Pre-clinical experience
The literature is rich with data referring to the benefits of titanium and titanium oxides used for medical applications such as implantable medical devices.
In 1981, Simpson et al1 reported in a study involving implants made of stainless steel, cobalt and titanium, that clinical symptoms of pain, swelling and inflammation were observed with the first two metals but none with titanium. Steinmann et al2,3 reported that tissue impregnation by corrosive products is equivalent to a chemical insult, and that metal ions can unite with protein to form an antigen. Such complex formations are known to occur with nickel, cobalt and chromium but are absent from titanium. Irena Gotman4 reported that local tissue response to metal implants is closely related to the amount and toxicity of the corrosive products. Thus minimal fibrosis is typically observed around titanium alloys, whereas fibrous layers as much as 2 mm thick are encountered with Co-Cr and especially with 316L implants.
In 1998, Zhang et al5 published the results about titanium oxides in vitro examinations showing that titanium oxides were able to inhibit platelet aggregation and fibrin growth (Figure 1).

Figure 1. Platelet aggregation in coated and uncoated groups.
In 2006, Yeh et al6 reported that titanium-NO was able to promote and accelerate endothelial cells growth versus other materials like stainless steel or nitinol assuming that a stent coated with titanium NO could achieve earlier re-endothelialisation and gain benefits in terms of thrombosis and cell proliferation reduction. Koster et al7 demonstrated in 2000 that stent induced inflammation could trigger restenosis, therefore a material acting like an unbreakable barrier against the release of toxic ions could have an effect on inflammation and therefore on restenosis reduction.
In 2001, Windecker et al8 demonstrated the efficacy of the titanium-NO compound, showing a neointimal hyperplasia reduction close to 50% compared with 316L stents of identical design in the porcine model at six weeks follow-up (Figure 2).
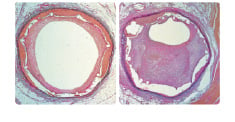
Figure 2. Comparison of coated and uncoated neointimal proliferation.
The antiproliferative effect was comparable to that reported by Suzuki9 for sirolimus-eluting stents in pigs.
Clinical experience
Efficacy
The efficacy of titanium-NO stents was confirmed by three randomised studies versus BMS enrolling 279 patients.
First, Windecker et al10 reported that titanium-NO stents were able to significantly reduce mean late loss (0.55 mm±0.63 mm) in comparison to uncoated 316L stents with same design showing a 40% reduction (p=0.03) at six months follow up. Second, Sansa et al11,12 reported a binary restenosis reduction of 71% for titanium-NO stents (p<0.05) versus bare metal stents (BMS) of other designs in acute coronary syndrome (ACS) patients with no acute or late thrombosis at six months angiographic follow-up. Third, Mendes et al13 reported that titanium-NO stents could significantly reduce the overall rate of MACE at six months follow-up showing a reduction of 88% (p=0.013) versus BMS in a cohort without any exclusion criteria.
Interestingly, these three randomised studies were performed without sponsorship from Hexacath.
Safety
The safety of titanium-NO stents was further confirmed by nine clinical studies enrolling more than 2,500 patients in both unselected populations as well as in the most complex indications such as diabetics or small vessels.
Grenadier et al14 reported a prospective non-randomised analysis of Titan BAS versus DES in small vessels (2.4 mm), and showed a target lesion revascularisation (TLR) at 19 months of 7.3% for the BAS group, 12.3% at 14 months for SES and 6.1% at 12 months for PES.
Valdes et al15 reported a prospective multicentre registry in diabetics with a MACE of 10.3% and a TLR of 7.09% at six months follow up. QCA and IVUS were performed at follow-up in a sub-group and confirmed a low late loss of respectively 0.56 mm and 0.50 mm.
Mosseri et al16 reported a multicentre and prospective experience with the Titan BAS in a cohort of 296 patients with 36% DM, 81% ACS, 61% of B2/C lesions and a mean lesion length of 17.5 mm. The MACE was 7% (gIobal) and 6.3% (patients) with a low TLR of 5.4% at six months. Karjalainen et al17-19 reported a single centre prospective, non-randomised, comparative registry of 405 patients treated with BAS and PES.
Patients characteristics were similar in both groups but in the BAS cohort patients were older (67 vs 64 p=0.022), more had hypercholesterolaemia (90% vs 82% p=0.017) and hypertension (66% vs 54%, p=0.016), more primary MI (44% vs 32% p=0.010) and more acute STEMI (31% vs 20% p=0.013). In the DES cohort, patients had more previous PCI (14%.vs 24% p=0.015). Lesions characteristics were similar in both groups except more type C lesions (25% vs 8% p<0.001) in the BAS group; the DES group had longer stented segment (15.6 mm vs 21.2 mm p.<0.001) while the lesion lengths were similar (13.1 mm vs 13.5 mm p=0.21).
Valdesuso et al20 reported a multicentre prospective registry in small vessels indications with a MACE at eight months of 8.6% in mean vessel diameter of 2.46 mm. Calvo et al21 reported preliminary results of a prospective multicentre registry enrolling 221 patients with acute STEMI. The rate of rescue PCI was 24%, diabetics 27%, B2/C lesions 78%, visible thrombus 28% and TIMI 0/1 in 58% of the cases. At six months, the MACE was 10.1%.
Baglini et al22 reported a case of a 67 year old male with type II diabetes treated for a 80% type C lesion with 2 BAS (2.5x 18/2.25x13 mm). At two months follow up the patient revealed that he had never taken ASA nor clopidogrel which had been recommended. The patient was asymptomatic and follow up angiography showed a patent LAD stent with TIMI III flow.
Most recently, Karjalainen et al presented during the Late Breaking Coronary Trials at EuroPCR 2006 in Barcelona, the results of TITAX AMI24, a multicentre prospective randomised study enroling 425 patients treated either with a Titan 2 BAS or a Taxus PES in AMI. At six months, the rate of MACE was 7,5% for the BAS group versus 7,1% for the PES group (P=1), with a rate of stent thrombosis of 0.5% in the BAS group versus 3,3% in the PES group.
Device description
Titan 2 BAS is a laser cut slotted tube, made of medical grade 316LSS and coated with a thin atomic layer of titanium-NO. The stent is mounted on a Hexacath 140 cm long PTCA balloon carrier, rapid exchange, compatible with 0.014” guidewires and made of semi-compliant balloon material. The catheter benefits from a hydrophilic coating and its shaft characteristics enable kissing balloon procedures in 6Fr guiding catheters.
The stent platform is the BMS Helistent benefiting from a unique helicoidal design providing excellent flexibility and conformability (Figure 3, 4).
Figure 3. Stent design.

Figure 4. Stent design in lateral view.
These features result in stent navigation inside the curved arteries and minimal stent induced trauma and inflammation due to this highly conformable helicoidal structure. The unique design of Titan 2 stent also provides ultra-low recoil available while maintaining collapse pressure of 1.2 bars, 50% higher than other tubular stent structures known to have an excellent radial force. This platform enables also the treatment of lesions located in bifurcations and gives an easier side branch access. Titan 2 is available in various diameters and lengths and provides an “Extra Small” and “Extra Large” design with a stent strut thickness adapted to each vessel diameter starting at 70 microns.
A proprietary process has been developed to coat titanium-NO on a stent surface based on a plasma technology using the nanosynthesis of gas and metal. A special custom made chamber forms a vacuum with the help of cryogenic pumps working at – 200°C. Pure titanium (99.99%) is used to obtain a titanium atomic vaporisation further the injection of noble gas. The combination of magnetic fields, vacuum and simultaneous injections of nitrogen and oxygen are necessary to create ionisation and a plasma formation of titanium-NO. Plasma is a unique state of the matter considered to be the 4th state after liquid, solid and gas. Titanium-NO is then coated on all the surfaces of the stent both inside and outside, through a unique and patented process which results in NO-particles presence on the stent surface23. This coating is extremely dense and hard, making titanium-NO coated stents extremely well adapted for direct stenting where the most calcified lesions cannot damage at all the external layers of the coating (Figure 5).
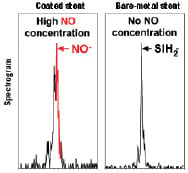
Figure 5. The coated stent shows a large NO-peak on its surface which is not present in the bare-metal stent.
In addition, the titanium-NO coating is adding strength to the stainless steel substrate making possible the use of thinner struts. However, the biggest challenge was to develop a process where the coating, which is comparable to a ceramic, would adhere homogeneously to the stainless steel substrate without any fracture after the stent expansion. Fatigue tests simulating 10 years of stent implantation with scanning electron microscope analysis have proven the integrity of the Hexacath titanium-NO coating. This has been shown to remain intact after stent implantation without any sign of micro fractures, thus ensuring all the demonstrated benefits of titanium-NO bio-coating which is specifically active against both restenosis and thrombosis, as well as being an accelerator of re-endothelialisation.

