Abstract
Abstract: Cardiovascular disease is the leading cause of death for women worldwide, with mortality rates due to cardiogenic shock (CS) remaining exceedingly high. Sex-based disparities in the timely delivery of optimal CS treatment contribute to poor outcomes; addressing these disparities is a major priority to improve women’s cardiovascular health. This consensus statement provides a comprehensive summary of the current state of treatment of CS in women across the spectrum of cardiovascular disease states and identifies important gaps in evidence. As sex-based data are limited in contemporary literature, clinicians may use this document as a resource to guide practice. Further investigations are necessary to inform best practices for the diagnosis and treatment of women with CS.
Cardiovascular disease is the leading cause of death for women worldwide, claiming 8.94 million lives annually, representing a global age-standardized mortality rate of 204 deaths per 100,000 women in 20191. While cardiovascular disease–related mortality rates have decreased over the past 2 decades, there has been no meaningful improvement in the dismal 30% to 50% in-hospital mortality rate of patients who experience cardiogenic shock (CS)2. The burden of CS is recognized as one of the most relevant, and improving CS outcomes has been identified as a priority to reduce women’s mortality associated with cardiovascular disease by 20303. Current evidence points to significant sex-based disparities in the timely delivery of optimal treatment for CS in women, which contributes to persistent poor outcomes4. Not only do women encounter delays in treatment, but they are less likely to receive guideline-recommended coronary interventions or device therapies compared with men, independent of disease severity56. Furthermore, there are limited data to guide management of CS in women despite biologic and pathophysiologic differences in disease presentation. Clinical research and randomized trials in CS pose significant ethical challenges, and women are consistently underrepresented, limiting our ability to evaluate the risks and benefits of cardiovascular drugs or devices in women. Accordingly, current society practice guidelines do not have sex-specific recommendations and do not highlight instances where evidence is insufficient for the diagnosis or management recommendations to optimize outcomes in women with CS. Therefore, this consensus statement aims to provide a comprehensive summary of available evidence on CS in women, identify knowledge gaps, and suggest directions for future clinical investigation.
Methodology
This statement has been developed according to Society for Cardiovascular Angiography & Interventions (SCAI) Publications Committee policies7 for writing group composition, disclosure and management of relationships with industry, internal and external review, and organizational approval. The writing group has been organized to ensure diversity of perspectives (including representation from heart failure [HF], interventional cardiology, cardio-obstetrics, and critical care cardiology) and demographic characteristics, and appropriate balance of relationships with industry. Relevant author disclosures are included in Supplementary Table 1. The work of the writing group was supported exclusively by SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment from SCAI. This was done in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the Association for Acute Cardiovascular Care (ACVC), which appointed authors within their associations according to their expertise. Literature searches were performed by group members designated to lead each section and were balanced to reflect differences or similarities in findings and noting risk-adjusted outcomes when available to address potential confounding between sexes. Initial section drafts were authored by the section leads in collaboration with other members of the writing group. Consensus tips were discussed and voted on by the full writing group using a modified Delphi method. Electronic surveys were sent to members of the writing group and responses discussed in teleconference format. Consensus was defined as 75% agreement with at least an 80% response rate. All advisements are supported by a short summary of the evidence or specific rationale. The draft manuscript was independently peer reviewed both by SCAI and EuroIntervention in March and April 2024 and revised to address comments. The writing group unanimously approved the final version of the document. ACVC approved the document in May 2024. EAPCI approved the document in October 2024. SCAI Publications Committee and Executive Committee endorsed the document as official Society guidance in October 2024. SCAI statements are primarily intended to help clinicians make decisions about treatment alternatives. Clinicians also must consider the clinical presentation, setting, and preferences of individual patients to make judgments about the optimal approach.
Sex-based differences in CS: etiology and presentation
The incidence and etiology of CS differs in women and men (Figure 1). Acute myocardial infarction (AMI) is a major cause of CS, accounting for 20% to 30% of CS in both women and men28. The majority of CS complicating AMI (AMI-CS) is due to atherosclerotic disease; however, spontaneous coronary artery dissection (SCAD) is an important contributor to CS (SCAD-CS) in women, occurring in up to 10% of SCAD cases9. Nonischemic HF–related CS (HF-CS) is more common than AMI-CS, accounting for 50% to 55% of CS in both women and men28. Within HF-CS, women are more likely to have de novo HF-CS (incidence, women 26% vs men 19%)8, Takotsubo syndrome (TTS) (1% vs 0.2%)10, and myocarditis (13% vs 3%) compared with men11. Peripartum and postpartum cardiomyopathy–related (PPCM)-CS uniquely affects women, and valvular heart disease (VHD)-related CS (VHD-CS), specifically aortic stenosis (AS), is more common in men but remains an important consideration for women12. Hormonal differences between the sexes may account for some of the observed differences in CS etiologies and outcomes. Estrogen has anti-inflammatory effects that protect against cardiac cell death, oxidative damage from ischemic/reperfusion injury, endothelial dysfunction, and adverse cardiac remodeling13; however, these hormonal differences may have paradoxical harmful effects by decreasing ischemic preconditioning in women compared with that in men13. Furthermore, varying estrogen levels throughout reproductive development and life transitions (ie, pregnancy and menopause) may contribute to disease states, such as PPCM and SCAD, which can progress to CS13.
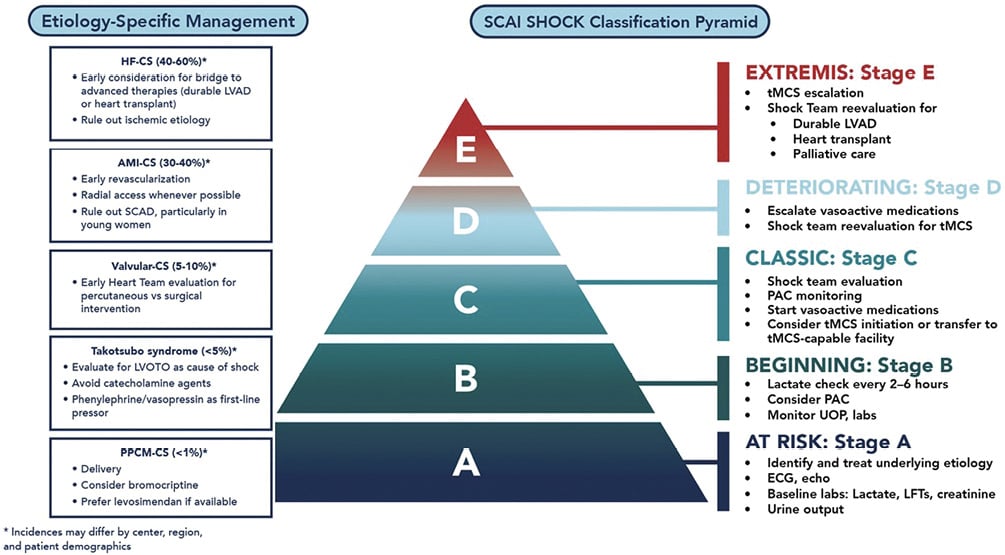
Figure 1. Etiology-specific management of cardiogenic shock in women. AMI-CS: acute myocardial infarction–related cardiogenic shock; HF-CS: heart failure–related cardiogenic shock; LFT: liver function test; LVAD: left ventricular assist device; PAC: pulmonary artery catheter; PPCM-CS: peripartum cardiomyopathy–related cardiogenic shock; STEMI: ST-elevation myocardial infarction; tMCS: temporary mechanical circulatory support; valvular-CS: valvular-heart disease–related cardiogenic shock.
Beyond the different underlying CS etiologies, the clinical presentation of CS differs based on sex. Women with AMI-CS tend to present with higher left ventricular ejection fraction (LVEF) and similar or lower rates of renal/liver insufficiency compared with men14. Despite this, hemodynamic studies have shown that women have worse cardiac contractility (lower cardiac index or cardiac power output) and a higher risk of death with AMI-CS as predicted by Society of Thoracic Surgeons mortality scores14. As a consequence, women with AMI-CS can be mischaracterized as being clinically stable despite ongoing systemic hypoperfusion, leading to delays in the initiation of appropriate advanced care. Sex-based differences in HF-CS also exist, and women are more likely to present with cardiac arrest, higher vasopressor requirements, and advanced SCAI SHOCK stages D and E8.
Contemporary shock management
The cornerstones of CS treatment include (1) early identification of CS with timely initiation of hemodynamic support to maintain systemic perfusion and end-organ function and (2) early diagnosis and targeted treatment of the underlying cause of CS. SCAI SHOCK classification, initially released in 2019 and updated in 2021, provides a 3-axis model that integrates shock severity, clinical phenotype, and risk modifiers across both men and women15. Building on SCAI SHOCK classification, we provide a consensus on best evidence-based practice pathways of care to optimize early diagnosis, monitoring, and treatment recommendations for women with CS (Figure 1).
Diagnosis of CS in women
Early assessment of end-organ damage and perfusion status is essential for establishing early the diagnosis and prognosis of CS as a continuum, as there is growing evidence that preshock and at-risk patients can be further risk stratified to inform management and outcomes16. Lactate is an objective biomarker that correlates with mortality in all types of shock and helps appropriately risk stratify patients; it is available as point-of-care testing with immediately available results. Despite universal society and expert guideline recommendations for frequent measurement of lactate levels for patients in or at risk of CS17181920, only 1 in 4 women and 1 in 2 men in the global RECOVER III study of AMI-CS had lactate levels measured before percutaneous coronary intervention (PCI), likely resulting in diagnostic delays of AMI-CS for both sexes and particularly for women21. Invasive hemodynamic monitoring provides important diagnostic and clinical information in the setting of CS to guide phenotyping (univentricular or biventricular shock), characterize severity, and guide pharmacologic and temporary mechanical circulatory support (tMCS) escalation (Table 1)20. Characterizing CS phenotypes predicts prognosis and may improve short-term outcomes by initiating earlier management guided by real-time data and serial assessments, thus accelerating end-organ perfusion and reducing progression to CS22. While randomized trials of pulmonary artery catheters (PAC) in acute HF and critical illness have failed to show a reduction in mortality, these trials evaluated routine, unselected use of PAC and excluded patients in whom clinicians thought a PAC was required for treatment23. Retrospective studies have shown that early targeted PAC use in CS prior to initiating tMCS is associated with lower mortality across all SCAI SHOCK stages24. Women with CS remain less likely to receive PAC monitoring8 despite observational evidence of survival benefit with a standardized PAC-guided CS pharmacologic and tMCS treatment protocols25. Thus, PAC monitoring is advised early for women with persistent symptoms or worsening end-organ function despite initial treatment17.
Table 1. Invasive cardiac hemodynamics and indicators of cardiogenic shock.
| Left ventricular metrics | Calculation | Indicator of cardiogenic shock |
|---|---|---|
| Cardiac index (CI) | CO/body surface area | ≤2.2 L/min/m2 |
| Cardiac power output (CPO) | (MAP × CO)/451 | <0.6 W |
| Cardiac power index | (MAP × CI)/451 | <0.4 W/m2 |
| Pulse pressure | Systolic blood pressure − diastolic blood pressure | <25 mm Hg |
| Systemic vascular resistance | ([MAP − CVP]/CO) × 80 | Variable |
| Right ventricular metrics | Calculation | Indicator of RV dysfunction |
| RAPs | >10/15 mm Hg | |
| RAP/PCWP ratio | >0.86 (in AMI) >0.63 (after LVAD) |
|
| Pulmonary artery pulsatility index | (PASP − PDP)/RAP | ≤0.9 (in AMI) <1.85 (after LVAD) |
| Right ventricular stroke work index | 0.0136 × SVI × (mPAP − RAP) | <6 g/m/beat/m2 |
| AMI: acute myocardial infarction; CO: cardiac output; CVP: central venous pressure; LVAD: left ventricular assist device; MAP: mean arterial pressure; mPAP: mean pulmonary artery pressure; PADP: pulmonary artery diastolic pressure; PASP: pulmonary artery systolic pressure; PCWP: pulmonary capillary wedge pressure; RAP: right atrial pressures; SVI: stroke volume index. Adapted from Geller, et al20. |
||
Consensus tips for contemporary shock management
• Early and frequent assessments of end-organ function including lactate measurements (ie, serial testing every 2-6 hours) are useful to improve early CS diagnosis and risk stratification and to guide the need for early invasive monitoring and advanced therapies.
• Early PAC use in women to assist early CS diagnosis and management may improve survival.
• PAC should be strongly considered in all patients on tMCS.
Management of CS in women
Inotropes and vasopressors are first-line treatment in CS due to their rapid onset of action and ease of use. Sex-based data are sparse, and the optimal pharmacologic agent for hemodynamic support for CS in women is unknown. Society recommendations suggest using norepinephrine or dobutamine as first-line vasoactive support in hypotensive patients1819. Inodilators (milrinone, dobutamine, and levosimendan) may be appropriate in patients with low cardiac output who are normotensive. A study comparing dobutamine with milrinone in CS showed no difference in outcomes overall or based on sex between the 2 medications26, and the calcium sensitizer levosimendan has not been shown to reduce mortality in the context of preshock compared with dobutamine27. In women with CS, aggressive escalation of vasopressors and inotropes at the expense of delays in tMCS should be avoided, as retrospective evidence suggests higher mortality in women compared with that in men. Although speculative, it is possible that women may have greater susceptibility to the toxic effects of vasopressors, including increased myocardial oxygen consumption, arrhythmias, and reduced end-organ microcirculatory perfusion202128.
Beyond pharmacologic support for CS, several tMCS options are available, including the intra-aortic balloon pump (IABP), the Impella family of pumps (Abiomed), and venoarterial extracorporeal membrane oxygenation (VA-ECMO). The TandemHeart device (LivaNova) is no longer marketed. While these devices are advised early to avoid the toxic effects associated with inotropes/vasopressors escalation in women, device-specific complications including vascular complications, limb ischemia, hemolysis, and stroke should be weighed against potential benefits29. Support strategies and their differential hemodynamic and physiologic effects are summarized in Table 2. Protocols for device selection, utilization, and deescalation and the advantages/disadvantages of each device have been previously detailed20.
Although the use of tMCS in CS has increased over the past 2 decades, prospective randomized controlled trial (RCT) evidence clearly establishing the clinical benefit of any tMCS device in CS is limited, and our ability to generalize results to women is further limited by underrepresentation of women in shock trials1718313233. Most contemporary randomized and observational tMCS trials are focused on AMI-CS, and data specific to non-AMI causes of CS, including HF, VHD, peripartum cardiomyopathy, myocarditis, and TTS, are limited34. Available sex-specific evidence for tMCS strategies are detailed in the disease-specific sections further (specifically AMI-CS and HF-CS).
Table 2. Summary of temporary mechanical circulatory support strategies.
| RV support | LV support | Biventricular support | ||||
|---|---|---|---|---|---|---|
| Impella RP (Abiomed) | TandemHeart RVAD (±Protek Duo) (LivaNova) | IABP | Impella (Abiomed) | TandemHeart LVADa (LivaNova) | VA-ECMOb | |
| Mechanism | Axial-flow continuous pump (RA to PA) | Centrifugal-flow continuous pump (RA to PA) | Balloon inflation-deflation (aortic counterpulsation) | Axial-flow continuous pump (LV to AO) | Centrifugal-flow continuous pump (LA to FA through transseptal cannula) | Centrifugal-flow continuous pump (RA to AO) |
| Support | RV | RV | LV | LV | LV Oxygenator may be added to the circuit |
RV and LV Includes oxygenator |
| Insertion/placement | Femoral vein | IJ vein | Femoral artery Axillary artery |
Femoral artery or axillary artery (2.5, CP) Axillary artery (5.5) |
Femoral vein to LA Femoral artery |
Femoral vein Femoral artery |
| Cannula size | 22F venous | 29F/31F venous | 7F-8F arterial | 2.5-12F arterial CP-14F arterial 5.5-21F arterial |
21F venous 12F-19F arterial |
17F-28F venous 14F-22F arterial |
| Flow, L/min | 2-4 | Maximum 4.5 | 0-1 | 2.5-5.5 | Maximum 5-8 | 2-7 |
| Maximum pump speed, rpm | 33,000 | 7,500 | NA | 2.5c/CP 51,000/46,000 5.0c/5.5 33,000/33,000 |
7,500 | 5,000 |
| LV unloading | — | — | ↑ | ↑-↑↑↑ | ↑↑ | ↓↓ |
| RV unloading | ↑ | ↑ | — | — | — | ↑↑ |
| Cardiac power | ↑ | ↑ | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Coronary perfusion | — | — | ↑ | ↑ | — | — |
| CVP | ↓ | ↓ | ↔ or ↓ | ↔ or ↓ | ↔ or ↓ | ↓ |
| MAP | — | — | ↑ | ↑↑ | ↑↑ | ↑↑ |
| LVEDP | ↑ | ↑ | ↓ | ↓↓ | ↓↓ | ↔ |
| PCWP | ↑ | ↑ | ↓ | ↓↓ | ↓↓ | ↔ or ↑ |
| Myocardial oxygen demand | ↓ | ↓ | ↓ | ↓↓ | ↔↓ | ↔ or ↑ |
| Surgical tMCS considerations | Pump options include Centrimag (Abbott), Cardiohelp (Getinge), and Rotaflow (Getinge). These can be used with or without an oxygenator in multiple configurations, including the following: (1) a temporary RVAD can have a drainage cannula in the femoral vein or RA with a return cannula from the IJ into the PA; (2) a temporary central RVAD can have a drainage cannula in the RA or RV with a return cannula into the PA; (3) a temporary central LVAD can have a drainage cannula in the LA or LV with a return cannula into the aorta; or (4) multiple central and percutaneous BiVAD configurations are possible. | |||||
| aTandemHeart LVAD is no longer commercially available. bOther percutaneous cannulation sites and multiple cannulation sites can be used: arterial access (axillary, subclavian, or carotid) or venous access (IJ). Central configurations are also possible. cImpella 2.5 and 5.0 are no longer commercially available. Adapted from Tehrani, et al30. AO: aorta; BiVAD: biventricular assist device; CS: cardiogenic shock; CVP: central venous pressure; FA: femoral artery; IABP: intra-aortic balloon pump; IJ: internal jugular; LA: left atrium; LV: left ventricle; LVAD: left ventricular assist device; LVEDP: left ventricular end-diastolic pressure; MAP: mean arterial pressure; NA: not applicable; PA: pulmonary artery; PCWP: pulmonary capillary wedge pressure; RA: right atrium; RV: right ventricle; RVAD: right ventricular assist device; tMCS: temporary mechanical circulatory support; VA-ECMO: venoarterial extracorporeal membrane oxygenation. |
||||||
Consensus tips for the management of CS in women
• tMCS is advised early for women in CS on inotropes/vasopressors, with persistent low cardiac output, rising lactate levels, or other signs of end-organ hypoperfusion, based on disease-specific and device-specific risk-benefit assessment.
Evidence gaps in the management of CS in women
• Randomized evidence is needed to inform the benefit of tMCS, the optimal tMCS device selection, and timing for women with CS based on CS etiology to determine device-specific complications and outcomes.
Specific etiologies and management of CS in women
AMI-related CS
Atherosclerotic AMI-CS
AMI is a common cause of CS in women. Approximately 12% of patients with ST-elevation myocardial infarction (STEMI) and 4.5% of patients with non-ST-elevation myocardial infarction (NSTEMI) develop CS according to a National Cardiovascular Data Registry report; overall, women comprised 45% of patients presenting with AMI-CS35. Women with AMI-CS are older with a higher prevalence of hypertension, diabetes, previous HF, atrial fibrillation, cerebrovascular disease, and renal disease36373839404142. Women have greater hemodynamic compromise at the time of AMI-CS presentation, characterized by more profound hypotension, lower cardiac output, and more acute complications such as acute severe mitral regurgitation and ventricular septal defects compared with men4042. Despite this, sex-specific substudies of the IABP-SHOCK II, SHOCK, and CULPRIT-SHOCK trials have shown consistent results based on sex, namely women with AMI-CS derive the same survival benefit as men with culprit-only revascularization without benefit from IABP support404142. Thus, early culprit-only revascularization with PCI is the mainstay of therapy in AMI-CS and improves mortality in selected patients of both sexes37383943. Despite that fact, women are less likely to receive aggressive AMI-CS treatment and undergo less primary PCI compared with men44. A study of 9,750 patients with AMI-CS (including 44% women) from the Ontario Myocardial Infarction Database showed that compared with men, women with AMI-CS were more likely to be admitted to hospitals without revascularization capabilities (16% vs 19.2%; P < .001) and less likely to be transferred to PCI-capable centers (11.3% vs 14.2%; P < .001)36. Even when admitted to PCI-capable centers, women experience delays in AMI-CS care. A National Inpatient Sample study of AMI-CS admissions showed that young women (age, 18-55 years) compared with age-matched men were less likely to receive early coronary angiography (49.2% vs 54.1%), PCI (59.2% vs 64.0%), and tMCS (50.3% vs 59.2%) and experienced higher in-hospital mortality (23.0% vs 21.7%; adjusted odds ratio [aOR], 1.11; 95% CI, 1.07-1.16; P < .001)44. Furthermore, women are more likely to present with NSTEMI-related CS41 and thus are disproportionately affected by the longer delays to PCI or coronary artery bypass grafting (CABG) experienced by patients with NSTEMI, regardless of sex, as compared with patients with STEMI3544. The newly developed SEX-SHOCK score to predict CS in AMI, using machine learning and incorporating ST-segment elevation, creatinine, C-reactive protein, and LVEF, outperformed other risk scores for both sexes in external validation (AUC females: 0.81 [0.78-0.83]; males: 0.83 [0.82-0.85]; P < .001) across the spectrum of ACS. The importance of a gender-specific risk prediction approach for CS, could mitigate sex inequities in early risk stratification of contemporary shock management45.
Sex-specific evidence for tMCS use in AMI-CS is summarized in Supplementary Table 2. IABP use for AMI-CS has declined over the past decade (29.8% in 2005 to 17.7% in 2014)46 after the randomized IABP-SHOCK II trial failed to show a benefit of IABP in reducing 30-day mortality overall31 or for women314047. At the same time, the use of Impella for AMI-CS has increased46. Nevertheless use of tMCS remains lower in women than in men with CS44 and in-hospital mortality is higher in those women who do receive tMCS, which is likely related, in part, to a higher burden of comorbidities and older age at presentation and lower rates of pulmonary artery catheter use48. While small-scale trials comparing Impella 2.5 or CP with IABP failed to show a reduction in mortality4950; subsequent registries have suggested a mortality benefit from earlier Impella use (either before or early in PCI)2151. Women, in particular, appear to have a greater survival benefit with early Impella support pre-PCI in AMI-CS as suggested by the international cVAD registry (survival in women: early 68.8% vs late 24.4%; P = .005) compared with men (early 43.2% vs late 40.3; P = .1)52. A subsequent sex-specific analysis of the global RECOVER III registry showed that women with AMI-CS on ≥2 inotropes before tMCS had significantly higher adjusted mortality (odds ratio [OR], 3.03; 95% CI, 1.26-7.29) compared with men (OR, 1.18; 95% CI, 0.89-1.56)21.
The recent landmark randomized DanGer Shock trial comparing the Impella CP with standard of care alone, enrolled 360 patients with STEMI-CS, excluding comatose patients or those with overt right ventricular HF. Impella reduced all-cause mortality at 180 days compared with standard of care (45.8% vs 58.5%; hazard ratio [HR], 0.74; 95% CI, 0.55-0.99; P = .04)33. Notably, 55.3% of patients underwent Impella implant before percutaneous revascularization, and median time from randomization to tMCS placement was 14 minutes. The subgroup of women in DanGer Shock did not show a benefit with Impella use (HR, 1.01; 95% CI, 0.58-1.79); however, randomization was not stratified by sex, the trial enrolled only 20% women and was underpowered to assess sex differences, and no formal interaction test was performed. The overall mortality benefit of Impella in DanGer Shock was offset by a 2-fold increase in bleeding and a 5-fold increase in vascular complications. The numbers needed to treat for survival was 8 and number needed to harm was 6 for a composite safety outcome (severe bleeding, limb ischemia, hemolysis, device failure, and worsening of aortic regurgitation). Complications were not reported by sex, and the overall risk benefit of Impella in women with AMI-CS remains difficult to assess. The need for additional randomized evidence on the use of Impella in women with AMI-CS is an imperative. Until then, based on the totality of evidence, Impella should be considered selectively but early in women with AMI-CS while weighing the risk of potential complications53.
VA-ECMO is used infrequently in AMI-CS compared with Impella and IABP46. Recent RCTs have failed to show a mortality benefit with early VA-ECMO use in AMI-CS and is associated with significantly higher bleeding and vascular complications. The extracorporeal life support (ECLS)-SHOCK trial54 randomized 420 patients (19% women) to early ECLS vs standard of care. There was no difference in 30-day all-cause mortality overall (ECLS 47.8% vs controls 49%) or among women (ECLS 59.5% vs control 56.4%). Moderate and severe bleeding (ECLS 23.4% vs control 9.6%; relative risk, 2.44; 95% CI, 1.50-3.95) and peripheral vascular complications requiring surgery (ECLS 11% vs control 3.8%; relative risk, 2.86; 95% CI, 1.31-6.25) were significantly higher with ECLS. A patient-level meta-analysis of 4 VA-ECMO RCTs including 567 patients (19% women) with AMI-CS failed to show a mortality benefit at 30 days with early VA-ECMO (46% vs control 48%), including in women (OR, 1.09; Pinteraction = .65)55. Major bleeding (VA-ECMO 25% vs control 12%; OR, 2.44; 95% CI, 1.55-3.84) and vascular complications (OR, 3.53; 95% CI, 1.70-7.34) were 2-4 fold higher with VA-ECMO.
A meta-analysis of registries in mixed CS populations suggests possible improved mortality with left ventricular (LV) unloading primarily with IABP in VA-ECMO (54% all-cause mortality with LV unloading vs 65% without); women were less likely to receive LV unloading (women 25.5% unloading vs 31.9% no-unloading). Whether women benefit more from unloading remains speculative56. The ongoing randomized study evaluating VA-ECMO with Impella unloading vs VA-ECMO alone in a mixed CS population will provide further insight on the potential clinical impact of LV unloading (UNLOAD-ECMO; NCT05577195). Until then, the lack of a mortality benefit and an increased risk of vascular complications does not support use of VA-ECMO in women with AMI-CS.
A recent patient-level meta-analysis of 9 RCTs including 1,114 patients (20.1% female) of mixed tMCS vs controls in AMI-CS, including 4 VA-ECMO randomized trials (611 patients), demonstrated that in aggregate, independent of sex, early routine use of tMCS did not reduce mortality at 6 months (HR, 0.87; 95% CI, 0.74-1.03; P = .10) and increased vascular complications compared with controls5357. In contrast, early tMCS use significantly improved survival in patients with AMI-CS but without hypoxic brain injury (HR, 0.77; 95% CI, 0.61-0.97; P = .024) independent of sex, age, and tMCS type53.
Based on the 2025 American College of Cardiology or American Heart Association guidelines, selective use of Impella for severe refractory CS is reasonable in patients with AMI-CS and without coma, and the routine use of IABP or VA-ECMO provides no benefit and is not recommended58.
Spontaneous coronary artery dissection-related CS
SCAD is an important underlying cause of nonatherosclerotic myocardial infarction (MI) in women and accounts for 20% to 25% of AMI in women younger than 50 years. In contrast with atherosclerotic AMI, the majority of SCADs will heal within 30 days. A conservative management is the preferred approach59 as revascularization for SCAD is associated with >50% acute procedure failure, high complication rates (iatrogenic dissection and abrupt vessel occlusion), and high reintervention rates (30% vs 19% with conservative management)59. Selective revascularization is reserved for patients with SCAD and ongoing ischemia, high-risk lesions (eg, left main involvement), or multivessel disease and, as a consequence, is more likely to be associated with shock60. In an analysis of 664,292 patients from the US National Readmission Database from 2015 to 2018, SCAD AMI was associated with higher rates of CS compared with non-SCAD AMI (9% vs 5%; P < .01), even after adjusting for younger age at presentation and lower baseline comorbidities (aOR, 1.5; 95% CI, 1.2-1.7). Patients with SCAD-CSCS were more likely to receive tMCS support with IABP (45% vs 28%, P < .001), percutaneous left ventricular assist device (LVAD) (17% vs 10%, P < .01), or ECMO (2.7% vs 1.2%, P = .03) compared with patients without SCAD and had lower in-hospital mortality (31% vs 39%, P < .01)9. This suggests that tMCS use is feasible in the setting of SCAD-CS and may allow for myocardial rest during coronary healing. There are no sex-specific data regarding outcomes or treatment strategies in patients with SCAD-CS.
Consensus tips for the treatment of AMI-CS in women
• Early revascularization with PCI and/or CABG is the mainstay of therapy in AMI-CS.
• In patients presenting with SCAD-CS, tMCS support to recovery and selective revascularization strategies in high-risk lesions may be appropriate.
• Selective early Impella use (either before or early in PCI) in women with AMI-CS without coma is reasonable; however, additional randomized evidence in women is needed.
• Current evidence does not support routine use of VA-ECMO or IABP in AMI-CS due to lack of mortality benefit and increased risk of vascular complications.
Evidence gaps in the treatment of AMI-CS in women
• Addressing local barriers and delays to care access in women with AMI-CS are institutional imperatives.
• RCT evidence in women to evaluate the risk benefit of Impella use in AMI-CS is an imperative.
• Evidence is needed to determine the optimal timing of tMCS in women with AMI-CS.
• Studies are needed to determine whether a complete revascularization approach and its timing improve outcomes in women with AMI-CS.
CS in the pregnant/postpartum patient
CS is rare in pregnancy and occurs in 3.8 of 100,000 antepartum and postpartum hospitalizations; however, CS in this context is associated with high maternal mortality (18.8% in peripartum CS vs 0.02% peripartum without CS) and higher rates of intrauterine fetal death (1.4% in peripartum CS vs 0.1% peripartum without CS)61. Peripartum cardiomyopathy is the most common cause of shock related to pregnancy, accounting for 56% of cases during pregnancy and 82% of cases postpartum. Other etiologies include acute coronary syndrome (either from plaque rupture or SCAD), pre-existing dilated cardiomyopathy, pulmonary arterial hypertension, severe VHD, and amniotic fluid embolism6263.
Similar to the nonpregnant patient with CS, invasive hemodynamics are critical to early identification of shock in the setting of pregnancy, and when identified, hemodynamic support is a priority (Figure 2). Levosimendan, where available, is considered the preferred inotropic agent, as it does not increase myocardial oxygen demand. Otherwise, dobutamine and norepinephrine may be used as first-line inotropic/vasopressor support agents64. Consideration for tMCS is advised early after starting intravenous therapy because medical therapy may be insufficient. Registry data suggest that early use of tMCS in pregnancy-related CS (defined as ≤6 days from onset) is associated with greater survival (18% mortality with support ≤6 days vs 38% with >6 days)61. Successful tMCS support during pregnancy has been described using IABP, temporary percutaneous or surgical LVADs (Impella, Tandemheart, and Centrimag [Abbott]), and VA-ECMO, but there is little evidence regarding a preferred device64. Need for tMCS support during birth further complicates device selection, with anticoagulation considerations (discussed further) and obstetric recommendations for assisted vaginal delivery (necessitating flexion at the hips) or cesarian section, both contributing toward device and access site selection.
Targeted therapies for the specific condition underlying the CS are advised. SCAD is the most common cause of MI in pregnancy, and patients with pregnancy-related SCAD have more severe disease compared with those with nonpregnant SCAD as evidenced by more frequent presentation with STEMI (57% vs 36%; P = .009), multivessel or left main disease (24% vs 5%, P < .001), and severe LV dysfunction, with LVEF of ≤35% (26% vs 10%, P = .007)65. For severe symptomatic VHD, especially stenotic left-sided lesions, cardiac surgery is an option, although it is associated with high fetal mortality rates up to 30%66. Catheter-based approaches may be appropriate (eg, mitral balloon valvuloplasty, aortic balloon valvuloplasty, and transcatheter aortic valve replacement [TAVR]); however, data in this population are limited to case reports and case series67.
Care of the pregnant patient with CS during cardiac procedures poses unique challenges68. In the supine position, the gravid uterus may cause aortocaval compression, which can further reduce preload and cardiac output. Placing the patient with a slight left lateral tilt can help relieve this and is especially important if tMCS is used. Meticulous attention to anticoagulation is imperative, as pregnancy is a hypercoagulable state with increased risk of thromboembolism compared with the nonpregnant state69. Both unfractionated heparin (UFH) and low-molecular weight heparin (LMWH) can be used during pregnancy; however, presence of anticoagulation at the time of delivery affects candidacy for epidural or spinal anesthesia, and close coordination with obstetrical anesthesia is required. Additionally, UFH use may be associated with higher rates of postpartum hemorrhage compared with LMWH69. Thus monitoring to maintain therapeutic anticoagulation is critical-UFH doses should be adjusted to within a therapeutic activated partial thromboplastin time range (1.5-2.5 times control), and LMWH doses should be adjusted to maintain anti-Xa levels of 0.6 to 1.0 units/mL70. Measures should be taken to reduce fetal radiation exposure include using external abdominal shielding, reducing fluoroscopy time, lower magnification and frame rates, and careful collimation68. Iodinated contrast is also associated with potential risk of fetal congenital hypothyroidism but does not preclude its use when lifesaving68. All measures to reduce fetal exposure are warranted, but these should not take precedence over procedures to preserve maternal life. Special considerations for the management of cardiac arrest in the pregnant or postpartum patient are in Supplementary Table 3.
Most importantly, a multidisciplinary team collaboration among cardiology, obstetrics, anesthesiology, and critical care are paramount to maternal and fetal/neonatal safety71. A pregnancy-heart team is advised for the evaluation and management of high-risk cardiac disease in pregnancy and is required for rapid decision making in pregnant patients with CS72, especially in conditions with high maternal mortality where pregnancy termination may be appropriate73. Other considerations such as choice of medications and anesthesia should be made based on the individual clinical situation, maternal benefit, and fetal exposure. Managed anesthesia care improves maternal airway and hemodynamic control while limiting maternal and fetal anesthetic exposure. Continuous fetal monitoring is advised if the gestational age is at ex utero viability (typically ≥23 weeks of gestation) and emergent cesarean delivery is an option; thus, the decision to implement fetal monitoring should be made in collaboration with obstetrics74. Timing and mode of delivery depends on maternal stability and fetal status and requires multidisciplinary coordination between cardiac and obstetric teams.
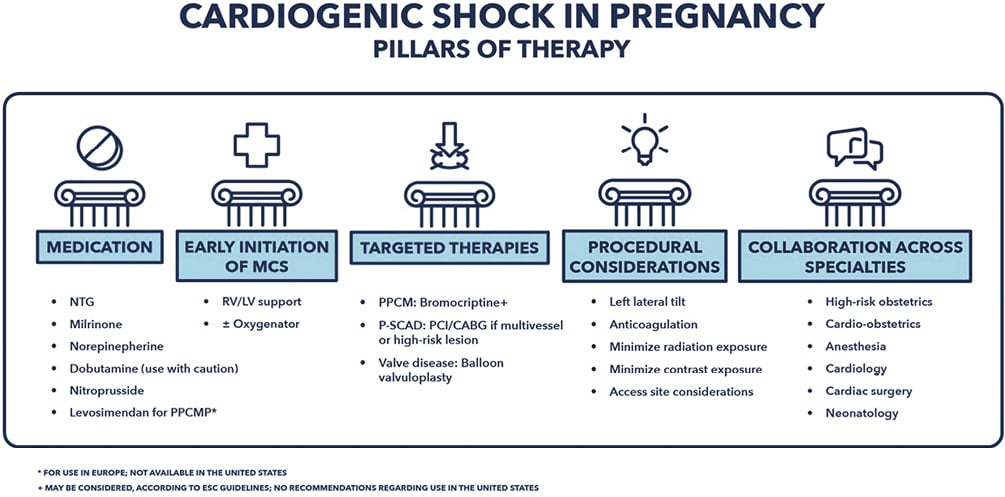
Figure 2. Cardiogenic shock in pregnancy. CABG: coronary artery bypass grafting; LV; left ventricle; MCS; mechanical circulatory support; NTG: nitroglycerin; PCI: percutaneous coronary intervention; PPCMP: peripartum cardiomyopathy; RV; right ventricle; P-SCAD; pregnancy-related spontaneous coronary artery dissection.
PPCM complicated by CS
CS complicates ~4% of PPCM, which is defined as idiopathic LV dysfunction (LVEF ≤ 45%) that presents toward the end of pregnancy or in the months following delivery75. The etiology of PPCM is thought to be multifactorial, with contributions from genetic factors, autoimmune responses, fetal microchimerism, and excessive prolactin production75. As with patients afflicted by CS during pregnancy, a multidisciplinary pregnancy-heart team is paramount to rapid decision making for patients with peripartum or postpartum CS72.
In addition to the general principles of CS treatment with typical pharmacologic therapies, bromocriptine may have a role as targeted treatment of PPCM-CS. Bromocriptine is a dopamine agonist that inhibits prolactin release and has been associated with higher rates of LV recovery in mostly pilot and observational studies76. While bromocriptine may be considered according to the 2018 European Society of Cardiology guidelines on the management of cardiovascular diseases during pregnancy71, bromocriptine is considered experimental in the United States and Canada. Accordingly, its clinical benefit is being investigated in a randomized, double-blind, placebo-controlled clinical trial (Randomized Evaluation of Bromocriptine in Myocardial Recovery Therapy [REBIRTH]; NCT05180773), comparing bromocriptine therapy vs placebo in women with PPCM (LVEF ≤ 35%)76. If used, bromocriptine has been associated with thrombotic complications and should be accompanied by at least prophylactic anticoagulation7176.
As with other CS etiologies, tMCS is advised in patients with PPCM-CS who cannot be stabilized on medical therapy alone. A small study reported excellent short-term survival (100% at 30 days and 80% at 6 months) with early use of tMCS and bromocriptine therapy77. Increased prolactin levels during ECMO treatment have been reported, which may be detrimental in PPCM-CS, and higher bromocriptine doses may be appropriate if used64. Because many patients have at least partial LV recovery, a bridge-to-recovery strategy is the preferred approach64; however, the evaluation for long-term advanced HF therapies-durable mechanical circulatory support (MCS; surgical LVADs or biventricular assist devices) and/or cardiac transplantation-should be initiated soon after implantation of tMCS, with plans to transition to long-term strategies if temporary support cannot be weaned after 7 to 10 days. Surprisingly, LV recovery with durable MCS is uncommon. An Interagency Registry for Mechanically Assisted Circulatory Support registry analysis of 1,258 women, including 99 women with PPCM, showed similarly low rates of recovery/explant in both patients with PPCM and patients without PPCM (6% for both), which may be due to variability in patient selection or recovery protocols between centers78. Cardiac transplantation is considered for patients for whom durable MCS is not an option or who do not exhibit substantial LV recovery on durable MCS after 6 to 12 months. Nevertheless, it should be noted that patients with PPCM have worse postheart transplant outcomes compared with women with other cardiomyopathies79.
Consensus tips for treatment of pregnant patients with CS, including PPCM
• An established multidisciplinary cardio-obstetrics team, including cardiology, obstetrics or maternal fetal medicine, anesthesiology, critical care, and nursing, is paramount to rapid decision making in pregnant patients with CS and may require transfer to a center with a dedicated cardio-obstetrics program.
• Early invasive hemodynamics assessment and consideration for early tMCS are critical to maternal survival.
• Measures to reduce fetal exposure to radiation and medications are warranted but should not take precedence over treatments to preserve maternal life.
• For patients with PPCM-CS, a bridge-to-recovery strategy is the preferred approach because of high rates of at least partial LV recovery.
Evidence gaps in the treatment of pregnant patients with CS, including PPCM
• Further data are needed to clarify the safety and efficacy of bromocriptine on LV recovery in PPCM and PPCM-CS.
Heart failure-related CS
HF-CS is the most common etiology of CS in the modern cardiac intensive care unit, with women representing one-third of these patients3480. The most common etiology of HF-CS is acute decompensation of chronic HF, accounting for >70% of HF-CS cases in women. De novo HF causes such as myocarditis and TTS are also more likely to occur in women compared with men (26.3% vs 19.3%) (see Supplementary Table 3 for Acute and Fulminant Myocarditis)8.
A sex-based analysis by the Cardiogenic Shock Working Group (CSWG) showed that women with HF-CS have higher baseline SCAI SHOCK stage compared with men (stage E 26% vs 21%) and have worse survival at discharge (69.9% vs 74.4%)8. This is, in part, related to the fact that women with chronic HF are more likely to be older, have more cardiovascular comorbidities (hypertension and diabetes mellitus)14, and have less evidence-based pharmacologic therapy and implanted device (internal cardiac defibrillator and cardiac resynchronization) therapies compared with men81. Despite presenting with higher clinical acuity, women with HF-CS were less likely to receive pulmonary artery catheterization (52.9% vs 54.6%), more likely to be treated without tMCS support (26.2% vs 18.8%), and less likely to receive heart replacement therapy with durable LVAD (7.8% vs 10%) or cardiac transplantation (6.5% vs 10.3%) when compared with men in a study8. Accordingly, there is a distinct need to develop care pathways to ensure that women have equal and timely access to durable LVAD and cardiac transplantation.
The use of tMCS for HF-CS has increased over the past 2 decades46 and is most commonly used as a bridge to advanced HF therapies (durable LVAD or cardiac transplantation). A retrospective analysis from the CSWG registry showed that for HF-CS, IABP is the most commonly used initial device, being used in 45% of the overall CSWG cohort, followed by Impella in 12% and VA-ECMO in 7%74. The CSWG registry sex-specific analysis showed that IABP and ECMO use is similar based on sex within the first 24 hours of admission, but women were less likely to receive an Impella83446. There are no randomized trials evaluating tMCS efficacy in HF-CS, and thus, there is no informed guidance for device selection or timing24. Early initiation of tMCS in HF-CS has a small but incremental benefit on mortality based on observational studies. A retrospective National Inpatient Sample database analysis of ~85,000 patients with HF-CS (30% women) supported with either IABP or Impella showed a modest mortality benefit with earlier support (within 48 hours of admission) compared with later support (after 48 hours), with an improved adjusted all-cause in-hospital mortality of 23.67% vs 27.67%82. Similarly, a retrospective Extracorporeal Life Support Organization registry analysis evaluating timing of VA-ECMO in ~8600 patients with predominantly non-AMI CS showed a small but significant improvement of in-hospital mortality with early (within 24 hours) vs later (after 24 hours) support (mortality 51.6% vs 54.7%; aOR, 1.2 with late ECMO) and that each 12-hour delay increased mortality (aOR, 1.06); the results were consistent across the sexes83. Additional sex-specific studies are needed to guide device selection and timing.
Takotsubo syndrome
TTS is a specific, acute, nonischemic cardiomyopathy that can present as CS in 5% to 10% of cases. TTS classically follows an intense emotional or physical stress and tends to present similar to MI but without plaque rupture10. Approximately 90% of TTS occur in women, and it is particularly prevalent in post-menopausal women. Younger patients (<50 years) account for 11.5% of TTS and are more likely to present with CS (15.3% vs 9.1%; P = .004) compared with older patients (age, 51-74 years)84. TTS with CS (TTS-CS) is associated with substantially higher mortality rates compared with TTS without CS (23.5% vs 2.3%)85, with the majority of death occurring in the first 24 hours after presentation when patients are most severely hypotensive86. The development of CS in TTS is likely multifactorial-LV systolic dysfunction may be exacerbated by RV dysfunction, and LV outflow tract obstruction from hyperkinetic basal ventricular segments may contribute to poor cardiac output86. As a result, the administration of catecholamines should be avoided in TTS and their potential to exacerbate hemodynamic instability86. Consequently, tMCS is frequently used as a bridge-to-recovery strategy for TTS-CS (38% of TTS-CS cases in 1 series10), aiming to reduce acute stage mortality85. A propensity score–matched analysis of the International Takotsubo Registry showed lower in-hospital mortality for patients with CS who received tMCS when compared with patients who did not receive tMCS (OR, 0.34; 95% CI, 0.12–0.95; P = .04)85.
Consensus tips for the treatment of HF-CS and use of advanced HF therapies in women
• There is a need to develop pathways of care to address the treatment disparities in women with HF-CS and ensure equal and timely access to durable LVAD and cardiac transplantation.
• Clinical evidence is needed to inform optimal tMCS selection (Impella, VA-ECMO) and timing in women with HF-CS.
VHD-related CS
Aortic stenosis
CS associated with severe AS occurs in up to 12% of patients and has been associated with an extremely high mortality rate in the absence of a corrective valve procedure87. Often, patients are treated with MCS or percutaneous valvular intervention (either balloon aortic valvuloplasty or TAVR) to stabilize CS, as immediate surgical intervention portends a higher risk of mortality in this context.
Sex-specific data regarding the outcomes and treatment of AS-CS are very limited and are mostly derived from the TAVR population. An analysis of 15,071 patients with AS treated with TAVR (2,200 of whom presented with CS) in the Transcatheter Valve Therapy (TVT) registry demonstrated that men presented with CS at a higher rate compared with women (17.5% vs 12.3%; P < .001)12. Despite potential differences in incidence of AS-related CS, a TVT registry study of 5,006 patients (~35% women) with AS-related CS showed that sex was not an independent predictor of 1-year mortality in patients with AS-CS treated with TAVR88. Although studies using first-generation and second-generation TAVR prostheses have demonstrated higher rates of bleeding or vascular complications in women treated with TAVR8990, recent studies have demonstrated no sex-specific differences in survival or stroke9192, which may reflect the changing demographic characteristics of the patient population being treated with TAVR (eg, lower risk) as well as advances in device technology and procedural techniques. Hence, although sex-specific data for the treatment of AS-related CS are lacking, TAVR may be appropriate as a viable treatment option for women with this condition93.
Aortic regurgitation
As for AS, there are no sex-specific data on outcomes or management of acute aortic regurgitation in the setting of CS. For additional information, see Supplementary Table 3.
Mitral regurgitation
Both acute and chronic mitral regurgitation (MR) can lead to CS either due the acute rupture of chordae or papillary muscle caused by AMI (which accounts for 22.5% of MR with CS) or worsening of chronic MR from leaflet restriction in the setting of decompensated HF94. While treatment of the underlying CS pathology (whether AMI-CS or HF-CS) remains paramount, shock may persist without management of the MR, and so, early intervention is advised if clinically feasible95. Studies have demonstrated that tMCS, particularly IABP, is useful in stabilizing patients with MR-CS and can act as a bridge to definitive mitral valve intervention, be it surgical or percutaneous9697.
Similar to AS-CS, there are minimal sex-specific data regarding the outcomes and treatment of MR-CS, and the data available are largely derived from patients treated with transcatheter mitral edge-to-edge repair (mTEER). A TVT registry analysis of 3,797 patients with MR-CS (40.5% women) showed that successful mTEER was associated with lower in-hospital mortality (9.1% vs 16.1%; P < .001) and 1-year mortality (34.6% vs 55.5%; P > .001) compared with patients with unsuccessful procedures. Similarly, a propensity score–matched analysis of 596 US Medicare beneficiaries (43.1% women) with CS who received mTEER had lower in-hospital mortality (OR, 0.6; 95% CI, 0.47-0.77; P < .001) and 1-year mortality (HR, 0.76; 95% CI, 0.65-0.88; P < .001) compared with patients who did not receive a mTEER98. Neither of these studies identified sex as an effect modifier of outcomes in patients with MR-CS treated with mTEER9899, thereby suggesting that appropriately selected men and women alike may benefit from mTEER in the setting of MR-CS.
Evidence gaps in the treatment of VHD-CS in women
• Sex-specific analyses of outcomes and treatment strategies are needed in patients with VHD-CS.
• Inclusion of an adequate subset of women in percutaneous valve intervention trials is paramount to understanding the sex-specific benefits and complications of these devices in the setting of CS.
Advanced HF therapies: limitations in care for female survivors of CS
Patients with CS who fail to recover with medical therapy or tMCS may be appropriate for advanced HF therapies (LVAD and cardiac transplant). While pivotal durable LVAD trials have shown mortality benefit for patients with chronic end-stage HF100101102, women have been underrepresented in these trials, so evidence regarding sex-specific differences in outcomes is indeterminate. For example, in the recent MOMENTUM 3 trial, which compared the Heartmate III and Heartmate II devices, only ~20% of enrollees were women103. While early generation pulsatile-flow durable LVADs were associated with higher mortality for women (OR, 2.13; 95% CI, 1.45-3.10; P < .0001), current generation continuous-flow LVADs show similar survival between the sexes104. There have also been specific concerns about an excess risk of neurologic events in women receiving durable MCS. In a Heartmate II cohort, the risk of hemorrhagic stroke was greatest in women younger than 65 years, whereas the risk of thromboembolic events was greatest in women older than 65 years105. With the contemporary Heartmate III LVAD, risk of stroke overall is much lower, but women continue to be at higher risk. A sex-specific analysis of the MOMENTUM 3 trial showed that women had an increased risk of stroke (adjusted incidence rate ratio [aIRR], 1.52; P = .12) in addition to higher risk of major bleeding (aIRR, 1.28; P < .0001) and infection (aIRR, 1.14; P = .01)106; however, this analysis also showed that there were no sex-based differences in overall survival or in the primary outcome (survival free of disabling stroke or need for pump replacement or removal at 2 years postimplant). In the context of the limited number of women enrolled and the lack of power, these findings highlight the need for additional studies in women specifically to establish the outcomes associated with durable LVADs.
Cardiac transplantation remains the gold standard treatment option for patients who develop end-stage HF and prolonged CS107. Women remain less likely to undergo transplant compared with men, accounting for only 23% of heart transplant patients108. In a United Network for Organ Sharing analysis, women receiving a durable LVAD as a bridge to transplantation had lower rates of heart transplantation (55.1% vs 67.5%), greater waitlist mortality (7.0% vs 4.2%), and more delisting for clinical deterioration (8.5% vs 4.7%) at 2 years of LVAD support, compared with men (all P < .001)109. Another sex-based analysis evaluating patients at the highest heart transplant urgency strata (status 1) found similar trends for women with lower rates of transplant and higher rates of delisting for death or clinical deterioration110. Contributing factors identified in these studies include higher allosensitization in women (which makes finding suitable donors more difficult) and/or MCS-related complications, but precise reasons underlying lower transplant rates in women remain unclear109110. Women who do proceed to cardiac transplantation have a similar posttransplant survival rate compared with men111. These findings underscore the importance of developing best practices in post-CS care to ensure women with HF have equal and timely access to transplant.
Barriers to care for women with CS upon presentation to medical attention
Vascular and bleeding complications remain major obstacles to the adoption of cardiac interventional treatments, including tMCS, in women. In the US multicenter CSWG research consortium of 5083 patients (30% women) with CS of any etiology, women had higher rates of adjusted vascular complications requiring intervention (10.4% in women vs 7.4% in men; P = .06) and vascular complications predicted mortality in women but not in men8. Further analysis of the CSWG registry identified that acute limb ischemia occurs in 3% to 19% of patients with CS and is associated with a near-doubling of in-hospital mortality. This analysis further identified female sex as a significant risk factor for development of acute limb ischemia in CS29. Nevertheless, major bleeding and vascular complications with tMCS devices have significantly improved over the past decade, particularly for women213352. Guidance for best practices for large-bore access for tMCS should be followed to minimize complications and include ensuring ideal femoral arteriotomy access (ie, using palpation, fluoroscopy, ultrasound, and micropuncture techniques), consideration of alternative tMCS implantation sites with experienced proceduralists and institutions, appropriate tMCS device care (ie, routine monitoring for acute bleeding or limb ischemia), and ensuring safe device removal with successful hemostasis (ie, use of vascular closure devices with or without balloon tamponade for large-bore closure)112. Potentially lifesaving procedures should not be avoided in women for fear of complications, rather improved vascular access techniques and device innovation should be implemented to mitigate risks of bleeding and vascular injury.
Low enrollment of women in clinical trials of CS spanning revascularization3741, tMCS47, and advanced HF therapies100 remains a major impediment to establishing best practices in this high-risk population (Figure 3). Approaches to improve enrollment of women in clinical trials should address age limits and exclusions that impact women specifically. Facilitated consent should be adopted in shock trials to better determine risks and benefits of novel treatments in women.
Lastly, standardization of shock treatment protocols can also help improve early diagnosis and recognition of shock in women and reduce sex-based disparities. Multidisciplinary shock teams (inclusive of advanced HF, cardiothoracic surgery, interventional cardiology, and cardiovascular critical care)113 can quickly identify and define the severity of CS, establish the etiology, rapidly implement measures for hemodynamic support, and initiate etiology-specific treatments. A standardized team-based CS treatment protocol including mandatory hemodynamic assessment, timely diagnosis, and early, appropriate tMCS use may reduce sex disparities and improve outcomes in CS outcomes14. Established shock teams and treatment algorithms have demonstrated faster and more appropriate treatments for patients with CS and improvements in survival in multiple centers25113114.
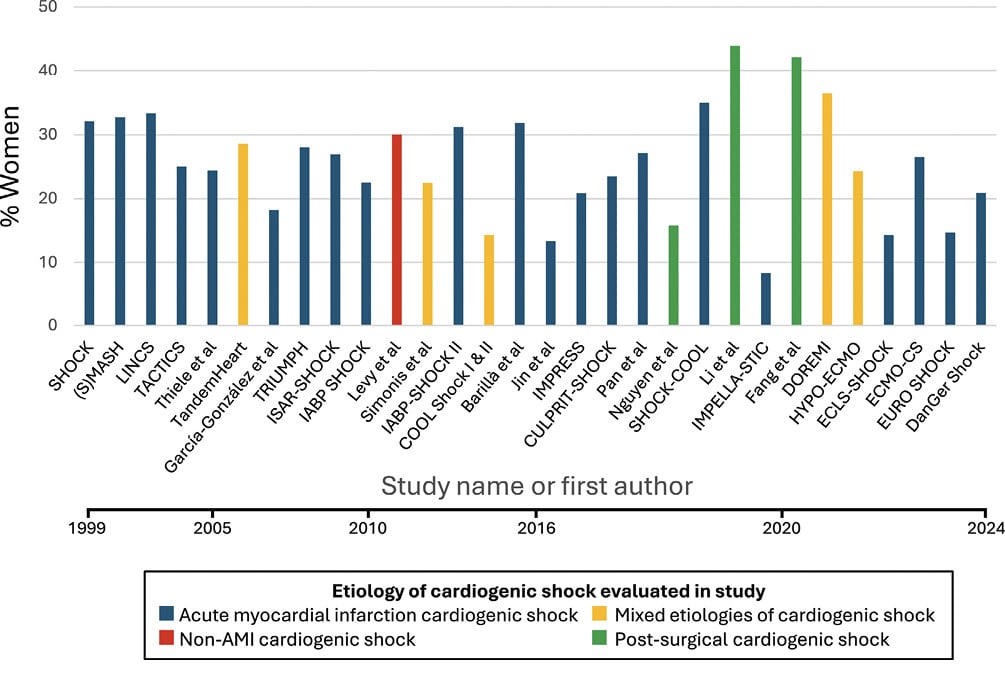
Figure 3. Rates of women enrollment in randomized clinical trials of cardiogenic shock. AMI: acute myocardial infarction.
Consensus tips to address barriers to care for women with CS
• Anticipated vascular complications should not deter use of potentially lifesaving tMCS; rather, risks should be mitigated with improved techniques for vascular access and follow best practices for indwelling devices.
• A standardized, team-based CS treatment protocol including mandatory hemodynamic assessment, timely diagnosis, and early, appropriate tMCS use may reduce sex disparities in CS outcomes.
Evidence gaps in addressing barriers to care for women with CS
• Improve enrollment in CS trials by setting a prespecified quota of women in ongoing and future CS clinical trials to determine risks and benefits of novel treatments in women.
• Device innovation for smaller profile devices and new approaches to mitigate vascular complications should be a priority.
• Validation of SCAI SHOCK classification in women is necessity.
Future directions and conclusions
Early identification of CS and its etiology and early referral for mechanical support are paramount to improving mortality outcomes in women. A standardized approach to CS diagnosis and early treatment as proposed (Figure 1) will help address disparities in current clinical care. The importance of a gender-specific approach is also underscored by the recent SEX-SHOCK score45, which could mitigate sex inequities in early risk stratification of contemporary shock management. Future trials in CS must enroll an appropriate number of women to inform the balance of risk and benefit in this population. Beyond this, dedicated randomized trials of women are necessary to determine the best treatment strategy to improve outcomes.
This consensus provides a comprehensive summary of the current state of treatment of CS in women in relevant disease states and identifies important evidence gaps. As there are limited sex-based data in contemporary literature, clinicians may use this document as a resource to guide practice. Further investigations are necessary to inform best practices for women with CS.
Peer review statement
Suzanne J. Baron, Cindy L. Grines, and Alexandra J. Lansky hold editorial roles in JSCAI. As lead authors of this consensus document, they participated in drafting and review of the consensus and in all pre-submission responses to reviewers; however, after submission, they had no involvement in the peer-review process or decision for publication.
Acknowledgements
The authors thank Ashley Radparvar, MD, for the preparation of Figure 2.
Funding
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Suzanne J. Baron leads the economic substudy of the STEMI-DTU trial for Abiomed. Josephine Chou is a consultant for Abiomed. Tayyab Shah is a consultant for Abiomed. Cristina Aurigemma is a speaker for Abiomed. Anna Bortnick reports honoraria from ClearView Healthcare and is a site PI for AEGIS-II trial (funded by CSL Behring). Alaide Chieffo is a speaker for Abiomed and is the PI for prospective, multicenter registry on INOCA-Italian MOH. Cindy L. Grines is in the advisory board of Abiomed. Navin K. Kapur is a consultant/speaker and the principal investigator for Abiomed clinical trials and registries and Getinge clinical trials and registries. Jacqueline Saw is the principal investigator for Hispanic Scholarship Fund—Spontaneous Coronary Artery Dissection (SCAD) genetics, and for Canadian Institutes of Health Research-SCAD genetics. Alexandra J. Lansky is in the advisory board and a consultant for Abiomed and in the echocardiography core laboratory of Abiomed clinical trials. Amanda R. Vest, J. Dawn Abbott, Mirvat Alasnag, Emanuele Barbato, Lavanya Bellumkonda, Robert-Jan van Geuns, Sigrun Halvorsen, Christian Hassager, Srihari Naidu, and Vivian Ng: none.
Supplementary data
To read the full content of this article, please download the PDF.

