Abstract
Realisation that the heart is not a post-mitotic organ has resulted in great interest in the field of myocardial stem cell therapy. In animal and early phase human infarct studies, the use of cellular therapy was associated with improvement in markers of myocardial function. More recent randomised studies have shown conflicting results which may, in part, be related to differences in trial design. Despite these discrepancies, this emerging technique has seen rapid translation into clinical trials, but has left an ongoing requirement for further research into the mechanisms of any effect observed. In this regard, the long term fate of administered cells is of critical importance. This review outlines the numerous non-invasive imaging modalities used in cell tracking. In particular, it highlights how magnetic resonance imaging is ideally suited to longitudinally track the migration and persistence of appropriately labelled cells.
Introduction
Progenitor stem cell transplantation is being evaluated as a potential adjunctive therapy to regenerate infarcted myocardium1,2. Preclinical studies have confirmed the beneficial effect of stem cell therapy in preserving ventricular function, reducing infarct size and scar formation and improving tissue perfusion. These early studies have been translated into a rapidly increasing series of clinical studies assessing the feasibility, safety and efficacy of cellular transplantation in patients with cell-death-related heart disease3-22. This rapid translation from bench to bedside has unfortunately left many unresolved issues in almost all aspects of myocardial stem cell therapy23-30. For example, the optimal cell type, minimum (and maximum) cell number to achieve effect, method of administration, timing of administration following ischaemic insult and timing and method of assessing effect all remain unknown.
The ultimate distribution and survival of the transplanted stem cells and the method by which their beneficial effect is achieved also have to be elucidated. In an attempt to answer these questions, imaging will have a vital role to play. Magnetic resonance imaging (MRI) has excellent tissue resolution properties, in certain specialised circumstances can be used to guide cell delivery and is the method of choice to assess various aspects of myocardial functional improvement31,32. MRI is also an excellent modality to longitudinally track appropriately labelled cells. In this review we have attempted to summarise the use of MRI in tracking the migration, persistence and engraftment of progenitor stem cells.
The ‘Ideal’ Cell Label
For an imaging modality to gain widespread acceptance as a method of tracking cells, certain criteria must be met. The ideal cell label/imaging modality would have the following properties:
1 Non-invasive
2 High spatial and temporal resolution
3 Label persistence to allow repeated imaging
4 Safety of label - both to cells and recipient patient
5 Imaging modality is safe and acceptable to patients
Within the field of cardiac imaging there are numerous methods to assess various parameters of cardiac function. Unfortunately none of these clinically available investigations are currently able to visualise unlabelled transplanted cells. Indeed, there are currently no in vivo imaging modalities which are able to visualise transplanted cells without prior ex vivo modification of the cells. Consequently, a number of contrast agents and imaging detectors being developed to allow in vivo sequential non-invasive imaging of transplanted cells33.
Currently available imaging methods for cell tracking
Nuclear imaging
Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) have become widely accepted and validated techniques throughout medical imaging. Despite lower spatial resolution than other modalities such as MRI, lower background noise results in higher sensitivity with this technique and it has been used to investigate initial homing, distribution and extra-cardiac sequestration following cell delivery. Using these imaging techniques, intra-cardiac retention has been disappointingly low. Aicher et al34 found that in rats following intra-ventricular injection, 111indium-labelled human endothelial progenitor cells accumulated within the myocardium, but the intra-cardiac radioactivity was only 2.69% of the total measured dose. In the presence of myocardial infarction, this percentage significantly increased but was still only 4.7% of the injected dose. Immunohistochemistry confirmed the localisation of these cells predominantly within the infarct border zone. Notably, most activity was seen out with the heart, initially in the lungs and later in the liver, kidneys and spleen. Assessment of the effect of radio-labelling these cells with 111indium showed complete impairment of proliferation and differentiation which may impact on any effect observed35. Similar homing to the myocardium was witnessed with intravenous delivery of 111indium-labelled allogeneic myocardial stem cells (MSCs) in a canine model of MI36. In this study, the intracardiac MSCs were tracked out to one week following MI. Interestingly, the MSCs were also co-labelled with an MR contrast agent. Despite intracardiac activity seen on SPECT, no signal hypo-intensity (due to the iron label) was visible on MRI, presumably due to the lower sensitivity of MRI when compared with SPECT. In a similar model of MI, this time in swine, the same group was unable to demonstrate labelled MSCs within the myocardium due to significant lung uptake which obscured assessment of myocardial uptake37. Using the positron emission tomography (PET) tracer F18-fluorodeoxyglucose (F18-FDG), similar findings have been seen in humans. In a small substudy of the BOOST clinical trial22,38, unselected bone marrow mononuclear cells (BMNCs) were labelled with 18F-FDG and administered via either the infarct related artery or peripheral veins. Intracardiac activity was demonstrated following intracoronary delivery, but not after intravenous delivery. Only 1.3-2.6% of total activity was detected within the infarcted myocardium although, by immuno-magnetically selecting the CD34-positive cells; this was increased to 14% to 39% of total recorded radioactivity. More recently, another group, using Tc-99m exametazime (HMPAO) to label bone marrow mononuclear cells (MNCs), have demonstrated the dynamic nature of initial homing. Two hours following intracoronary delivery of these MNCs, myocardial radioactivity was recorded in all patients with recent MI and four of five patients with chronic MI. In the acute MI group, total myocardial activity accounted for 1.3% to 5.1% of total radioactivity. Twenty hours following injection, none of the chronic MI patients exhibited any residual myocardial activity, while three of five in the acute MI group had persistent activity39,40.
To calculate the minimum amount of cells which can be reliably counted using SPECT, Jin et al41 assessed the maximum dose of 111indium-oxine which did not affect cellular function, and then calculated the minimum detectable activity (MDA) of BM MNCs. In a human torso phantom (i.e. immobile and fixed) they found that for a 16 minute scan the MDA was 10,000 cells but this could be reduced to a MDA of 2,900 cells with a 64 minute scan. Interestingly, they found that in vitro cellular survival, proliferation and migration were affected at radiation doses in excess of 0.14Bq per cell. In previous studies, cellular doses of 15Bq per cell34, 30Bq per cell35 or even 42 Bq per cell37 were used with viability only measured for 2-4 days. This therefore raises the possibility that the poor intra-cardiac retention of cells seen in these studies may simply represent radionuclide-induced cellular death and leeching out of the radionuclide to the lymphoreticular system.
Thus, although giving an important insight into early homing kinetics, these studies have been somewhat limited by radioactive decay precluding long-term tracking, relatively poor spatial resolution and potential toxicity of the label itself. Further work must include proven safe doses of radionuclide, preferably with longer half-lives to enable longer tracking.
Reporter gene techniques
Fluorescent and/or genetic manipulation of cells can be undertaken with the subsequent gene expression imaged using SPECT, PET, or optical techniques. The introduction of reporter genes into cells has previously been used to image gene transfer into the myocardium42. This study demonstrated the ability to image rat cardiomyocytes, previously infected with adenovirus modified to carry the HSV1-sr39tk and luciferase reporter genes. They found that in vivo cell tracking using optical and micro-PET imaging techniques was possible, although leaking of the virus from the myocardium and subsequent hepatic transduction resulted in bright signal being seen in the liver. Similar techniques have been used to allow subsequent imaging using confocal fluorescent microscopy or fluorescent imaging43,44. Although not yet applied to the heart, labelling of cells with fluorescent semiconductor nanocrystals (or ‘qdots’) may allow in vivo imaging of labelled cells45 which can be visible for up to 4 months after transplantation46.
Although useful research tools, by their very nature these techniques either require sacrifice and explantation of tissue or are confined to use in small animal models. They have little current use in clinical settings.
Cellular magnetic resonance imaging
Basic physics of MRI
MRI relies on the nuclear properties of protons and, in particular, the charge, mass and spin of the proton. Given that water contains two protons (or hydrogen atoms) and the human body contains up to 70% water, there is obvious potential to utilise this vast number of protons. When placed in a magnetic field, these protons will align themselves along the main direction of the magnetic field (called Bo, or the ‘z’ axis). While spinning, the protons will ‘wobble’ along the Bo axis (a useful analogy is to think of a spinning top). Externally applied radiofrequency (RF) pulses, when applied to the protons, will cause deviation from the low energy alignment along Bo and the protons will naturally try to return to this low energy state, giving out energy as they do so. The spin-lattice or longitudinal relaxation time (also called T1) represents the exponential recovery of the proton spin to align with Bo. Spin-spin or transverse relaxation time (also called T2) is the loss of phase coherence or dephasing of the spins at an angle to Bo due to multiple interactions between local proton spin induced magnetic fields. T2* (or T2 star) represents the loss of this phase coherence of the proton spins in the Bo field and represents a combination of local magnetic field inhomogeneities and T2.
MRI contrast agents
In addition to intrinsic MRI tissue contrast due to magnetic relaxation, certain external agents can be introduced to improve contrast to noise ratio. Paramagnetism refers to the ability of certain metals (gadolinium, manganese and iron) to shorten nuclear magnetic resonance (NMR) relaxation times resulting in an enhancement, or brightening, on T1-weighted images. Preparations of these are commercially available and tend to shorten T1 relaxation time more than T2 and T2*. Contrast agents utilising iron oxide are termed supreparamagnetic and are due to sufficient unpaired spins exerting a net magnetic effect which exceeds the magnetism from unpaired electrons. In high concentrations, superparamagnetic iron oxide (SPIO) nanoparticles will shorten T1, T2 and T2* relaxation times of tissues. Although gadolinium chelates have been used to label and subsequently track endothelial progenitor cells in the mouse kidney, the required dose to image groups of cells is high and would likely alter cellular function and may even be lethal to them47,48.
The potential toxicity of paramagnetic agents has meant that their use in cell tracking has been largely superseded by SPIO, particularly in the field of myocardial stem cell tracking.
The use of SPIO agents in myocardial cellular imaging
TYPES OF SPIO
Although differences do exist between various SPIO and ultrasmall (U)SPIO nanoparticles, they have in common an iron oxide core and some form of coating which allows them to exist in aqueous suspension. Types of coatings include starch, dextran and polystyrene beads and care has to be taken with post mortem tissue fixation to ensure the capsule is not inadvertently dissolved out.
Dextran-coated SPIO nanoparticles such as ferumoxides (Feridex®, Berlex inc., Wayne, NJ, USA) or ferumoxtran-10 (Combidex®, Advanced Magnetics, inc., Cambridge, MA, USA) are approved for clinical use, particularly in hepatic and lymphatic imaging in malignancy. Currently, they are only approved for intravascular use and are not licensed for clinical intra-cellular labelling. Larger micron-sized SPIO particles conjugated with fluorescent polystyrene beads which also allow fluorescent microscopy (iron fluorophores – Bangs Particles, Bangs Laboratories, Fishers, IN, USA) are not available clinically but have been used experimentally.
ENTRY INTO CELLS
SPIO particles can be incorporated into cells by various processes including phagocytosis, pinocytosis, endocytosis (clathrin or caveolin-mediated, or independent). Some of these, such as clathrin-mediated endocytosis require specific receptor-ligand bonding to enable internalisation to occur49. More mechanical processes using a gene gun, which directly ‘fires’ SPIO particles into the cell, or electroparation, which opens up pores in the cell membrane by passing electrical current through it, are also available, but concerns exist regarding the long-term viability and function of cells following such treatment50,51. Although entry into cells can occur spontaneously, certain SPIO nanoparticles (e.g. ferumoxides) require the presence of a transfection agent to facilitate entry. Initially these transfection agents (TA) were commercially synthesised for this specific purpose but were not approved for clinical use52,53. The finding that a clinically available drug (protamine sulphate) also acts as a highly efficient transfection agent is increasing research with the ultimate aim of moving labelling into the clinical realm54.
Effect of intra-cellular iron on parameters of cellular function
With the realisation that radionuclide labelling may be adversely affecting cellular function, there has been justified concern regarding the effect large quantities of intracellular iron may have on these cells. Hinds et al55 demonstrated that micron sized iron-fluorophores could be readily taken up by mesenchymal stem cells and haematopoietic progenitor cells and did not affect viability, proliferative capacity or differentiation. However, Kostura et al56 discovered that labelling of MSCs with ferumoxides resulted in an inability in these labelled cells to differentiate into chondrocytes. This finding has not been repeated when using protamine sulphate as the TA and it is likely that this failure in chondrogenesis represented interaction between ferumoxide-PLL (the TA used in the initial study) thus inhibiting chondrogensis57,58.
Therefore, it would appear that these progenitor stem cells can be efficiently labelled with iron with no significant effect on normal cellular proliferative and differentiation ability.
Imaging stem cells using MRI
Using both MSCs and hematopoeitic stem cells, Hinds et al55 demonstrated that it was possible to label these with iron fluorophore Bangs particles and then image cells using MRI. These superparamagnetic divinyl benzene inert polymer microspheres are approximately 0.9 µm in size and contain both a magnetite iron oxide component and a fluorescent label. Although they used a high field strength magnet (11.7T) and scan time was >4 hours, the voxel sizes were sufficiently small that, using a T2*-weighted sequence, single cells could be imaged as a signal void which corresponded to microscopic cellular position59. The same group was then able to deliver, via direct intra-myocardial injection and under MRI fluoroscopic guidance, magnetically labelled MSCs to the infarct border region in swine and successfully track these cells within the myocardium for three weeks60,61. Following sacrifice, sections taken from regions where MRI hypo-intensities were seen contained the fluorescent label from the Bangs particles, indicating cellular retention at these sites. Although Kraitchman et al had previously demonstrated the success of SPECT but failure of MRI to detect dual (Feridex® and 111indium) labeled MSCs in the infarcted canine myocardium following peripheral intravenous delivery36, the same group went on to demonstrate that following MRI-guided, direct intramyocardial injection (with a high local concentration of cells) of Feridex® labeled MSCs into the canine infarct border region, the resultant signal hypo-intensity could be followed out to 8 weeks and that the cells left small hypo-intensity tracks extending into the infarct itself which presumably represents migration into the infarct itself62.
Although these pre-clinical studies have utilised direct intra-myocardial injection of cells, most of the clinical studies so far have employed intracoronary infusion of cells, usually via an over the wire percutaneous trans-arterial coronary angioplasty (PTCA) balloon catheter which is inflated to low pressure in the artery at the site of previous angioplasty, and the cells are slowly infused over a short period of time. By its very nature, this will result in a more diffuse pattern of cell distribution, with less concentration of cells, which may make tracking of these problematic. Baklanov et al demonstrated that, using a novel double contrast technique, Feridex® labelled autologous MSCs could be imaged immediately following infusion via either the infarct related artery or via retrograde infusion using the anterior intracardiac vein63. Again, using a T2*-weighted sequence, they found signal hypo-intensity within the anteroseptum which, when compared with infarct imaging following gadolinium-DTPA, corresponded to the infarct region itself. Post-mortem histological analysis using Prussian blue staining confirmed iron containing cells within this infarct region. We have recently demonstrated that, in a porcine MI model, iron-fluorophore (Bangs particles) labelled endothelial progenitor cells, when infused down the infarct related artery, are visible as a signal hypo-intensity but, in contrast to Baklanov’s findings, the cell signal hypo-intensity is initially located around the outside of the infarct, in the infarct border region64. Although Aicher et al reported similar peri-infarct homing features in humans34, we believe that our findings relate to the more severe myocardial injury resulting from our longer balloon inflation which we have shown invariably results in intra-myocardial haemorrhage and microvascular obstruction65. Interestingly, serial imaging of our animals reveals that, at six weeks following cell delivery, the cell signal hypo-intensity appears more within the infarct region itself, which may indicate migration of the cells into the infarction (Figure 1).
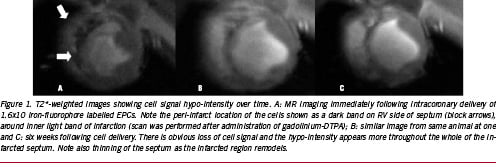
The above studies have invariably utilised either steady-state free-precession or T2* sequences to image the cell signal as a hypo-intensity. In tissues with short T2 and T2* relaxation times, it may be difficult to determine whether iron-labelled cells are present. In an attempt to improve tracking of iron-labelled cells, several investigators have attempted to image the cells by concentrating on the magnetic dipole associated with SPIO particles and display the images as a positive contrast or bright image. In phantom and in vitro experiments, Cunningham et al were able to excite and refocus water molecules surrounding iron-labelled cells and view this as a bright image66. In a rabbit model of hind-limb ischaemia, Gilson et al have demonstrated that positive contrast imaging of injected iron-labelled cells is possible and that, when compared with control animals injected with ferumoxide alone, the volume of positive contrast persisted for longer in animals receiving the ferumoxide labelled cells67.
With current techniques, it is extremely difficult to image labelled cells as a positive contrast in a moving image (i.e. the beating heart). The field is relatively young and it is likely that further advances will be made to enable positive contrast tracking of these cells.
Cellular retention and assessment of retained cell number
The earlier reports utilising SPECT/PET have demonstrated very low rates of intra-cardiac cellular retention in multiple mammalian species. The signal hypo-intensity seen when imaging iron labelled cells under MRI may be erroneously enlarged due to signal averaging within voxels, an effect which may be more pronounced with T2*-sequences (T2* ‘blooming’). Although this may allow small numbers of cells to be visualised, relating cell signal hypo-intensity to retained cell number is still under evaluation. Freyman and colleagues have shown highest long-term retention following direct intra-myocardial delivery (which was greater than intra-coronary arterial, which was greater than peripheral intravenous) but did not evaluate cell number. The relation of the injection site to the infarct zone also affects cell retention. In a dog model of MI, Soto et al demonstrated that, following intra-myocardial injection, most cells persisted following injection into the infarct region itself, followed by cells injected into the peri-infarct border region, with little retention of cells when injected into remote myocardium68. By comparing signal intensity ratios from infarcted:remote/normal myocardium, we have demonstrated that, following intra-coronary delivery of iron-fluorophore labelled EPCs, the relative signal hypo-intensity from the cellular label returns toward baseline over a six week period with the vast majority of this change occurring in the first week following cellular delivery64. Although the signal hypo-intensity does not reach baseline (indicating some iron label still persisting), this loss of hypo-intensity presumably relates to rapid loss of viable cells and subsequent removal of iron label by scavenger macrophages.
Since signal hypo-intensity does not accurately reflect retained cell number and, with the recent interest in positive contrast techniques, Foltz et al attempted to correlate injected cell number with MRI signal (both positive and negative contrast) area and found good correlation using positive contrast area and injected cell number (r2=0.68)69. Using two minute scans on a clinical 1.5T magnet, they were able to identify cells down to a minimum injected number of 25,000 cells.
Although promising, these recent advances are still at an early stage. Further work is necessary before MRI can be widely accepted as a robust method of assessing retained cell number.
Cellular fate
Obvious concerns when imaging cells using non-invasive methods, are whether the images obtained actually reflect living, viable cells in vivo and, if they do reflect viable cells, whether these cells have retained original phenotype or have altered somehow (by either transdifferentiation or fusion). Radionuclide and iron-oxide labels will still be visible following cellular death, although as phagocytic cells remove them to the reticuloendothelial system, the signal intensity will drop. The benefits of MRI in long term tracking are thus evident.
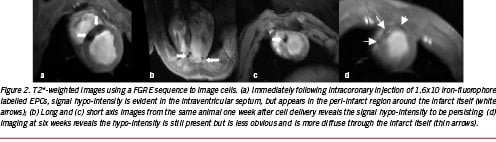
Kraitchman et al demonstrated that one week following intramyocardial injection, ferumoxide-labeled MSCs persisted within areas of infarction70. Over a longer time course, Hill et al demonstrated that three weeks following injection of iron-fluorophore labeled MSCs, the cells remained intact although their appearance had elongated61. DAPI staining and histological assessment was used to ascertain cell viability. We have recently demonstrated that, in a pig infarct model, following intra-coronary infusion of iron-fluorophore labelled EPCs, at six weeks following cell delivery, viable iron-containing cells were visible in the infarct core region (Figure 2). Immunohistochemical analysis using macrophage specific antibodies demonstrated that these iron containing cells were not macrophages and, most likely, represent persistence and engraftment of the original cells64.
The paucity of adequate antigens to cell surface markers in many of these large animal models has meant that it has not been possible to definitively ascertain the ultimate fate of injected cells. These studies would suggest that the persisting viable cells are not simply macrophages which have phagocytosed iron and most likely represent a small fraction of the originally injected cells.
Future work
The use of MRI in stem cell therapy is continually changing. The gradual introduction of higher field strength magnets will see increase in their use in this regard, although the problems encountered with susceptibility remain to be fully resolved. Efforts to improve immediate cell survival are ongoing and involve attempts to encapsulate cells in their own micro-environment in an attempt to improve survival rates during actual injection. One additional added benefit of this technique is that the capsules themselves may act as another source of MRI contrast and may allow us to non-invasively track cells without prior iron labelling.
Conclusions
There has been much recent interest in the subject of myocardial regeneration. The rapid translation from basic science to clinical trials has left many unanswered questions, of which long term migration, survival and engraftment of cells is of critical importance. There are issues with all current imaging modalities which make them less than ideal in this regard. We believe that MRI fulfils all necessary criteria to be successful in cell tracking: constant refinements and improvements in pulse sequence design has improved the tempero-spatial resolution of MRI, the cellular labels used are non-toxic to both cell and patient and persist for many weeks and the imaging itself is safe and involves no ionising radiation.
The fact that MRI can be used to characterise which patients require stem cell therapy, in certain cases can be used to guide cell delivery and is excellent at assessing response to cell therapy are added bonuses which will ensure it is at the forefront of this emerging field for a long time to come.

