Shortly after the so-called fire-storm of the 2006 European Society of Cardiology congress where alarming data on long-term outcome of drug-eluting stents (DES) was presented, an FDA panel was convoked to review in-depth the outcome of randomised trials and a myriad of registries conducted in Europe and the United States. At the panel meeting in December 2006, the Swedish SCAAR registry was for the first time publicly presented and stirred up a lot of controversy. Shortly after, the results were published in the New England Journal of Medicine and the authors concluded: “Drug-eluting stents were associated with an increased rate of death, as compared with bare-metal stents”. It is likely that our Swedish colleagues did not realise the profound impact of their clinical research on the common practice of American and European physicians. A wave of suspicion emerged within the medical interventional and cardiological community, triggering an official reaction from the European Society, medical authority of Sweden, the United Kingdom and other European countries. In our own institution, the board of the directors requested that we analysed in an epidemiological fashion the outcome of those patients treated over the last seven years with either DES or bare metal stents (BMS). A European task force on DES was established in order to provide general guidelines to the medical community.
One year later, I had the privilege to be the discussant of a late breaking trial session, again at the European Society of Cardiology congress, and I was assigned to comment on a presentation entitled “Long-term outcomes with drug eluting stents vs. bare metal stents in Sweden - one additional year of follow-up”.
I’ve always been fascinated by countries such as Sweden, Denmark and the Netherlands, that are able to collect and control the quality of the medical practice on a national scale. As a trialist, I still consider survival and quality-of-life in unselected patient populations as the ultimate endpoints when judging the benefits of a new treatment modality. The Swedish clinical follow-up obtained from national registries in a real world patient population without scheduled angiographic and clinical follow-up is a reflection of real world clinical practice and a true observation of the real course of the disease and treatment. Therefore my enthusiasm for the work of our Swedish colleagues.
In preparing the discussion, I received the draft of their lecture and quickly realised that something altered the outcome of their follow-up. Obviously, the addition of one single year of follow-up had profoundly modified the content and conclusion of the paper published in the NEJM. Through continuous dialogue with the investigators we obtained the separate landmark analysis of the patients recruited in 2003, 2004 and 2005. At first sight it appeared that the patients enrolled in 2005 had a very different “behaviour” as far as the freedom of death and myocardial infarction were concerned. The landmark analyses of 2003 and 2004 showed no difference in death and MI over the first 6 months, and an increasingly detrimental long-term effect in the DES patients over the ensuing years of follow-up (Figure 1a).
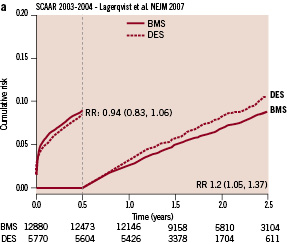
Figure 1 a. Six-month landmark analysis – cumulative incidence of death and myocardial infarction of the 2003-2004 cohort of the SCAAR registry.
In contrast, the 2005 landmark analysis, showed a risk reduction of 31% with DES over the first six months and no late unfavourable effect over the two subsequent years (Figure 1b).
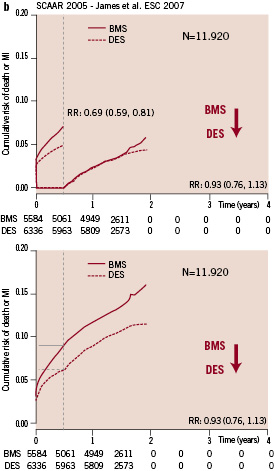
Figure 1b. Six-month landmark analysis – cumulative incidence of death and myocardial infarction of the 2005 cohort of the SCAAR registry.
The fundamental question is what happened over these three years, which could explain the dramatic change in outcome. Some assessment of the baseline characteristics of the original 2003-2004 cohort published in the NEJM compared to the baseline characteristics of the cohort recruited between 2003 and 2005 did not reveal spectacular changes in baseline characteristics. The incidence of diabetes and the treatment of small vessels and in-stent restenosis remained very similar over time. It was mainly the incidence of myocardial infarction and the use of three or more stents or long stents that slightly increased. Although we were not provided with statistical information or propensity score data, it would be reasonable to assume that the risk profile of the patient did not decrease. At the time of their presentation no additional information was provided on procedural changes in their practice as well as on the duration of the dual antiplatelet therapy. Undoubtedly, the major changes between 2003-2004 and 2005 was the increasing use of DES (from 22% in 2003 to 37% in 2004 and reaching 53% in 2005) [see Figure 2].
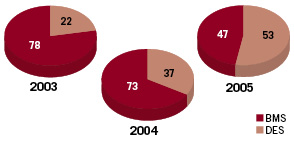
Figure 2. Percentage of drug-eluting stent (DES) and bare-metal stent (BMS) use per year in Sweden.
On an ironic note, at the time of the comments on their presentation, the senior author on this editorial explained to the audience how the outcome following DES implantation was actually improving over time, while the investigators were continuing to use more of these “killing instruments” – as a reference to the last line of the their NEJM paper. Still today, we don’t have a clear explanation for this erratic change in outcomes. From the statistics published by the Swedish authorities, it appeared that nine out of the 26 centres involved at that time in the registry were performing less than 400 interventions per year. This might become relevant when considering the well-known relationship between short and long-term outcome of PCI and volume load. In addition, it appears that the use of DES ranged from 0.4% to 62% in the centres involved in the data-collection. As far as we know, no statistical adjustment for these rather large difference in DES penetration has been published. Today it is striking to put side-by-side the cumulative events curve for mortality observed in the West-Denmark1, Dutch2 and Swedish registry (Figure 3).
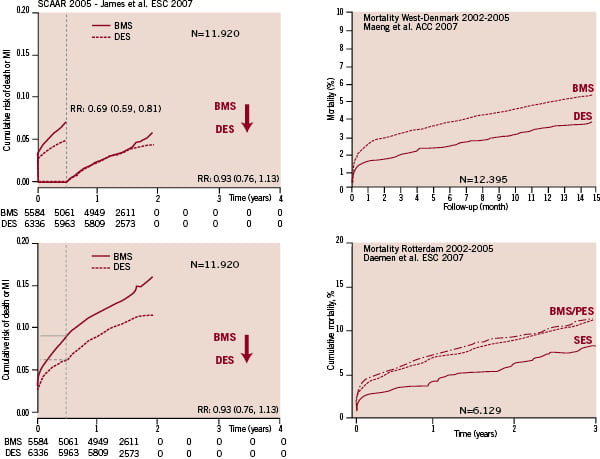
Figure 3. Comparison of the 2005 analysis of SCAAR with the comparative real-world data from West-Denmark and Rotterdam.
They are all characterised by an early (1 month) difference in mortality. This factual information has re-comforted many of our interventional colleagues, who felt already guilty having implanted a DES in most of their patients. In the mean time, the impact of the NEJM on the Swedish medical practice resulted in a drop of the DES use to less than 20%, a phenomenon which has been sarcastically coined the Swedish yo-yo. It is a heavy responsibility for our Swedish colleagues to assess the result of this drop in DES-use. The vintage in Swedish interventional cardiology of 2007 will only be properly evaluated in 2010. It is unclear whether the low use of drug-eluting stents will again increase or decrease the mortality and the morbidity of percutaneous interventions in Sweden. Anyway, these results will always remain in the annals of interventional cardiology.

