Abstract
Conventional tubular balloon expandable (BE) stent designs are poorly suited to coronary bifurcations, yielding highly variable results. For this reason, intense interest remains and industry development continues for dedicated bifurcation coronary stents to make treatment of such lesions more straightforward. Innovative designs have emerged, some balloon expandable, but also self-expandable, based on the premise that nitinol based designs may conform more favourably than other metals to non-tubular vascular structures such as bifurcations. Through discussion of one of the early implantations of the first of these new age self-expanding designs, the Devax coronary bifurcation system, we outline the state-of-the-art use of self-expanding designs in percutaneous intervention to coronary bifurcations, and revisit an old paradigm with contemporary applications. The Devax system was the first of a new generation of self-expanding coronary stents after a more than 15 year lull in their development and was also the first dedicated drug eluting stent (DES) for bifurcations. It was first used in man in 2003. Other self-expanding designs have followed and shown promise in this setting, including Stentys and the Capella Sideguard. New indications have also emerged, including small vessels, addressed by the Cardiomind Sparrow and soft, unstable lesions, treated by the vProtect luminal shield. Of note, all the novel devices presented have a relative paucity of clinical data with no randomised clinical trials to date, but – if the contemporary results continue to be favourable – their indications may become more widespread in the future of coronary intervention.
Introduction
Conventional tubular balloon expandable (BE) stent designs are poorly suited to coronary bifurcations, yielding highly variable results1-5. Early BMS BE dedicated devices failed to improve outcome6-8. A return to conventional tubular BE stents with Cypher DES for bifurcations in the Nordic9 study showed that a simple one stent approach could yield good clinical outcomes – perhaps with the best results published to date for tubular BE technology (3% MACE) – but with the suboptimal result of a high rate of residual side-branch stenosis (19%). Moreover, while the Nordic group have shown that DES use with the culotte approach may improve these SB results dramatically10, concerns exist with regard to the long term safety of this complex multi-stent approach with conventional stent designs. For this reason, intense interest remains – and industry development continues– for dedicated bifurcation coronary stents to make treatment of such lesions more straightforward. Innovative designs have emerged, some balloon expandable11,12, but also self-expandable, based on the premise that nitinol based designs may conform more favourably than other metals to non-tubular vascular structures such as bifurcations13,14.
Through a discussion of one of the early implantations of the first of these new age self-expanding designs, the Devax coronary bifurcation system, we discuss the use of self-expanding designs in percutaneous intervention of coronary bifurcations, and revisit an old paradigm with contemporary applications.
The Axxess™ system
The Axxess™, or Devax, System15-17 (Devax, Irvine, CA, USA) is a dedicated 7 Fr compatible bifurcation stent system. It is a self-expanding stent, comprised of a flare-shaped – also described as conical – nitinol stent platform and the DES version, the Axxess Plus, has a bioabsorbable polymer coating which releases Biolimus A9, a sirolimus analogue (Figure 1).
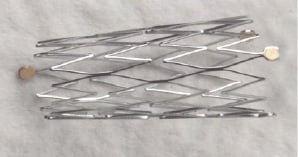
Figure 1. The Axxess Plus Stent. Conical design with 1 gold marker proximally, 3 distally.
It is designed to be deployed precisely at the carina of a bifurcation, and can be used alone if the lesions at the bifurcation are short, or with overlapping stents downstream in the presence of longer lesions in the tributary branches, which have generally been balloon expandable DES. The Devax system was the first of a new generation of self-expanding coronary stents after a more than 15 year lull in their development and was also the first dedicated DES for bifurcations. It was first used in man in 2003.
Devax: an early case
This case was performed in late 2003, and now has been followed clinically for almost five years. This was a 59 year old man with dyslipidaemia who presented with unstable angina. He had normal LV systolic function with an ejection fraction by ventriculography of 65%. There was single vessel coronary disease with a 75% stenosis, Medina 1,1,1 classification involving the LAD and a large first diagonal (Figure 2), but without lesions extending deeper into the LAD and diagonal, making it ideal for the Axxess stent alone.
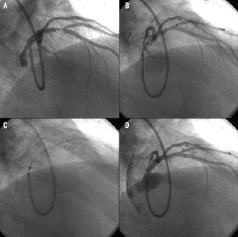
Figure 2. The Axxess Plus procedure. 2A. Baseline 1,1,1 lesion, with a large diagonal. Both vessels are wired and predilated. 2B. The delivery system is positioned with the distal markers at the level of the carina. 2C. Partial release brings the distal (flared) part of the stent proximal to the carina and advanced slightly within the ostia of both branch vessels. 2D. The system is advanced to allow positioning at the carina with full coverage of the ostia of both branches, and finally fully deployed.
This lesion was treated with a Devax 3.5 x 10 mm stent with excellent angiographic result at the bifurcation, and mild residual disease in the LAD, just proximal to, and distinct from, the stent. Optimal deployment of this stent is obtained using a technique that differs to that of balloon expandable tubular stents. The stent is positioned proximal to the bifurcation with the distal markers at the level of the carina (Figure 2b) then partially deployed by withdrawing a covering sheath (Figure 2c), and advanced slightly within the ostia of both branch vessels (Figure 2d) before complete release. The procedure of the case described is illustrated stepwise (Figure 2) for optimal deployment, which is essential to facilitate the benefits of its design.
The patient presented again four months later with exertional angina and coronary angiography demonstrated no disease in the Devax stent, but progression of the proximal disease in the LAD, which was treated with an overlapping short 3.5 x 8 mm cypher DES. Routine study follow-up angiography four months later demonstrated good patency in the Cypher™ and the Devax stents. Repeat angiography two years later revealed no restenosis and no progression of disease. A follow-up exercise stress test in November 2007 was negative and he remains asymptomatic almost five years post-procedure.
Overall results with the Devax system
The Axxess™ Plus trial15,17 was a prospective multicentre single-arm study that enrolled 139 patients. Each patient received an Axxess Plus stent at the level of the carina. Depending on the lesion anatomy, additional non-study stents were placed distally if necessary. Angiographically driven TLR was highly favourable at 7.5% at six months with a late loss of 0.09, comparable to sirolimus eluting stents in non-bifurcated lesions17. The Diverge study was a subsequent multicentre registry and was completed in December 200718, recruiting 302 patients and results, although as yet unpublished, were presented by Verheye et al at the Transcatheter Cardiovascular Therapeutics (TCT) meeting in 200818 and showed a 4.3% ischaemic driven TLR in the overall population at nine months; also presented was a substudy of 140 patients in the Diverge trial with angiographic follow-up showing an overall binary restenosis rate of 6.4%, with only 0.7% in the Axxess SE stent, 4.8% in a SB stent and 2.1% in a distal MB (Cypher™) stent. To date, over 500 patients have been treated with this device worldwide in the Axxess pilot, Axxess plus, Diverge and Axxent LMS trials collectively.
Results from the Axxent LMS trial, including IVUS data, have recently been published19. Although this was a study of only 28 patients, it provided important data through the modality of intravascular ultrasound. Interestingly, at six months there was a 12.4% increase in Devax stent volume, leading to a numerical increase in lumen area. Importantly, with regard to the daughter vessels, which were stented with cypher stents, the investigators did report only a 4.8% restenosis rate at the LAD ostium, but a 19% rate at the Cx ostium, which may reflect the difficulties of overlapping BE with SE technology. There were, however, no cases of stent thrombosis and a TLR rate of 9%.
Other self-expanding stents applied to bifurcations and beyond
Stentys™
Stentys™ (Stentys SAS, France) (Figure 3) is a nitinol self expanding tubular stent, available in drug (paclitaxel) eluting and bare metal form20,21.
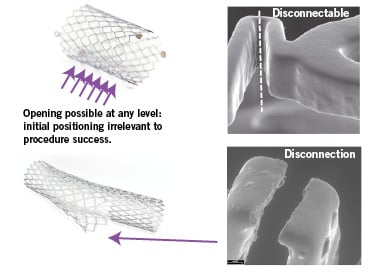
Figure 3. Stentys. Small detachable interconnections allow opening at any level, allowing optimal coverage of even multiple side-branches.
It is compatible with 7 Fr delivery systems, although it is soon to be 6 Fr, and has the novel ability of being able to disconnect its stent struts by angioplasty at any point along its length20. This is facilitated by a Z-shaped mesh that is linked by small detachable interconnections. At the ostium of a SB, gentle balloon mediated disconnection, coupled with ongoing self-expansion by the nitinol frame, is designed to maximise conformability and coverage. Stentys is currently under evaluation in the OPEN-I trial, a phase I multicentre non-randomised study, which has completed recruitment and has ongoing follow-up.
Despite only two stent sizes available at this time, it is currently indicated for proximal vessels of dimensions 3-4.5 mm and distal MB 2.5-4 mm and SB 2.5-3 mm, illustrating the versatility of the self-expanding nitinol design to conform to lumen dimensions. This stent essentially maintains a provisional T approach, but with the added advantage that if SB ostial postdilatation is performed, there is full coverage of the SB ostium. Optimal SB postdilatation, as in tubular BE stents, mandates distal crossing of the SB ostium.
Cappella Sideguard™
The Cappella Sideguard™ (Cappella Inc, Auburndale, MA, USA) (Figure 4) is a 6 Fr compatible trumpet-shaped nitinol self expanding stent22.
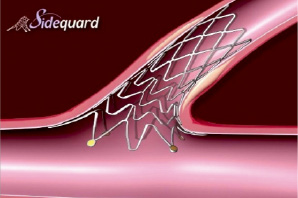
Figure 4. Cappella Sideguard. Nitinol side branch stent with trumpet shaped design allowing full ostial coverage.
It is a SB access device with a strut thickness of 65 µm; this compares to 140 for Cypher™, 132 for Taxus™ and 81 for Xience V™. Unusually, it has a balloon mounted delivery system, the first for a self-expandable device. The stent is deployed using a nominal pressure balloon, which helps tear a protective sheath that keeps the Sideguard in place until deployment23. Once released from this “balloon-release sheath technology”, the Sideguard self-expands into place.
The device has the benefit of ease-of-use for interventionists habituated to balloon expandable devices; it has the theoretical added benefit of nitinol mediated ongoing self-expansion to adhere more closely to the side-branch ostium. A conventional MB stent is then required. The procedure is completed with rewiring both stents and kissing balloon inflation. The first-in-man (FIM) study investigators recently reported early experience with Cypher™ DES as MB stenting 16 patients22. Technical success was 12/16 (75%). One month MACE was 1/16 (6.25%). Essentially this is a device that optimises the mini-crush approach to bifurcation stenting, with the incorporation of self-expanding technology.
The Sparrow™ stent
The Cardiomind Sparrow™ (Cardiomind Inc, Sunnyvale, CA, USA) (Figure 5) is a self-expanding nitinol stent designed to provide unprecedented access to distal and small vessels, regardless of tortuosity or calcification24-27.
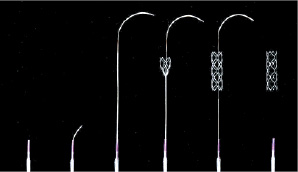

Figure 5. CardioMind Sparrow. Self-expanding stent mounted on an 0.014” wire and delivered through an over-the wire balloon. Two-step, electronically mediated, catch release delivery system.
The stent is loaded on a 0.014” guidewire platform and deployed through a uniquely specialised two stepwise delivery system which enables electrolysis of mechanical latches holding down each end of the stent; this technology enables the guidewire / stent platform to be delivered through an over-the-wire balloon. While the first version was bare metal, a DES version has been developed incorporating rapamycin eluted by a biodegradable PLA/PGLA block copolymer matrix. This technology is being evaluated in the CARE II study, an international multicentre randomised trial which is ongoing, and will compare the DES Sparrow to the BMS version and the MicroDriver BMS in a 4:4:3 respective allocation27.
vProtect™ Luminal Shield
The vProtect™ shield (Prescient Medical Inc., Doylestown, PA, USA) (Figure 6) is a self expanding nitinol stent28,29 which aims to treat soft, high-risk atherosclerotic lesions, even those that are non-obstructive, with the rationale that gentle self-expansion may cause less vascular injury with sufficient radial force of expansion in this setting.
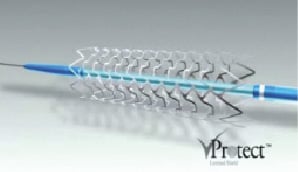
Figure 6. vProtect self expanding nitinol stent. Designed to complement a Raman spectroscopy based plaque characterisation device. Proposed for stenting of soft, high-risk, unstable lesions; whether obstructive or not.
It is designed for use with an accompanying novel plaque characterisation system, the vPredict™ Optical Catheter System30, which promises to provide information on plaque composition in vivo to unprecedented accuracy. The vProtect Shield™ system was first used in man in June 2008 and is currently being evaluated in a phase I study, the SECRITT I trial.
Nitinol and structural design of SE stents
All the novel devices described are manufactured from nitinol based materials. Nitinol has some unusual metallurgic properties which merit discussion. A nearly equiatomic composition of nickel and titanium, it is one of the few biocompatible alloys that displays the property of super-elasticity: unlike stainless steel, nitinol can be crushed fully flat and still recover its original shape31. Moreover, the narrow temperature range within which nitinol’s super-elasticity is exhibited includes body temperature. It is unique among metallic alloys in that it exists in two crystalline phases called martensite and austenite32. The austenite, or ‘parent’ crystal structure, is cubic in nature; the martensitic ‘daughter’ structure is a complex monoclinic structure; the transition between these phases is influenced by temperature and stress31. Within a limited range, alloy producers can control the temperature at which the change in nitinol’s molecular structure may occur, which can, in turn, have dramatic effects on the chronic outward force on self-expansion.
Perhaps a deficiency of self-expanding designs in general is that diametric expansion may result in geometric shortening and potentially, if there is uncertainty of the direction of foreshortening, this can lead to inaccuracies of deployment. However, this is a design rather than a material issue and can – and has – been overcome in the contemporary nitinol designs by incorporating compensatory bridges or diamonds in the strut patterns31. Thus, the clinical efficacy of nitinol SE stents could be dramatically altered by manipulation of these metallurgic and design properties.
From historical to contemporary applications
Bare metal and self-expanding coronary stents were born almost simultaneously in the mid-1980s. While Palmaz et al introduced the use of balloon mounted stents (as used in coronary arteries today) in peripheral arteries in 198533, Schatz et al subsequently modified the Palmaz® stent, which led to the development of the first commercially successful coronary stent, the Palmaz-Schatz™ stent34. However, Puel was the first to implant a coronary stent in humans in Toulouse, France in March 1986 using a self-expanding mesh device, the first generation Wallstent™35,36. The two technologies were used with almost equal frequency at that time. Early SE designs, in particular the first generation Wallstent™, had problems with high incidence of stent thrombosis (24%), mostly within the first two weeks37. But subsequent SE designs, particularly nitinol designs, displayed comparable results to BE stents in many studies38-42. Unfortunately, their additional benefit of positive vessel remodelling and continued stent expansion was offset by an excess of neointimal hyperplasia, leading to similar late loss to BE designs41,42. Even with old self-expanding designs, potential benefits were seen over BE stents in randomised studies, with a trend to a lower incidence of edge tears with SE stents38,42 and a lower incidence of other procedural complications such as slow flow and side-branch occlusion42. The advent of drug eluting balloon expandable stents in the late 1990s spelt the decline of self-expanding stents in the field of coronary intervention, as large companies withdrew investment and marketing in favour of their balloon expandable siblings which they also manufactured, and which were generally felt to be easier to use and position accurately.
There is an increasing return to the paradigm of self-expansion for coronary stents. Incorporation of narrower strut technologies, drug elution, the use of nitinol and improved delivery systems may improve results. It will be of paramount interest to contemporise direct angiographic and clinical comparisons of the new age of SE designs with the latest generation BE stents in randomised studies. Newer designs are becoming increasingly user friendly, but will still require acknowledgement by interventionists that they are a different technology requiring different handling in the cathlab and new skills, even for the most skilled interventionists, to optimise deployment. For instance, an important observation for SE stents is that more accurate and more aggressive predilatation is generally required to obtain a larger lumen and enable optimal self-expansion during deployment of this technology, as the radial forces at the time of stent deployment are considerably less than with BE stents. This may support the use of predilatation balloons with a balloon:artery ratio close to 1.
A number of studies have shown promise and are ongoing for highly innovative SE devices in niche coronary settings, such as Devax, Stentys and Capella in coronary bifurcations, Cardiomind in small vessels and the vProtect Shield in soft unstable lesions. However, up to now, all the devices presented have a relative paucity of clinical data and there is a lack of randomised trials demonstrating superiority of these new devices over standard BE stents. Thus, despite being potentially promising, SE coronary stent technology still needs to prove its efficacy. If the results continue to be favourable, the indications may become more widespread for coronary intervention.

