Abstract
Aims: Achievement of the angiographic gold standard TIMI 3 flow (Thrombolysis In Myocardial Infarction) flow grade during PCI (percutaneous coronary intervention) in the setting of AMI (acute myocardial infarction) is insufficient for attainment of optimal prognostic benefit, as there is a poor prognosis for patients with evidence of inadequate flow at the tissue level despite patent coronary arteries. PCI in lesions containing thrombus are associated with an increased risk of complications occurring through dislodgement of thrombotic material resulting and distal embolisation leading to slow flow or even no-reflow. Devices which remove thrombus from coronary arteries (thrombectomy devices) or protect from distal embolisation of thrombus (distal protection devices) are increasingly used in PCI.
Methods and results: We have performed a systematic review of the literature to investigate the role of these devices in PCI in the setting of AMI. Use of thrombectomy devices in randomised and multicentre trials in patients undergoing PCI during STEMI is associated with a significant benefit in a number of markers of myocardial perfusion including MBG (myocardial blush grade), ST segment resolution and improvement of distal embolisation, although no significant benefits in mortality have been observed.
Conclusions: There does not appear to be strong evidence for the use of embolic protection devices and distal filter devices in the setting of primary PCI in native coronary arteries, although evidence from trials such as the SAFER trial would make a strong case for their use in SVG interventions.
Introduction
The goal of percutaneous coronary interventions (PCI) in the treatment of AMI (acute myocardial infarction) is not only the restoration of coronary flow to TIMI 3 (thrombolysis in myocardial infarction) flow grade, which is the conventional angiographic gold standard for reperfusion, but also the achievement of optimal myocardial tissue perfusion which is a more reliable predictor of long-term outcome1. PCI in lesions which contain thrombus, such as in primary PCI, are associated with an increased risk of acute complications. This is thought to be due to dislodgement of thrombotic material causing distal embolisation leading to slow flow or even no-reflow, characterised by inadequate flow at tissue level despite patent coronary arteries (TIMI 3 flow). Patients with no-reflow have larger infarct sizes, more significant left ventricular dysfunction, a greater risk of cardiac death and non-fatal cardiac events2,3. For example, in patients with TIMI 3 flow, myocardial blush grade (MBG), an angiographic marker of myocardial perfusion, is a good predictor of short-term mortality, MACE, infarct size and residual left ventricular ejection fraction4. De Luca et al5 demonstrated a linear relationship between infarct size and a number of markers of reperfusion including MBG in STEMI (ST elevation myocardial infarction) patients who underwent PCI and had TIMI 3 flow post-procedure. ST resolution during coronary intervention is also thought to be an important marker of myocardial reperfusion, and many studies have demonstrated that in STEMI patients with TIMI 3 flow following PCI, ST resolution is a good predictor of infarct size, LV systolic function and mortality6,7. No-reflow has been demonstrated to be an independent predictor of long-term mortality post PCI8. Thrombus containing lesions are associated with an up to seven-fold increase in periprocedural myocardial infarction, requirement for emergency CABG (coronary artery bypass grafting) and death compared to lesions without thrombus9,10. The presence of thrombus is not always visible on angiography and only 1/3 of thrombus observed using angioscopy in patients undergoing PCI was seen on angiography11. Consequently the potential for adverse outcomes in PCI due to the presence of thrombus and distal embolisation is often underestimated. Distal embolisation may occur in up to 15% of patients in primary PCI12 and is associated with a significantly increased five year mortality of 44% compared to 9% in those patients without distal embolisation. With such an increase in morbidity and mortality through distal embolisation of thrombotic material during primary PCI, there has been a keen interest in reducing the burden of thrombus and distal embolisation during these procedures. New devices to remove thrombus (thrombectomy devices) and to prevent embolisation of thrombus and plaque during PCI (embolic protection devices) are becoming widely available and used in clinical practice. We have therefore reviewed the use of mechanical thrombectomy and embolic protection devices and the current literature regarding their use. An overview of available devices is presented in Table 1 and their use is discussed more fully in the subsequent sections.
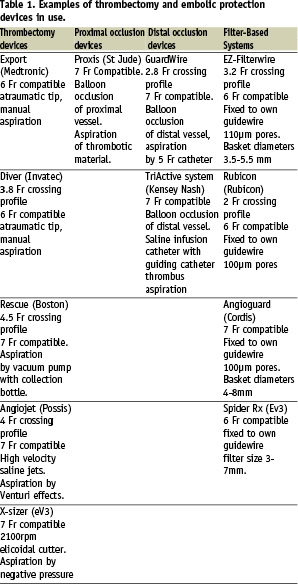
Thrombectomy devices
Thrombectomy devices are used to remove thrombus from coronary arteries. There are several thrombectomy devices available currently and these can be further subdivided into simple devices such as aspiration thrombectomy catheters, complex mechanical devices such as rheolytic thrombectomy devices, X-Sizer devices and finally laser devices.
Simple devices
Aspiration thrombectomy catheters
Aspiration thrombectomy catheters are simple devices which function to aspirate intra-coronary thrombus. There are several commercially available catheters for this purpose including Export Catheter (Medtronic, Minneapolis, MN, USA), Pronto Extraction Catheter, Diver CE aspiration catheter (Invatec, Roncadelle, Italy), QuickCat (Kensey Nash, Exton, PA, USA), Rio (Boston Scientific, Natick, MA, USA) and Fetch (Possis, Minneapolis, MN, USA). A number of trials using these aspiration thrombectomy catheters in the setting of ST elevation AMI have been published recently. In the REMEDIA trial13, in which patients undergoing primary PCI were randomised to either standard PCI or PCI plus thrombectomy using the Diver CE catheter, significantly more patients in thrombectomy arm had myocardial blush grade > 2 and ST segment resolution > 70% than those in control. Similar results have been observed in the DEAR-MI trial using Pronto catheter14. In this study only 3% no-reflow was observed in the Pronto catheter arm compared to 15% in the PCI control group arm and a significantly decreased cardiac enzyme rise post procedure. Furthermore, a significantly increased rate of direct stenting in the thrombectomy device arm was observed (70% vs 24%). Longer term studies15 in which STEMI patients were followed over six months following PCI demonstrated that there was significantly less LV dilatation in the Diver CE thrombectomy group than in the stand alone PCI group (19% vs 5%), although there was no difference in ejection fraction. This data would suggest that use of thrombectomy devices in primary PCI may be associated with a lower incidence of LV remodelling, which is a precursor of congestive cardiac failure and results in significantly worse prognosis post STEMI16. Several other randomised trials have been performed (Table 2) using aspiration thrombectomy catheters in the setting of STEMI, and in the majority of these trials a significantly greater ST segment resolution rate and myocardial blush scores >3 was observed in patients where thrombectomy devices were used.
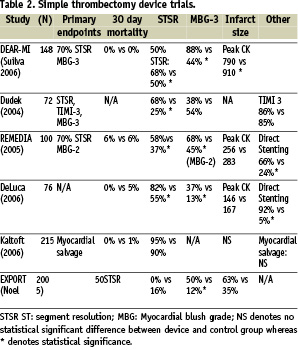
In some of these trials, significant improvements in direct stenting rates were observed13,15. There were no significant changes in 30 day mortality rates observed, although sample sizes in these trials were relatively small, and mortality in the control PCI groups seemed to be low as high risk PCI patients tended to be excluded from these studies.
Simple devices have a number ofQ advantages over more complex thrombectomy devices in that they are, as the name suggests, simple to use with little or no learning curve. Furthermore, use of simple thrombectomy devices does not significantly increase procedural time14 which is particularly important when time is of the essence in primary PCI. In contrast in the AIMI trial17 in which the complex angiojet thrombectomy device was used, the total procedure time was significantly longer in the thrombectomy device arm (76 minutes) compared to control (60 minutes). The potential disadvantages of simple thrombectomy devices include the non-negligible risk of distal delivery of the device for distal thrombus containing lesions, and the potential for dissection/perforation of the vessel. However, such a risk is relatively small as no such complications were described in either the REMEDIA13 or DEAR-MI14 trials. In situations where there is a large thrombus burden, it is possible that these simple thrombectomy devices are not as effective, which is why some advocate use of more ‘complex’ thrombectomy devices.
Mechanical devices
Rheolytic thrombectomy devices
Rheolytic thrombectomy devices such as the angiojet thrombectomy system (Possis Medical, Minneapolis, MN, USA) is a 4 Fr catheter system which is connected to a driver unit whose role is to generate high velocity saline jets at its distal end directed towards the proximal end which results in a central low pressure zone with a vacuum effect which draws debris into the catheter. These high velocity jets result in fragmentation of the thrombus which is then aspirated by the Venturi-Bernoulli effect into the catheter. Antoniuicci et al18 studied the effects of the angiojet device in 100 STEMI patients where significant improvements in ST resolution rates (90% vs 72%, P=0.02), TIMI frame count and smaller infarct size were observed in the thrombectomy group. In contrast, in the AIMI trial17 in which 480 patients with STEMI (but presence of thrombus on angiography not an entry requirement) were randomised to either angiojet thrombectomy and PCI or PCI alone demonstrated a significant increase in infarct size in the thrombectomy group, and no significant changes in either ST resolution or myocardial blush scores. Furthermore, the 30 day MACE score was significantly greater in the thrombectomy group. A number of points have been raised regarding this trial however, for example, there was a lower baseline TIMI flow grade, a lower rate of anterior MI and a far greater use of temporary pacing (58% vs 19%) in the thrombectomy group which may have contributed to the worse outcome. Of note, Antoniucci and colleagues19 have published the protocol for the JETSTENT multicentre trial in which 500 patients with STEMI and visible thrombus or a totally occluded vessel will be enrolled and angiojet device thrombectomy with PCI will be compared to PCI alone in this patient cohort. No restriction on clinical status or high risk coronary anatomy will be used – hence the population cohort in this study will reflect a more ‘real life’ cohort. Interestingly, in contrast to the AIMI trial, the Multi-Centre STENT registry where angiojet was utilised in 4% of procedures involving 9,707 patients, there was no significant differences in mortality at nine months compared with those patients with no thrombectomy, despite the higher clinical risk profile of the angiojet patient cohort due to a higher percentage of patients with cardiogenic shock and larger thrombus20. Similarly in the Florence Appraisal Study of Rheolytic Thrombectomy (FAST) in which the angiojet device was employed in 116 consecutive patients with AMI and extensive thrombus visible on angiography, angiojet use was associated with a significant improvement in reperfusion parameters when compared with a control population with similar thrombus burden21. Furthermore, in hospital MACE was relatively uncommon with around 8% quoted in this series. Further randomised controlled trials such as the forthcoming JETSTENT are required to clarify the role and safety of devices such as the angiojet device.
X-Sizer
The X-sizer thrombectomy catheter (Ev3, Plymouth, MN, USA) has an elicoidal cutter positioned at the distal end of the catheter which rotates at 2,100 rpm. Advancing the catheter results in fragmentation of the thrombus by the elicoidal cutter which is aspirated by means of continuous negative pressure maintained by the system. Napodano et al22 randomised 92 STEMI patients in a single centre trial to X-sizer thrombectomy and PCI or PCI alone. X-sizer use was associated with less distal embolisation and no-flow, better ST segment resolution (83vs 52%) and superior TIMI myocardial blush grade (72% vs 37%). The larger X-amine multicentre trial23 involving 201 STEMI patients with initial TIMI 0-1 flow demonstrated that ST segment resolution (68% vs 53%), distal embolisation occurrence (2% vs 10%) and angiographic composite endpoint of slow-flow, no-reflow, or distal embolisation (6.0% vs 19.8%) were significantly improved in the X-sizer device arm. Six-month mortality and MACE rates were however not significantly different between the two treatment groups. In this trial, no complications such as coronary perforation/dissection were noted with use of the X-sizer device, although one coronary artery-venous fistula was noted on follow-up angiography. In the X-Tract study24 in which 797 patients from 60 centres in the US and Canada with diseased saphenous vein grafts (SVGs) or thrombi containing native coronary arteries were randomised to PCI and thrombectomy with the X-sizer device or PCI alone. There was no significant difference in outcome between thrombectomy and control group in 30 day (16.8% vs 17.1%) and at one year for (31.3% vs 28.2%) major cardiac events (cardiac death, MI or target lesion revascularisation). Of note, only 70% of lesions contained thrombus in the thrombectomy group angiographically and 58% in the control group, and the majority of patients in both groups >70% had TIMI 3 flow at the start of the procedure. Interestingly, subgroup analysis of patients who had thrombotic lesions in this study demonstrated that the X-sizer device significantly reduced the occurrence of death or large MI by 30 days by 53% (4.7% vs 9.9%, P<0.04). Complication rates were not significantly different between the two groups, with dissection of coronary artery being reported at 1.2% in X-sizer device group and 0.2% in the control group (P=0.12). Newer devices, such as the ThromCat catheter (Kensey Nash, Exton, PA, USA), work on a similar principle although the helical cutter rotates at 95,000 rpm and the helix is enclosed within the catheter for no direct vessel wall contact. Preliminary data would suggest that this device is safe and effective in the setting of both acute and elective PCI, although further data in the form of randomised controlled trials are required to clarify the role of this device in STEMI.
Laser devices
Experience with laser therapy has been largely based on observational data arising from specialised centres. Topaz et al25 studied 59 patients with unstable angina (UAP) and AMI who underwent laser coronary angioplasty where all patients received adjunct balloon dilation followed by stent. A 96% laser induced reduction of thrombus burden area was achieved in the AMI group, whilst 97% was achieved in the UAP group. There were no significant major complications, perforations or major dissection in either patient group. In a recent small randomised trial 26 where 27 consecutive STEMI patients were randomised to either PCI or PCI and adjunct excimer laser coronary angioplasty (ECLA), target vessel diameter stenosis, TIMI flow, TIMI frame count and myocardial blush score did not differ between the groups. The authors argued that ECLA is feasible and safe for the treatment of patients with STEMI, and that results of procedure were at least on par with conventional treatment. Clearly these are small trials and the role of laser devices in STEMI remains uncertain.
Meta-analysis data and the role of thrombectomy devices
A number of meta-analyses27-29 have been published investigating the role of thrombectomy devices on angiographic and clinical outcomes in primary and rescue PCI, although in all of these meta-analyses the thrombectomy devices were not subdivided into simple or complex devices (Table 3).
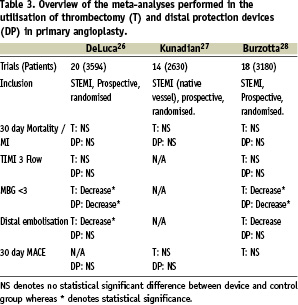
In the meta-analysis of Burzotta et al29, the use of thrombectomy devices was associated with almost a significant reduction in the rate of occurrence of angiographically evident distal embolisation, the rate of MBG<3 and failure to achieve ST resolution. However, neither MACE, death or early MI were significantly altered through use of thrombectomy devices. Similarly, no benefit in 30-day mortality, re-infarction rates or MACE was observed in meta-analysis performed by Kunadian et al28 following use of thrombectomy devices. In the meta-analysis of De Luca et al27, MBG 3 rates post-procedure and distal embolisation were significantly improved with adjunct thrombectomy although, as in the other meta-analyses, 30 day mortality was not significantly improved.
A number of studies have demonstrated that MBG, corrected TIMI frame count and persistent ST elevation are predictors of mortality and MACE post-revascularisation following myocardial infarction30. Given that these recent meta-analyses have shown that the parameters of myocardial perfusion such as MBG, ST segment resolution, etc., significantly improve post-mechanical thrombectomy, it is rather surprising that no mortality/MACE benefits are observed. Patients included in these trials are often not the most high risk patients for PCI and have a very low mortality rate, so observation of any further potential reduction in such a low mortality would require far larger studies. Thrombectomy devices have been observed to have beneficial effects on left ventricular remodelling14, but for benefits in mortality to be observed longer term follow-up of patients would be required – however the majority of these studies have only studied mortality at 30 days. Mechanical devices can themselves induce distal embolisation when crossing the thrombotic lesions, evidence of this can be seen in the increased infarct size in the AIMI study17,31. Furthermore, distal embolisation is not the only determinant of infarct size and poor reperfusion, other mechanisms such as myocardial oedema, lymphocyte obstruction of microcirculation and myocardial injury after reperfusion are thought to have an important role in low flow and no-reflow32. Clearly, thrombectomy devices would not be expected to influence myocardial perfusion mediated through these mechanisms.
Furthermore, the selection of patients that require adjunctive thrombectomy techniques is of paramount importance. Not all the trials which have used mechanical thrombectomy devices have required patients to have angiographically visible thrombus on angiography; for example, in the AIMI trial17, 15-20% of patients included had either no thrombus or only “possible” thrombus. Certainly, one may expect that the patients with the greatest intracoronary thrombus burden may achieve the greatest benefit from the use of thrombectomy devices.
Embolic protection devices
There are a number of embolic protection devices which are characterised according to their mechanism of action including distal occlusion, distal filter and proximal occlusion. The role of these devices is to prevent the distal embolisation of material from the target lesion into the coronary microcirculation.
Distal occlusion devices
Distal occlusion devices work by occluding the vessel distal to the PCI target lesion, thereby preventing distal embolisation of debris and clot during the angioplasty procedure. At the end of the procedure, the stagnant column of blood contained between the target lesion and the distal occlusion device can be aspirated prior to the distal occlusion being relieved and so preventing the distal embolisation of debris. These systems include a guidewire which contains a distal inflatable occlusive balloon passed distal to the lesion and inflated to prevent antegrade flow. Intervention is performed over this wire, and any liberated debris released during the intervention is aspirated using an aspiration catheter system (see Figure 1).
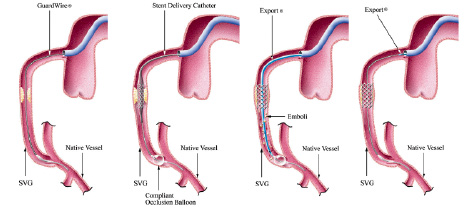
A
B
C
D
Figure 1. GuardWire distal occlusion device. Panel A illustrates how the guidewire is used to pass the lesion and the device is positioned distal to the lesion. Panel B shows the balloon as it is inflated and occludes the distal vessel and the intervention at the site where the target lesion begins. Panel C illustrates an export catheter being used to aspirate the column of blood and debris between target lesion and distal balloon. Panel D illustrates the distal occluding balloon being deflated and removed from the vessel.
Distal occlusion devices have the advantage of being able to trap both large and small particulate matter in addition to soluble mediators, in contrast to filter devices which may allow both small particles and soluble mediators to pass through them. Examples of this system include GuardWire (Medtronic, Minneapolis, MN, USA) and TriActive system (Kensey Nash, Exton, PA, USA). Studies using these devices have been performed in angioplasty trials in both native coronary arteries as well as saphenous vein grafts. In the multicentre randomised controlled EMERALD trial33, a total of 501 patients were randomised to distal occlusion with GuardWire (n=252) or conventional angioplasty (n=249) in patients undergoing primary PCI. No significant differences between ST resolution rates, infarct size measured by technetium Tc 99m sestamibi or MACE at six months was observed between control groups and distal protection groups. No significant differences were observed in intraprocedural complications in the distal protection and control groups, including sustained ventricular tachycardia or fibrillation (7.9% vs 6.8%) respectively and heart block or bradycardia requiring treatment (18.3% vs 20.5%) respectively. Emergency coronary artery bypass graft surgery was required in no distal protection patients and in two control patients. There were no intraprocedural deaths or strokes. Similarly, in the prospective randomised multicentre MICADO study, no significant differences in TIMI perfusion grade, incidence of no reflow or MACE were observed34. In contrast, studies using distal protection devices in saphenous vein grafts (SVGs) have been more optimistic35,36. In the multicentre SAFER trial (Saphenous Vein Graft Angioplasty Free of Emboli Randomised)36, 801 patients were randomised to either routine angioplasty with a guidewire or use of a distal protection device. A significant improvement in MACE was observed using distal protection driven primarily through a reduction in myocardial infarction and no-reflow phenomenon. Indeed, even in low MACE risk procedures, there was still a significant benefit in 30 day MACE through the use of GuardWire distal protection devices36. There are a number of possible explanations for why distal protection does not appear to be beneficial in the primary PCI setting in native vessels, even though there are significant benefits observed using distal protection in SVG. Firstly, distal protection would not protect from embolisation down side-branches between the lesion and distal protection device. SVG’s do not have side-branches and hence this would not be a problem. Furthermore, in the SAFER trial35, one of the exclusion criterion was myocardial infraction, and over 85% of patients included in this trial had TIMI 3 flow and 1.5% TIMI 0,1 at the start of the procedure. In contrast, in primary PCI trials such as EMERALD33, over 65% of patients had TIMI 0,1 flow and so positioning of the distal protection device distal to potentially thrombogenic lesions could result in distal embolisation by itself.
Distal filter
Distal filters are non-occlusive protection devices which are positioned distal to the target lesion in order to filter macro debris embolisation to the distal myocardium, although small molecules and humoral mediators are able to pass through (See Figure 2).
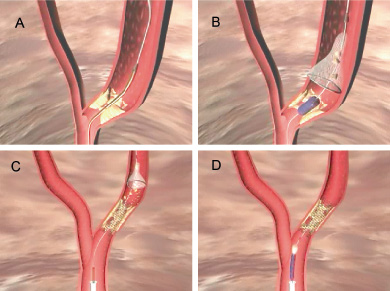
Figure 2. Panel A shows the distal filter, mounted on a 0.014” guidewire and constrained by a delivery sheath being placed distal to the lesion. Retraction of the sheath releases the microporous filter, which allows uninterrupted antegrade blood flow. PCA and stenting is then performed over the guidewire (Panel B). The forward blood flow drives the liberalised particulate debris into the filter, where the larger particles are caught (Panel C). A sheath is then passed over the wire, across the stent and then used to close the filter, removing the captured debris (Panel D).
They have the advantage over distal occlusion devices in that they preserve antegrade flow down the vessel, and so ischaemia during prolonged occlusion of a vessel is not problematic and the operator is still able to perform an angiogram whilst using this device. Disadvantages of distal filter systems include relatively large diameter sheaths (approx 0.040 to 0.050 inches) required to keep the filters in a collapsed state during advancement across the lesion, with the potential dislodgement of debris. Furthermore, there is the potential for small molecules of debris to pass through the filter pores as well as between an incompletely opposed filter support ring and vessel wall. Finally, distal filter devices are unable to remove humoural mediators which can pass downstream to the distal myocardium through the filter pores. Smaller sized 100 µm embolic particles are tolerated in far larger number before interfering with micro-circulatory function than are larger particles37, consequently smaller particles are thus less likely to cause no reflow.
Examples of this system include EZ-FilterWire (EPI, Boston Scientific, Natick, MA, USA), Spider and Microvena Trap (Ev3, Minneapolis, MN, USA) and Interceptor (AVE, Medtronic, Minneapolis, MN, USA). In the multicentre Protection Devices in PCI Treatment of Myocardial Infarction for Salvage of Endangered Myocardium (PROMISE) trial38, 200 patients with chest pain and either ST segment, elevated cardiac markers or angiographic evidence of thrombotic occlusion were randomised to either filter wire distal protection or routine angioplasty. No significant improvements were observed in 30 day mortality, infarct size or maximal adenosine flow induced velocity. Similar observations have been recorded with the SPIDER Rx filter device which was evaluated in a randomised trial of 140 primary PCI patients (PREMIAR study39). Use of the SPIDER Rx filter was not associated with any improvement in ST segment resolution, myocardial blush, LV ejection fraction or six months MACE.
Analogous to the situation regarding distal occlusion devices, distal filter systems have been shown to be efficacious in SVG interventions in non-inferiority trials with distal occlusion devices (SPIDER trial40, FIRE trial41, PRIDE study42).
Proximal occlusion
Proximal occlusion devices work by occluding the vessel proximal to the target lesion, in very much the same way that distal protection device occlude the vessel distally. These proximal occlusion devices have an advantage over the distal occlusion devices in that they do not require distal landing zones and they do not have the problem of difficulty of placement of the device in the distal vessel because of vessel tortuosity etc. Furthermore proximal occlusion devices do not have the problem of inadequate protection of large side branches as in distal devices, where embolic material proximal from the target lesion can pass down large side branches located in the vessel wall proximally to the distal protection device. In addition proximal protection devices do not need to cross the target lesion like distal protection devices and so do not have the risk of causing distal embolisation during their deployment. The main limitation of proximal protection devices is that in very proximal target lesions, use of the device is limited because of problems with the proximal landing zone. Examples of proximal occlusion devices include Proxis embolic protection system (St Jude Medical, St Paul, MN, USA), Kerberos Rinspirator / Protection device (Kerberos Proximal Solutions, Cupertino, CA, USA) and Parodi Anti-emboliSation device (ArteriA Medical Devices, Inc., San Francisco, CA, USA).
The most studied proximal protection device is the Proxis device (St Jude Medical) and the Feasibility And Safety Trial for its embolic protection device during transluminal intervention in coronary vessels: a European Registry (FASTER) demonstrated that the Proxis device was safe to use during SVG and native coronary artery interventions to capture embolic material43. Furthermore, the multicentre PROXIMAL trial in which Proxis device catheters were compared to distal protection devices in patients undergoing PCI in saphenous vein grafts demonstrated demonstrated non inferiority in 30 day death, myocardial infarction and target vessel revascularisation44. However there are no multicentre randomised control trials investigating the role of the Proxis device in primary PCI, although the ongoing PREPARE study (PRoximal Embolic Protection in AMI Resolution of ST segment Elevation) aims to address this issue. Single centre registry data during primary PCI of 172 patients using Proxis proximal protection has shown use of this device to be feasible and safe in primary PCI with debris collected from 77% of cases, TIMI 3 flow in 96% of patients and complete ST resolution at 60 minutes close to 80% with no significant complications and 30 day MACCE rates of 4%45.
Meta-analysis data and the role of distal protection devices
A number of meta-analyses have been performed investigating the role of distal protection devices in primary PCI27-29 (Table 3). In the meta-analysis of De Luca et al27 in which seven trials were analysed involving 1,362 patients, no significant benefit in 30 day mortality, TIMI 3 flow, or distal embolisation was observed with the use of distal protection devices, although an improvement in the proportion of patients with MBG3 was seen. Similarly, the meta-analysis of Kunadian et al28, did not show any benefit of distal protection devices on either mortality, re-infarction/reintervention or 30-days MACE in primary PCI. Similarly, in the meta-analysis of Burzotta et al29 in 970 primary PCI patients, angiographic distal embolisation, TIMI 3, failure to achieve STR, MACE and death and MI outcomes were not significantly different in the distal protection and control group. Interestingly, as in the meta-analysis of De Luca et al27, myocardial blush scores where significantly improved using distal protection compared to control. Certainly the role of distal protection devices is uncertain in primary PCI with most trials showing little if any benefit at all, and they remain as yet unlicensed by the FDA for use in primary PCI. There does appear to be good evidence for the use of distal protection devices in SVG interventions, with trials such as the SAFER trial35 showing significant benefits in MACE scores driven primarily through a reduction in myocardial infarction and no-reflow.
Conclusion
In conclusion, distal embolisation and slow/no-reflow during percutaneous coronary angioplasty is associated with a significant increase in morbidity and mortality post procedure. Achievement of the angiographic gold standard TIMI 3 is not sufficient during PCI procedures for attaining an optimal prognostic benefit as there is still a poor prognosis for patients with evidence of inadequate flow at the tissue level, despite patent coronary arteries. The use of thrombectomy devices in a number of randomised and multicentre trials in patients undergoing PCI during STEMI is associated with a significant benefit in MBG, ST segment resolution, improvement of distal embolisation and longer term adverse left ventricular remodelling. As yet, no benefits in mortality have been observed although this may be related to the measurement of only 30 days mortality in the majority of these studies. Much of this data comes out of small studies which may be statistically underpowered to detect small but significant differences between the two treatment strategies. Further, larger studies are required to assess the role of these devices in a more representative and higher risk cohort of STEMI than used in many existing studies, and identification of the sub-group of patients who will derive the most benefit from the use of these thrombectomy devices. It appears that most of the data regarding a beneficial effect of thrombectomy devices is derived from studies using simple thrombectomy devices such as the Export Catheter (Medtronic, Minneapolis, MN, USA), Pronto Extraction Catheter, Diver CE aspiration catheter (Invatec, Roncadelle, Italy), QuickCat (Kensey Nash, Exton, PA, USA), Rio (Boston Scientific, Natick, MA, USA) and Fetch (Possis, Minneapolis, MN, USA) and indeed some trials using more complex thrombectomy devices (such as Angiojet) have shown a paradoxical increase in complications associated with their use (AIMI trial17). There does not appear to be strong evidence for the use of embolic protection devices and distal filter devices in the setting of primary PCI, although good trial data exists which would make a strong case for their use in SVG interventions, such as in the SAFER trial35 SPIDER trial40, FIRE trial41 and PRIDE study42.

