Abstract
Side branch (SB) occlusion is one of the most serious complications of main vessel (MV) stenting. Although plaque shift has been considered the major mechanism of SB occlusion, recent studies have suggested carina shift to be the more important cause. Considering the recent pressure wire as well as intravascular ultrasonography studies, the relationship between carina shift and plaque shift in SB occlusion can be described as follows. The anatomical compromise of the SB after MV stenting is not as functionally significant as it appears, because it is mostly explained by carina shift, which is not the major cause of functional compromise. Superimposition of plaque shift over carina shift appears to be the mechanism of haemodynamically significant SB stenosis. Plaque is shifted mostly from the proximal MV, which explains why the plaque burden of the proximal MV is a significant risk factor of SB functional compromise or occlusion.
Introduction
The most challenging part of coronary bifurcation stenting is protection of the side branch (SB) during the procedure. SB occlusion after main vessel (MV) stenting is one of the most serious potential complications of this procedure and may be the major reason why operators prefer the systematic two-stent technique for complex bifurcation lesions. SB occlusion is reportedly associated with increased risk of periprocedural myocardial infarction1. The manoeuvres used to protect the SB are complex and are also a source of procedural complications. Aggressive dilatation of the SB is sometimes associated with underexpansion of the stent in the MV, which results in in-stent restenosis or possibly stent thrombosis2.
Plaque shift vs. carina shift: two major mechanisms of SB occlusion
Since the development of balloon angioplasty, SB stenosis has been reported as the major cause of SB compromise3. In the stenting era, significant SB ostial disease has also been reported to be a major cause of SB occlusion after MV stenting4. Moreover, MV stenting in the presence of a large atheromatous plaque around the bifurcation is associated with plaque shift (the snowplough effect) to the SB, sometimes resulting in its occlusion5.
Most classifications of coronary bifurcation lesions are based on the plaque distribution of the main vessel and the side branch6,7, because plaque shift was considered the major mechanism of SB compromise8. True bifurcation lesions have significant plaque burden both in the main vessel and in the SB, and are at high risk of SB compromise. When plaque in the proximal main vessel or at the bifurcation carina is shifted and added to plaque in the SB ostium, the SB can be compromised.
A pathological study, however, demonstrated that atherosclerotic plaques mostly occur on the lateral wall, whereas the flow divider regions (carina) tend to be spared9, which may suggest that the contribution of plaque shift has been overestimated. Instead, the carina itself can be shifted to the SB, which may be the major cause of SB compromise (Figure 1). The first paper suggesting the critical role of carina shift was based on complex angiographic analysis of coronary bifurcation lesions10. The predicted SB minimal lumen diameter (MLD) was calculated using the geometric assumption that carina shift was a major mechanism of SB compromise. Predicted SB MLD was well correlated with observed MLD (r=0.91, p<0.001), which proved the initial assumption. That study, however, was limited by two-dimensional angiographic analysis. Intravascular ultrasonography (IVUS) is the best technique for evaluating plaque burden.
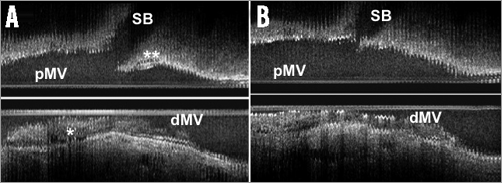
Figure 1. Plaque (*) and carina (**) morphology change before (A) and after (B) main vessel stenting. dMV: distal main vessel; pMV: proximal main vessel; SB: side branch (Intravascular image courtesy of B.K. Koo)
Koo et al published the first IVUS study specifically examining the relative importance of plaque shift and carina shift in 56 patients with a coronary bifurcation lesion11. IVUS examination of the MV before and after stent implantation showed that increase in the distal MV lumen was primarily due to enlargement of the vessel area, not to decreased plaque burden, suggesting that SB luminal narrowing is mostly related to carina shift, not plaque shift. Another interesting point is the morphology of the carina. Medina et al12,13 described the morphological pattern of the carina on IVUS, which consists of the presence of a spiky carina with variable length and orientation (“eyebrow” sign). This morphology is a powerful predictor of ostial SB compromise after MV stent implantation. However, these studies did not examine the SB. H-C. Gwon’s group analysed IVUS images of the SB as well as the MV before and after MV stent implantation in 44 patients14. SB compromise was defined as change in the lumen volume, while carina shift was defined as change in the vessel volume, and plaque shift as change in plaque volume in the 5 mm ostial segment of the SB. This study showed that SB compromise was well correlated with carina shift (r=0.94, p<0.001), but not with plaque shift (r=–0.02, p=0.90) (Figure 2). Moreover, carina shift accounted for 85% of SB compromise. Thus, it seems evident that carina shift is a more important contributor to anatomical SB ostial compromise.
Functional studies, however, showed opposing results. One study examined the MV and SB by pressure wire as well as IVUS in 40 patients15 and found that significant fractional flow reserve (FFR) in the SB after MV stenting was always accompanied by plaque shift, whereas carina shift was rarely associated with significant FFR in the SB. Anatomical significance is not well associated with functional significance in the SB after MV stenting16. Angiographic stenosis >75% was accompanied by functional significance in only 27% of the SBs examined.
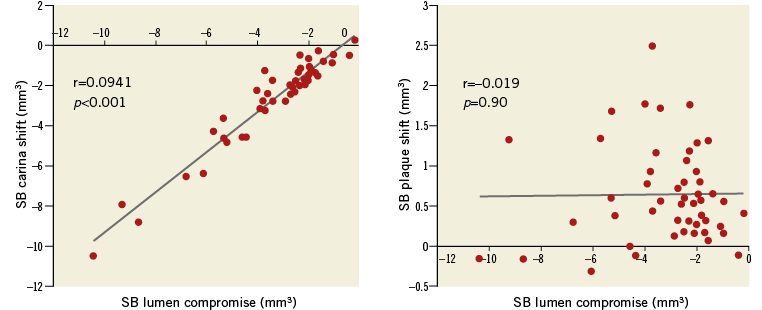
Figure 2. An intravascular ultrasonography study showed that SB compromise was well correlated with carina shift, but not with plaque shift, suggesting that carina shift is a more important contributor to anatomical SB ostial compromise.
Plaque shift as a major cause of SB compromise
Data from a large bifurcation stenting registry also showed the importance of plaque distribution. A subgroup analysis of the second Korean Coronary Bifurcation Stenting (COBIS II) registry analysed predictors of SB occlusion in 2,227 patients17. SB occlusion (TIMI flow <3) was noted in 187 patients (8.4%) just after MV stenting. Flow was restored spontaneously in 26 (13.9%) and by SB intervention in 103 (55.1%), but not in 58 (31.0%). A jailed wire in the SB was an independent predictor of SB occlusion, but was associated with flow recovery (p=0.02). This study found five independent predictors of SB compromise: significant SB ostial disease, SB lesion length, left main lesion (negative predictor), acute coronary syndrome, and significant proximal MV stenosis. Plaque shift associated with a large plaque burden is considered to be the mechanism for the last two predictors.
Similar findings were noted in a computed tomography angiography study18. This study examined predictors of SB occlusion with pre-procedural computed tomography (CT) angiography in 65 patients, and found two independent predictors: significant SB disease and plaque thickness in the proximal MV on the ipsilateral side of the SB. As shown in this study, plaque is shifted from the proximal MV.
An IVUS study also revealed that SB plaque shift is significantly correlated with plaque volume change in the proximal MV (r=0.465, p=0.002), but not in the distal MV (r=0.058, p=0.70)14.
Technical tips to reduce the risk of SB compromise
There are few studies demonstrating procedural predictors of SB compromise. Unfortunately, the jailed wire technique did not lower the risk of SB compromise, but was helpful for restoring flow after SB compromise in the COBIS II registry17. There has not yet been a randomised trial evaluating the impacts of the jailed wire technique on SB compromise. Predilation of the SB also did not protect against occlusion in the COBIS II registry. However, a recent randomised controlled trial including 372 patients with true bifurcation lesions indicated that predilation of the SB improved TIMI flow after MV stenting19. Stent overexpansion may facilitate plaque and carina shift. In an IVUS study, proximal MV stent expansion (defined as change in lumen volume) was significantly correlated with SB plaque shift (r=0.34, p=0.03), whereas distal MV stent expansion was significantly correlated with SB carina shift (r=0.41, p=0.003)14.
Based on those studies, the consensus from the European Bifurcation Club20 recommends the following procedures to optimise the provisional single-stent technique without SB compromise (Figure 3). 1) Start by wiring the MV and large SBs. 2) Predilate the MV and the large SB with severe ostial stenosis, if indicated. 3) Perform MV stenting with a size just optimal in relation to the distal main vessel, while avoiding stent overexpansion (distal optimisation). 4) Perform the proximal optimisation technique which may help wiring the SB and stent apposition in the proximal MV. 5) Rewire the SB followed by SB ballooning or stenting if further SB treatment is indicated.
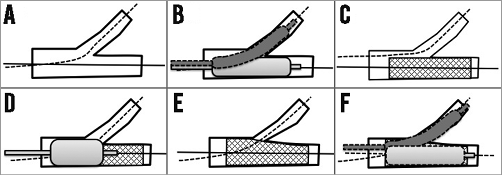
Figure 3. Procedures to optimise the provisional single-stent technique avoiding SB compromise. A) Start by wiring the MV and large SBs. B) Predilate the MV and the large SB with severe ostial stenosis, if indicated. C) Perform MV stenting with a size just optimal in relation to the distal main vessel, while avoiding stent overexpansion (distal optimisation). D) Perform the proximal optimisation technique which may help wiring the SB, and stent apposition in the proximal MV. E) & F) Rewire the SB followed by SB ballooning or stenting if further SB treatment is indicated.
Summary
The relationship between carina shift and plaque shift in SB occlusion is as follows. The anatomical compromise of the SB after MV stenting is not as functionally significant as it appears, because it is mostly explained by carina shift, which is not the major cause of functional compromise. Superimposition of plaque shift over carina shift appears to be the mechanism of haemodynamically significant SB stenosis. Plaque is shifted mostly from the proximal MV, which explains why the plaque burden of the proximal MV is a significant risk factor of SB functional compromise or occlusion.
Conflict of interest statement
H-C. Gwon has received research funds from Abbott Korea, Medtronic Korea, Boston Scientific Korea, and Biotronik Korea. M. Pan has received minor lecture fees from Abbott. Y. Bin Song has no conflicts of interest to declare.