Abstract
Aims: Percutaneous coronary interventions (PCI) in high-risk cardiac patients are preferentially referred to specialised myocardial intervention centres (MIC). Included in this group are patients with a haemodynamic collapse or high likelihood of haemodynamic collapse, either during balloon inflation or with acute vessel closure. The TandemHeart®, a percutaneous transseptal left ventricular assist (PTVA®) that can be introduced using standard catheterisation laboratory techniques, offers interesting perspectives to reduce procedural risks.
Methods and results: Between September 2000 to July 2006, The TandemHeart®, supported the circulation of 23 patients (age: range 46-74, mean 59) admitted to our centre for high risk, either emergency or elective, PCI. Successful implantation was achieved in 100% of patients. The mean time for implementation of circulatory support was 35 minutes (range 16-62). The index PCI was successful in all patients except two. A pump flow up to 4L/min was achieved with significant reduction of left ventricular filling pressures, pulmonary capillary wedge pressure and with significant increase of systemic arterial pressures. Duration of support ranged from 1-222 hours (mean 31±49.8 hours). Five patients died with the TandemHeart® in place, four of whom were in irreversible cardiogenic shock at admission. Mild to moderate access site bleeding was seen in 27% of patients. One patient experienced a loge syndrome of the leg. Core temperature (Ct) decreased to <36.5°C in six patients, profound hypothermia (Ct < 35°C) was observed in two patients. There was no technical device failure.
Conclusions: The TandemHeart® - PTVA® provides effective, total left ventricular support in very high risk PCI settings. The rate of device related cardiac and vascular complications was acceptable.
Introduction
With increasing operator experience, refinement in technology and adjunctive pharmacological treatment, percutaneous coronary intervention (PCI) is now considered the treatment of choice for many high-risk subgroups in which PCI was previously contra-indicated. PCI may even be a valuable option for those patients where coronary bypass grafting (CABG) is clinically contra-indicated1-3. Many of these procedures are elective.
In general the benefits of a specific (percutaneous) procedure should be weighed against the risks involved; taking into account alternative treatment strategies, the interventional and intensive care team experience4 and taking in consideration the individual patient risk scores, that may aid the operator in selecting or avoiding adjunctive pharmacotherapy or specific devices5-9.
Patients in whom it is considered that PCI poses a significantly high risk include these with high likelihood of haemodynamic collapse, either during balloon inflation or with acute vessel closure4. In general, these are the patients in whom a large amount of the viable myocardium is supplied directly or indirectly by the affected artery. The development of a percutaneous ventricular assist device (VAD) that could be easily and quickly inserted prophylactically or in case of an haemodynamic collapse proves invaluable in this setting.
The TandemHeart®, a percutaneous transseptal left ventricular assist device (PTVA®) system (CardiacAssist Inc., Pittsburgh, PA, USA), has been demonstrated to effectively reverse cardiogenic shock after myocardial infarction11 and to play an important role for bridging to another definitive therapy12. This system allows for rapid implementation of circulatory support using standard interventional techniques in the catheterisation laboratory and is designed to deliver up to 4.5 litres of blood flow per minute. In this report, we will report on a single centre six year clinical experience with the TandemHeart® in high risk PCI procedures.
Methods
Patients selection
Since 2000, the Thoraxcenter, Erasmus University Medical Centre, Rotterdam, The Netherlands started a program evaluating percutaneous left ventricular assist devices (LVAD) during high risk PCI. Between September 2000 to July 2006, twenty-three patients admitted to our centre for acute coronary syndromes (ACS)/ ST segment elevation myocardial infarction (STEMI) or for elective PCI, were treated with TandemHeart®.
In non-elective patients, the indication for TandemHeart® support was based on already established haemodynamic instability before the index PCI procedure, defined as typically with low cardiac output (cardiac index < 2.2 L/min/m2), peripheral signs of tissue hypoperfusion (decreased urine output and/or cold extremities), systemic hypotension (systolic blood pressure < 100 mmHg) despite vasopressor therapy and in the presence of appropriate left ventricular filling pressures (pulmonary capillary wedge pressure, PCWP > 15 mmHg). In case of presence of an intra aortic balloon pump (IABP) haemodynamic measurements were done with the IABP paused for 60 seconds.
In patients who underwent elective PCI, circulatory assist was indicated in procedures which had a presumed high risk of ischaemic/haemodynamic complications, based on the presence of severely depressed left ventricular (LV) function and planned treatment of ≥ one complex lesions in vessels supplying a large amount of myocardium. CABG was not considered a treatment option in any of these patients. Major exclusion criteria were predominant right ventricular failure requiring right ventricular support, severe aortic insufficiency, severe sepsis and anoxic brain damage.
System description
The TandemHeart® PTVA® (Figure 1-2) incorporates arterial perfusion cannula configurations ranging from 9 to 17 Fr, an unique 21 Fr venous transseptal cannula, and a centrifugal blood pump.
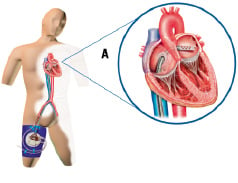


Figure 1. Schematic of the system deployed. The Tandemheart® removes oxygenated blood from the left atrium via a 21 Fr transseptal cannula that is advanced from the femoral vein trough the inter-atrial septum into the left atrium (A). The oxygenated blood is returned into the arterial vascular system via a 15 up to 17 Fr arterial cannula by means of a centrifugal pump (B). The centrifugal pump contains a single moving part (rotor/impeller) that is suspended by magnetic force on a thin lubricating film of fluid to reduce heat and friction, thereby reducing the risk of thrombus formation.
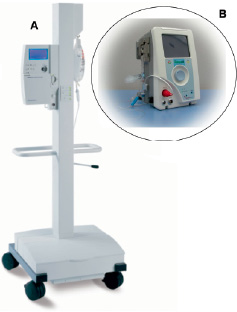
Figure 2. Microprocessor-based controller used in this series (A) displaying PTVA speed and flow. These parameters are controlled by adjustment of a single knob. The controller includes extensive self-diagnostic and alarm features to ensure patient support without the need for constant operator surveillance. Panel (B) shows the recently introduced Escort Controller. This light (21 pound or 9.53 kg) weight controller can be mounted at the patient’s bedside and includes an easy-load infusion system. The latter allows the lubrication fluid infusion line to be installed in seconds.
Oxygenated blood from the patient’s left atrium is supplied to the pump by the trans-septal cannula and than returned to the patient’s systemic arterial circulation. The centrifugal pump contains a single moving part (rotor/impeller) that is suspended by a thin lubricating film of fluid to reduce heat and friction, thereby reducing the risk of thrombus formation. The pump connects to a microprocessor-based controller that displays PTVA speed and flow. These parameters are controlled by adjustment of a single knob. The controller also provides automatic system monitoring and alarms indicating conditions that require action. The system is designed to deliver up to 4.5-5 litres (L) of blood flow per minute, depending on the size of the arterial cannulation and the filling conditions of the left atrium, while operating at a relatively low speed (7500 revolutions per minute, RPM).
Insertion technique
A standard transseptal puncture technique, using an Inoue guidewire, was used to gain access into the left atrium from the right femoral vein. Transseptal puncture was carried out by an experienced operator only. The inter-atrial septum was dilated with an 2-stage 14/21 Fr dilator and the venous inflow cannula was inserted (proper positioning in the left atrium was checked by either angiography and/or transesophageal echocardiography). One 15 Fr or 17 Fr perfusion catheter (Bio-medicus cannula, Medtronic, Minneapolis, MN, USA) was inserted into the femoral artery and advanced to the common iliac artery. An ileac angiography was performed before the placement of the perfusion catheter to delineate the arterial anatomy and size and to disclose eventual luminal obstructions. In three patients a dual femoral approach with Y connection was used for arterial access. Both arterial and venous cannula were fixed to the skin with multiple sutures in order to secure their position. The pump was placed on the upper leg of the patient. After checking the central venous pressure, priming and cautious de-airing of the entire system, the arterial and venous cannulas sets were connected to the pump by a heparin-coated tygon tubing (up to 30 cm length) and the pump was activated to its maximum rotation speed. Output pump flow was measured by an external electromagnetic flow meter (HT 311, transonic).
Device weaning and removal
A predefined weaning protocol was initiated at the moment vasoactive drugs were reduced to a stable minimal level (dobutamine up to 4 µg/kg/min., norepinephrine up to 0.1 4 µg/kg/min.) provided stable haemodynamic parameters (mixed venous or central venous saturation > 65%). A stepwise reduction of pump assist was performed, by reducing pump speed from 7500 to 3500 RPM (steps of 500-1,000 mL/min), adapted to the medical condition of each patient individually. The final removal decision was based on medical judgement. A weaning period up to six months was respected for most patients. The pump was not stopped until immediately before removal. No specific instructions on access site closure were issued.
Concomitant treatment
The index coronary intervention was performed after TandemHeart® implantation and functioning. Arterial access was obtained via the contra-lateral femoral artery and the interventional strategy was left to the operator, according to standard techniques. Standard intensive care was provided to each patient. Patient sedation and intubation was considered for clinical and/or comfort reasons. A balloon tipped pulmonary artery (PA) catheter was placed via the contra lateral femoral vein for haemodynamic monitoring purposes if clinically indicated. Cardiac output determinations were made using standard thermodilution techniques.
During the index procedure, a constant flow (10 ml/h) of heparinised infusate is maintained providing a localised concentration of heparin in the interior of the pump in order to obtain localised anti-coagulation thereby minimising systemic heparinisation, the risk of bleeding and thrombus formation. Additional boluses of heparin were administered peripherally to maintain the activated coagulation time to approximately 200 seconds during routine support (400 seconds during insertion) or an activated partial thromboplastin time of between 65 and 80 seconds.
Predefined safety endpoints and time periods
The following predefined clinical events related to the use of the TandemHeart® were assessed in each patient: any minor or major TIMI bleeding9, laceration of the insertion vessel/limb ischaemia, device related thromboembolic events. infective complications, residual atrial septum defect after device removal, device malfunction or failure.
The insertion time was defined as the time from providing of the Brockenbrough needle to the ‘full’ heparinisation following connection of the femoral cannula to the pump. The duration of support was defined as the time from ‘full’ heparinisation at time of connection to the pump till the removal of the femoral cannula.
Statistical analysis
Variables with normal distribution were analysed using parametric tests while variables with a non-normal distribution were analysed with non-parametric tests. Continuous variables are expressed as mean ±SD or median ±SD and differences are compared using Student t test or Mann Whitney test. Categorical variables are expressed as counts and percentages. Differences were assessed by Fisher exact test or chi-square test, as appropriate. All analyses were performed using SPSS version 12 statistical software (SPSS Inc., Chicago, IL, USA). A two-tailed p value < 0.05 was considered significant for hypothesis testing.
Results
Baseline characteristics
Baseline clinical and procedural characteristics are listed in Table 1.
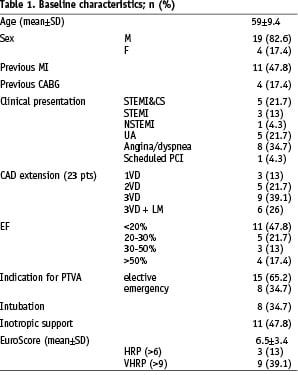
Mean age was 59 ± 9.4 years, 19 patients (83%) were men. The ejection fraction was <30% in 16 patients (70%). Fifteen patients (65%) suffered a three vessel disease, six of whom with left main involvement. The indications for TandemHeart® support were elective/high risk PCI in 15 patients and non-elective (emergency) in eight patients with ACS and acute heart failure. Eight patients experienced an evolving (< 36 hours) myocardial infarction, five of which were complicated by acute heart failure/cardiogenic shock (CS). Eight patients needed intubation and mechanical ventilation as part of their medical treatment and 11 were on inotropic support at the start of the implant procedure. Standard EuroScore in our patients ranged between 2 and 14 (mean 6.5) providing a surgical risk of mortality between 1.5% and 46.6% (mean: 11.3±11.6%). Very high risk patients (EuroScore > 9) were nine13.
Haemodynamic effects
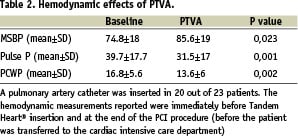
The TandemHeart® showed good performance, achieving flow rates up to 4.0 L/min. Mean systemic arterial pressure was 74.8±18 mmHg at baseline and 85.6±19 mmHg after pump functioning (p= 0.023). Pulmonary wedge pressure was 16.8±5.6 mmHg at baseline and 13.6±6 after pump functioning (P= 0.002). The pulse pressure was reduced on support from 39.7±17.7 to 31.5±17 (p<0.001) (Table 2).
In two patients the initial period after implantation of the TandemHeart® was characterised by a complete non- pulsatile arterial blood pressure (pulse pressure < 7 mmHg). In one patient with the recovery of the heart function < 24 hours after implantation, increasing pulse pressures became evident. Modulation of pulsatility with low amplitude to the non-pulsatile blood flow produced by the PTVA could be achieved by varying pump flow. One patient did not show recovery of pulsatile blood flow and he died with the TandemHeart® in situ.
Procedural and clinical outcomes
Procedural and clinical outcomes are summarised in Table 3.
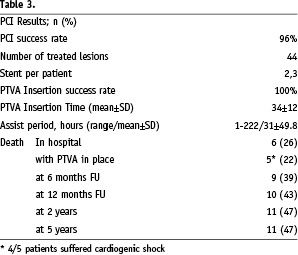
Index-PCI procedural success was achieved in 42 lesions (96%). A total of 44 lesions were treated (one up to four lesions per patient), all including stent implantation (mean stent per patient rate: 2.3).
The mean time for insertion of the TandemHeart® was 34±12 minutes (range 16-62), the procedural success was 100%. The circulatory assist period ranged from 1h to 222h, mean 31±49.8h One patient with a severe femoral artery stenosis needed PTA and stenting of the iliac artery vessel prior to cannulation.
Seventeen patients were successfully weaned from the TandemHeart®. In-hospital death occurred in six patients. In total five patients died with the device in situ, four of which were considered in irreversible CS at admission. Another patient admitted for CS was initially stabilised after 222 hours on TandemHeart® support. However, this patient developed abrupt circulatory failure after removal of the pump and died a few hours later. No further invasive treatment was possible due to the abrupt worsening of his clinical condition. During the follow-up period, three patients were lost and one patient died due to progressive heart failure < 30 days. An uneventful six months follow-up was observed in 13 patients.
Complications
Potential complications related with the use of TandemHeart® are summarised in Table 4.
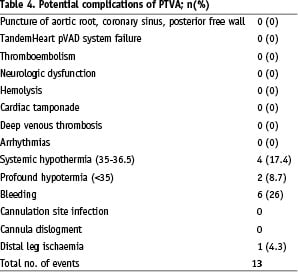

In this series of patients, no procedural complications during device insertion were noted and no cardiac tamponade, thromboembolic events nor device failure occurred. In two elective cases (8.7%) the weaning needed to be postponed due to the occurrence of profound hypothermia (core temperature 32.7 °C and 34.2 °C) and the need for active re-warming. Minor to mild access site bleeding complications occurred in 26% of patients (n=6), none required specific treatment. Lower limb ischaemia complicated by a loge syndrome occurred in one patient with pre-existing severe peripheral vascular disease. This patient needed a fasciotomy. Dislocation of the venous cannula occurred in one patient and required pump removal.
Most pumps were surgically removed in the operation theatre (n=12, 52%). A closure device (Prostar) failed to close completely the puncture site in two out of three attempts. A local compression device (Femostop, Radi) was used successfully in these patients as well as in three others without any complication.
Discussion
PCI in high-risk coronary patients with (potential) haemodynamic compromise is challenging and may be considered a special dimension of MIC14. Active circulatory support using VAD constitutes a valuable safeguard to reduce potential fatal intervention-related complications in this setting and is nowadays considered part of the current therapeutic armamentarium.
IABP is currently the most widespread mechanical device used to support and to improve coronary perfusion in patients with acute heart failure. IABP implantation is easy and its use is safe and associated with a low incidence of serious complications. IABP may provide a lot of diastolic augmentation of coronary flow in patients in shock, however only limited forward output augmentation (up to about 0.5 L/min). This level of support has been shown to be scarcely able to improve clinical outcomes in the setting of complete hemodynamic collapse15.
Active circulatory support using VAD combines the beneficial effects of the myocardial unloading and an increase in tissue perfusion pressure. Theoretically, this technique should also allow very early decompression (“unloading”) of the left ventricle in massive myocardial infarction, thus enhancing the chances of recovery of the jeopardised ischemic non-infarcted areas16. In animal models, LV unloading prior to revascularisation of the infarcted artery led to significant myocardial salvage in comparison to the implementation of unloading after reperfusion17. A trial testing this concept, including one patient reported in this series, was stopped early due to poor recruitment. However, the concept could nicely be illustrated by echo contrast imaging in one of our patients (Figure 3).
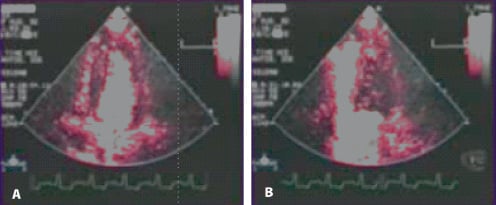
Figure 3. Echocardiographic contrast perfusion images in a patient on Tandemheart(r) circulatory support. Panel A: low circulatory support (high wall tension, poor myocardial perfusion, contrast remains in the ventricle cavity). Panel B: Full circulatory support with effective unloading of the left ventricle (low wall tension, good myocardial perfusion).
Our data confirmed that TandemHeart® support was associated with significant decreases in left ventricular filling pressures, mean systolic blood pressure and arterial pressures. The blood flow, up to 4.5 L/min, provided by the device may be sufficient to both optimally unload the LV and to prevent and even reverse organ dysfunction in cardiogenic shock patients11,18.
Patient selection is the single most crucial factor in determining a successful outcome in patients who receive temporary mechanical circulatory support. Rapid diagnosis and treatment are essential but are often based on limited information. The patient’s history and overall clinical setting should be considered in the decision process to initiate circulatory support. A pulmonary-catheter was used in the majority of the patients as adequate left ventricular filling conditions are essential for proper functioning of (preload to) the device. Haemodynamic data, including mixed (or central) venous oxygen saturation, may help to guide the overall patient selection, the management and potential weaning decisions. In our series five patients died while on TandemHeart® support, four were in profound CS at the time of treatment initiation. Patients beyond the ‘irreversible edge’ of CS prove bad candidates for this technique in this and other series11. Of interest, only three out of the successfully weaned patients (n=17) deceased during the FU period, suggesting this device may have a tangible impact on the long-term survival as well.
The TandemHeart® can be used prophylactically and in bail-out situations. In experienced hands cardiovascular support can be implemented in less than 35 minutes. The most important drawback of the technique may be the difficulty of cannula insertion. Substantial atherosclerotic changes in the iliac artery and the consequent peripheral vascular disease are most common in the group of coronary patients who most benefit from the effects of the pump. In this series, a total number of 13 device-related in-hospital adverse events rate were observed, 0.6 events per patient. Many of them were access site complications. One patient developed a loge syndrome requesting a fasciotomy, but no other iatrogenic arterial insufficiency was noticed. Mild to moderate groin bleeding was encountered in six out of 23 patients (27%), none needing a specific treatment. Most pumps were surgically removed in the operation theatre in order to allow optimal local haemostasis and proper inspection of the cannulation site. A local compression or closure device may provide a valuable alternative given a clean puncture technique. Access site complications may be minimised by prior iliac angiography and by implementing an ultrasound guided puncture technique of the (common) femoral artery13. When necessary, the use of distal perfusion and antegrade cannulation of the superficial femoral artery may obviate peripheral vascular complications as well.
As opposed to other modalities of percutaneous cardiopulmonary support (CPS) used in this indication19,20, the TandemHeart® system keeps the patient’s lungs as its own ventilator and may be used to support patients for a longer period of time without major haematological or pulmonary complications (up to 10 days in our series). No thrombi formation was noticed either during the support or after the removal of the device and no thromboembolic event occurred. Evidence of clinical relevant haemolysis, which was a particular concern, was looked for but absent in our series (data not shown). Also this observation is in line with the Leipzig series11.
Systemic hypothermia (<36,5°C) was observed in 25% of patients (n=6) but the temperature decreased below 35°C only in two cases. Contact of the system circuit with room temperature may have contributed to a cooling effect on the blood flowing through the pump. Other factors that may have contributed to heat loss are the use of general anaesthetic agents and neuromuscular blockers leading to vasodilatation and the loss of muscular tonus. Correcting measures may be straightforwardly introduced to avoid or rapidly detect this complication in the future.
Conclusion
Our results create optimism for more widespread introduction of the TandemHeart® in the MIC setting. The concept of closed chest left heart bypass, with left atrial to femoral artery bypass, constitutes a relatively safe and reliable safeguard in high risk PCI and acute heart failure. TandemHeart® PTLA® can be rapidly and percutaneously implanted in the catheterisation suite using standard interventional techniques in both a prophylactic or emergency settings. The TandemHeart® provides more haemodynamic support than the IABP and is effective in stabilising haemodynamics. Access site complications remain the Achilles’ heel of this technique.

