 2.50, 2.75, 3.0, 3.5, 4.0 and 4.5 mm.
At discharge, 100 mg of aspirin daily indefinitely and 75 mg of clopidogrel daily at least six months were prescribed for all patients. We recorded adverse events during hospitalisation and clinical follow-up was performed at 30 days, and six and 12 months.
Primary and secondary end points
The primary end point was the first occurrence of major adverse cardiac event (MACE) at 12 months defined as the composite of target lesion revascularisation (TLR), recurrent MI, or death from cardiac causes. TLR was defined as a repeat percutaneous intervention of the target lesion to treat a stenosis (>50%) within the stent or in the segments 5 mm distal or proximal to the stent, driven by clinical symptoms and/or signs of myocardial ischaemia, or bypass surgery of the target vessel due to the in-stent restenosis or other complications of the target lesion. Myocardial reinfarction during the follow-up was diagnosed when a rise in the myocardial injury marker level (troponin I or T) >upper reference limit was detected together with symptoms suggestive of acute myocardial ischaemia. For the diagnosis of myocardial reinfarction during the index hospitalisation, a new rise >50% above the previously measured injury marker level was required. Cardiac death was defined as any death due to cardiac causes, unwitnessed death or death of unknown causes. The secondary endpoints of the trial included all-cause mortality, composite of cardiac death or reinfarction and ST. According to the protocol, ST was diagnosed in the presence of an acute coronary syndrome with angiographic evidence of either vessel occlusion or thrombus within the study stent, or in autopsy. ST was categorised as acute (<24 hours after the stenting), subacute (1-30 days after the stenting), or late (>30 days after the stenting). Additionally, we agreed to use the definition of ST according to the Academic Research Consortium (ARC) classification as definite, probable, or possible.27 Blinded outcome assessment was performed by the independent clinical event committee.
Statistical analysis
In the current literature, there are no published data on the utilisation of TITANOX-coated stent or PES in the setting of acute MI comprising both NSTEMI and STEMI. Because of the exploratory nature of the study and the consequent lack of a prior knowledge about the effect size, the sample size calculation was based on subgroup analysis of the results of previously published registry data of TITANOX-coated stents and PES in the real world clinical practice.24 In this registry, the incidence of MACE at 12 months
in patients presenting with acute MI before the index PCI was ~7% in TITANOX group and ~15% in PES group. We estimated that a total of 200 patients would be required in each group to provide 80% power at the 5% level of significance to detect this difference of 8% in primary end point between the study groups. All analyses were done on the basis of intention-to-treat principle, i.e., the analyses were based on all patients, as randomised.
Continuous variables are presented as means (SD) and study groups were compared by Student’s unpaired t test. Categorical variables are presented as counts and percentages and were compared by the chi square or Fisher’s exact test. In order to identify the independent predictors for primary end point, TLR, and ST during the 12 months follow-up, first univariate logistic regression for each baseline clinical characteristics and procedural variables was applied. At the second stage, the variables significantly (P<0.05) associated with dependent variables in univariate analyses were included in multivariable analyses. A two-sided P value <0.05 was required for statistical significance. The rate of survival free from primary end point during the 12 months follow-up period were estimated with the Kaplan-Meier method. The 95% confidence interval (CI) for the relative risk was calculated with the use of standard errors from the Kaplan-Meier curve. The significance of differences between treatment groups was assessed by the log-rank test. All data were analysed with the use of SPSS version 11.28
The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Baseline and procedural characteristics
Between December 2005 and November 2006, a total of 425 patients were randomised to the two treatment groups (214 to the TITANOX group and 211 to the PES group). Baseline characteristics were comparable between the study groups, except by a higher incidence of previous PCI’s in the TITANOX group (Table 1).
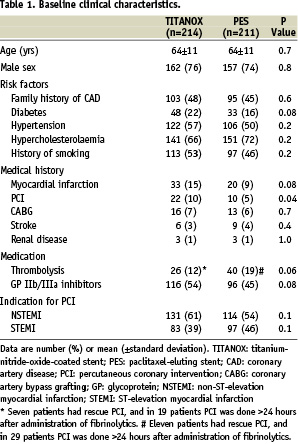
The baseline angiographic variables and procedural characteristics are presented in Table 2.
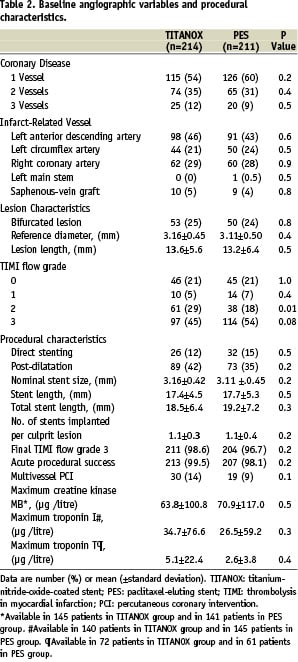
Procedural success was achieved in 99.5% of patients in the TITANOX group and in 98.1% in the PES group. The mean peak value of the creatine kinase MB isoenzyme (64±101 µg/l in TITANOX group vs. 71±117 µg/l in PES group, P=0.5) and troponin I (35±77 µg/l vs. 27±59 µg/l, respectively, P=0.3) were similar in both study groups.
Clinical outcome
At 30 days (Table 3), the incidence of recurrent MI was lower in the TITANOX group than in the PES group (P=0.03).
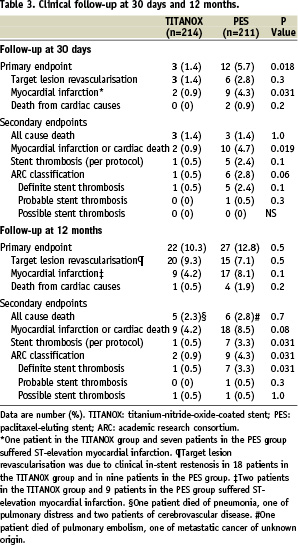
ST occurred in one patient (0.5%) in the TITANOX group and in 5 (2.4%) patients in the PES group (P=0.10). At 12 months, complete follow-up was obtained from all patients in both study groups (Table 3). The cumulative incidence of primary endpoint was 10.3% in the TITANOX group and 12.8% in the PES group (relative risk 1.28, 95% CI 0.70-2.33, P=0.5, Figure 1).
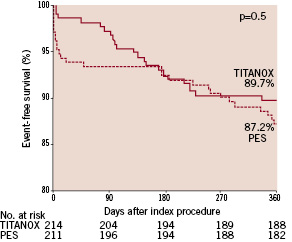
Figure 1. Kaplan-Meier curve of event-free survival in patients randomised to titanium-nitride-oxide-coated stent (TITANOX) vs paclitaxel-eluting stent (PES). P=0.5 by the log-rank test.
A total of 20 patients (9.3%) in the TITANOX group and 15 patients (7.1%) in PES group underwent TLR (P=0.5). Clinical in-stent restenosis requiring repeat intervention occurred in 18 patients (8.4%) in the TITANOX group and in nine patients (4.3%) in the PES group (relative risk 0.49, 95% CI 0.21-1.11, P=0.1). During 12 months follow-up, the incidence of ST according to the study protocol was 0.5% in the TITANOX group and 3.3 % in the PES group (P=0.03). When using ARC classification, we observed a lower rate of ST in the TITANOX group (0.9% vs. 4.3%, respectively, P=0.03, Table 3). Characteristics of individual cases of ST are shown in Table 4.
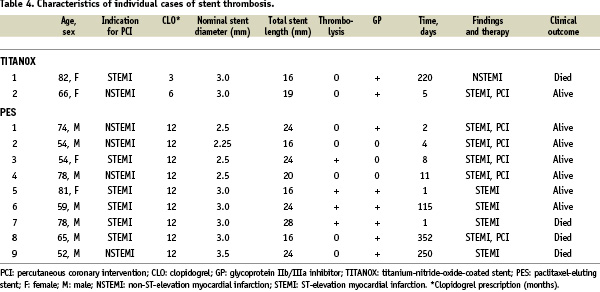
In the PES group, ST occurred in 4 (10%) patients who received thrombolysis therapy before index procedure, of which three had subacute ST (range 1-8 days), and one had late ST (day 115). In three patients, clopidogrel was prematurely discontinued before the event of ST, and all of these patients were in the PES group. Four patients out of 11 who suffered ST died.
Clopidogrel was prescribed after the discharge for a mean length of 7.6 months in TITANOX group and of 10.0 months in PES group (P<0.001). A total of 67 patients (31%) in TITANOX group and 138 patients (65%) in PES group were receiving dual antiplatelet therapy with aspirin and clopidogrel at the time of the twelve months follow-up (P<0.001).
In multivariable analysis, there were no independent predictors for the primary end point, TLR, cardiac death or ST. Recurrent MI was predicted by older age (OR 1.1, CI 1.0-1.1, P=0.02).
Outcomes in patients presenting with NSTEMI or STEMI
A total of 245 patients presented with NSTEMI before the index procedure (131 patients in TITANOX group vs. 114 in PES group). These patients were more likely to have diabetes (P=0.001), hypertension (P=0.04) and prior PCI (P=0.04) in their medical history than patients presenting with STEMI. The occurrence of the primary end point (12.2% in NSTEMI patients vs. 10.6% in STEMI patients, P=0.6), MI, cardiac death and TLR were similar in these two subgroups of patients during the 12 months follow-up. Furthermore, the rate of ST was comparable in these two subgroups of patients (2.0% vs. 3.3%, P=0.5, respectively). In addition, patients presenting with NSTEMI and receiving PES were more likely to have recurrent MI during the follow-up (P=0.03, Figure 2).
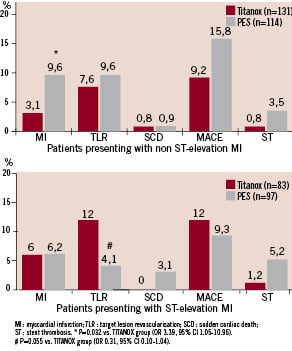
Figure 2. Effect of titanium-nitride-oxide-coated stent (TITANOX) and paclitaxel-eluting stent (PES) on subsequent outcome events in patients presenting with NSTEMI or STEMI before the index procedure. At 12 months follow-up, the incidence of myocardial infarction was increased in PES patients presenting with NSTEMI before the index procedure (A). In patients who presented with STEMI, there was a slight but non-significant difference in TLR for PES vs. TITANOX stent (B).
There was a slight but non-significant tendency to increased incidence in TLR in the TITANOX group compared with the PES group in patients who presented with STEMI before the index procedure (P=0.055).
Discussion
Main study findings
To our knowledge, TITAX AMI trial is the first head to head randomised comparison of TITANOX-coated stent and PES among patients with acute MI (NSTEMI or STEMI). The main finding of this trial is that both stent types resulted in comparable incidence of primary end point during 12 months follow-up. Secondly, although the overall rate of ST was fairly low, there was a trend towards a higher rate of ST in PES treated patients. However, in multivariable analyses, no independent predictors for primary endpoint or ST were found.
Previous studies
A total of six observational studies on DES implantation in acute STEMI patients have been published.29-34 Four of the studies compared sirolimus-eluting stent (SES) to BMS.29-32 At six months, the rate of MACE among SES treated patients was ~6-9%. Hofma et al conducted observational comparison of SES and PES implantation for the treatment of acute MI. After 12 months there was no difference between groups in MACE-free survival,34 but with the use of PES, the incidence of MACE at 12 months was higher than in present study.
At present, a total of five randomised trials comparing of DES and BMS in STEMI have been published.10-14 The STRATEGY trial is a prospective, single-blind and randomised trial with the main purpose of evaluating impact of single high-dose bolus tirofiban plus SES versus abciximab plus BMS in patients with MI.10 In this small trial (n=175), the rate of MACE was surprisingly high in both stent arms at 12 months follow-up (18% vs. 32%, respectively). Similarly, in MULTISTRATEGY trial in 745 STEMI patients, high-dose bolus tirofiban plus SES implantation was associated with a significantly lower risk of MACE (7.8% vs. 14.5%) than abciximab plus BMS within eight months after intervention.11 In addition, there are two moderately large, randomised trials comparing SES to BMS (TYPHOON and SESAMI) in the clinical setting of acute STEMI.12,13 In both of these studies, the incidence of MACE was ~7% during 12 months follow-up with the use of SES, which is distinctively lower than observed in present study. However, the main limitation with SESAMI trial was that the primary endpoint was binary restenosis instead of clinical endpoint such as MACE. As for the use of PES, the PASSION trial is the only randomised trial comparing PES and BMS for acute STEMI.14 PES tended to reduce the incidence of serious adverse cardiac events at one year and the use of PES was associated with a lower rate of MACE (8.8%) than the present study in both stent arms.
Recently, four meta-analyses of the clinical trials on the use of DES for treatment of acute STEMI have been published.15-18 These analyses showed, that the use of DES in patients with acute STEMI is safe and improves clinical outcomes mainly by decreasing the risk of reintervention compared with bare BMS.
Stent thrombosis
In the present study, the incidence of definite ST was 3.3% in the PES group. This rate is comparable to the previous observational and randomised studies with DES implantation in patients with acute MI (0-3.4%). However, since the sample size is small and patients with thrombolysis therapy were included in this analysis, no valid conclusions can be drawn. The incidence of definite ST in the TITANOX group was low (0.5%), given the thrombotic environment at the time of the stent deployment and the inclusion of patients with thrombolysis therapy in the present trial. It has been suggested that thrombolysis therapy preceding coronary stenting predisposes to ischaemic cardiac complications, such as early reinfarction, ST or urgent target vessel revascularisation. Facilitated regimens with thrombolytic therapy lead to urgent target vessel revascularisation more often compared with primary intervention (4% vs. 1%) and in the ASSENT-4 trial, a strategy of full-dose tenecteplase plus stenting within one to three hours, was associated with a higher incidence of MACE compared with stenting alone strategy in patients presenting with STEMI.35,36
Study limitations
There are various salient differences between the TITAX AMI trial and previous MI trials (TYPHOON/PASSION). The inclusion and exclusion criteria differed considerably. TITAX AMI trial included patients with NSTEMI and STEMI, which are usually considered to be different entities, whereas previous studies included only patients with STEMI. Therefore it may be difficult to compare the present study with the STEMI studies. However, recent reports suggest that the prognosis of NSTEMI and STEMI are similar despite different management strategies.19,20 Similarly, in the present study, 12 months outcome was comparable in these two subgroups of patients, despite the fact that NSTEMI patients tended to have more co-morbidities. In addition, the TYPHOON and PASSION trials excluded patients who received thrombolysis therapy, whereas the TITAX AMI trial included them. Notably, four patients (10%) with thrombolysis and PES experienced ST. In the present trial, PES was chosen to be a control stent, whereas most previous studies with positive results were performed with SES.
The sample size was based on a small real life cohort and the present trial is underpowered to reveal potential small differences in primary and individual end points, although we chose the setting of acute MI known to predispose to clinical complications. In addition, TITAX AMI trial did not reach its primary endpoint, and therefore much larger sample size is needed to demonstrate the non-inferiority of TITANOX stent compared with PES. In the present trial, the randomisation was performed after visualisation of culprit lesion, and this strategy may have had the flaw of possible selection bias by the operator who excludes patients with certain angiographic characteristics, e.g., large thrombus. The design of our study did not include angiographic follow-up or routine non-invasive testing for myocardial ischaemia and therefore we certainly under-estimated the incidence of silent or angiographic restenosis. On the other hand, by relying on clinical follow-up only, the chance of unnecessary TLR due to the “oculostenotic reflex” or patient’s unjustified anxiety was avoided. In addition, the stenting was performed in patients with relatively large infarct related arteries with low risk of in-stent restenosis.
Conclusions
In conclusion, in patients presenting with acute MI, both TITANOX coated stent and PES resulted in similar clinical outcome at 12 months. The overall risk of ST was low during dual antiplatelet treatment, but there was a non-significant trend towards less ST in the TITANOX group.
Clinical trial registration information
Clinical trial registration information: URL:http://www.clinicaltrials.gov. Unique identifier: NCT00495664.
Funding sources: This work (Dr Airaksinen) was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland.
Acknowledgements
The authors thank Tuija Vasankari, RN, Eija Niemelä, RN, and Marja-Liisa Sutinen, RN, for their support in the conduct of this study.

