Abstract
Aims: Sirolimus- and paclitaxel- eluting stents (SES and PES respectively) have been shown to produce a sustained reduction in restenosis and repeat revascularisations as compared to bare-metal stents (BMS) up to four years. There is still limited data about the long-term safety and efficacy of DES in high-risk subgroups.
Methods and results: A total of 6,129 consecutive patients were treated during three sequential periods with BMS (n=2,428; January, 2000 to April, 2002), SES (n=866; April 2002 to February 2003) or PES (n=2,835; February 2003 to December 2005). A stratified analysis (including age, gender, diabetes, clinical presentation, treated vessel, multivessel disease, AHA lesion class, bifurcation, in-stent restenosis, average stent diameter <2.5 mm and total stented length <30 mm) was performed to evaluate possible heterogeneities in treatment effect. At four years, all-cause mortality was identical between the drug-eluting stent (DES) and BMS cohorts (13.5% vs. 13.4%, respectively; Adjusted HR 1.10, 95% CI 0.90 - 1.34) without evidence of heterogeneity in the high-risk patient subsets. Both DES significantly reduced the risk for target vessel revascularisation (TVR) as compared to BMS (TVR: 11.9% vs. 15.7% respectively; Adjusted HR 0.69, 95% CI 0.58 - 0.82) along with a reduced risk for post-operative MI (adjusted HR 0.75, 95% CI 0.57 - 0.98), but counterbalanced by a non-significantly higher risk for stent thrombosis (3.1% vs. 1.6%; adjusted HR 1.26, 95% CI 0.82 - 1.95). DES failed to show superiority to BMS in patients with acute myocardial infarction (TVR 10.5% vs. 9.2% respectively; Adjusted HR 1.26, 95% CI 0.82 - 1.93).
Conclusions: In a real world patient population, after four years, the overall use of DES was associated with similar all-cause mortality rates and a significantly reduced risk for post-operative MI and TVR as compared to BMS.
Introduction
While the superior anti-restenotic properties of drug-eluting stents (DES) have been extensively demonstrated, their impact on hard clinical endpoints like death and myocardial infarction (MI) was never appropriately studied. Although pivotal randomised controlled trials and meta-analyses demonstrated similar rates of death, post-operative MI and stent thrombosis in DES as compared to bare-metal stents (BMS), they were underpowered to detect meaningful differences in these hard clinical endpoints, particularly when the relative safety and efficacy were questioned in high-risk subgroups.1-4 Although the randomisation was a key feature, the main limiting factors in the pivotal randomised trials were the highly selected patient populations (approximately 40% of the daily clinical practice) and the use of angiographic primary endpoints in many of them. These constraints limited the ability to generalise the conclusions to an all-comer population and precluded proper subgroup analysis. Meta-analyses of randomised controlled trials, including trials in higher risk patients, using aggregate data rather than patient-level data, partially resolved the long-term safety concerns but were again unable to study high-risk subgroups.
Registries including higher risk patients have recently shown a consistent trend towards an increased rate of late stent thrombosis, but an improved survival rate when DES are used.5-9 However, the majority of the registries suffer from a severe selection bias due to concomitant non-randomised use of DES and BMS. Thereby, the “DES” cohorts in these studies were often a mixture of different types of DES (often mixed with BMS), and ignored the fact that there is a clear difference in the safety and efficacy of different types of DES.4,10-12
In the present study, we analysed the relative safety and efficacy of three sequential cohorts of all-comers (n=6.129) treated with either BMS, sirolimus- or paclitaxel-eluting stents (SES and PES respectively). In particular, we performed a stratified analysis to study the efficacy of both types of DES among high-risk patient subsets.
Methods
Study design and patient population
Between January 1, 2000 and December 31, 2005, a total of 7,217 percutaneous coronary interventions were performed in our institution using BMS, SES or PES. From January 2000 until April 16th 2002, 2,681 percutaneous coronary interventions were performed using exclusively bare metal stents, from April 16, 2002, until February 23, 2003, 1,035 interventions were performed using SES (Cypher®, Cordis Corp., Johnson & Johnson, Warren, NJ, USA), as part of RESEARCH registry13, and from February 23, 2003 to December 31, 2005, 3,339 interventions using PES (TAXUS™ Express2™ or Liberté™, Boston Scientific, Natick, MA, USA), as part of the T-SEARCH registry.14 Procedures in which two different types of stents (BMS and either SES or PES; SES and either BMS or PES; PES and either SES or BMS) were used were excluded (n=162).
Although a total of 784 patients underwent multiple procedures, only patients initially enrolled in one of the sequential cohorts (BMS, SES or PES group) were maintained for analytical purposes throughout the follow-up period in their original cohort, even if a repeat intervention was performed using a different type of stent. A total of 6,129 patients fulfilled these criteria. (Figure 1)
This study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
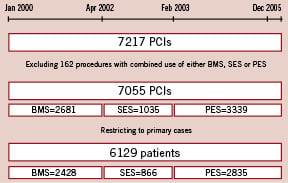
Figure 1. Flowchart depicting the number of procedures in the three sequential periods and the number of patients fulfilling the inclusion criteria in each cohort. Primary cases indicates the number of patients undergoing their first intervention in the study period (2000-2005). SES indicates sirolimus-eluting stent, BMS bare metal stent, PES paclitaxel-eluting stent, PCI percutaneous coronary intervention.
Procedures and post-intervention medications
All procedures were performed following previously defined current standard procedural guidelines.15 Baseline, clinical and procedural patient characteristics were prospectively entered into a dedicated database.
Patients were prescribed aspirin plus clopidogrel 75 mg/day (after a loading dose of 300 mg) before or during baseline coronary interventions. Patients treated with BMS received at least one month of clopidogrel (mean 2.4±2.3 months). Patients treated with SES, received at least three months of clopidogrel (mean 4.5±3.2 months), and patients treated with PES received at least six months of clopidogrel (mean 6.4±3.4 months). All patients were advised to remain on aspirin indefinitely.
Planned angiographic follow-up was performed in 12.0%, 25.9% and 14.3% in the BMS, SES and PES groups respectively.
Baseline definitions
Angina was categorised according to the Canadian Cardiovascular Society (CCS) classification for stable angina and according to the Braunwald classification for unstable angina.16,17 Hypertension was defined as a blood pressure >140 systolic or >90 mmHg diastolic or based on the current use of antihypertensive treatment. Dyslipidaemia was classified as a total serum cholesterol level >6.2 mmol/l or the use of lipid lowering drugs. Diabetes was defined as treatment with either an oral hypoglycaemic agent, insulin, or through diet. Complete procedural success was defined as the achievement of <50% diameter stenosis (visual assessment) and Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow in all lesions intended to treat. Clinical success was defined as procedural success without death or (re) infarction during the index hospitalisation.
Endpoint definitions and clinical follow-up
The primary safety endpoint was all-cause death and post-operative MI and the primary efficacy endpoint was target vessel revascularisation (TVR) at 4-years of follow-up. Secondary endpoints were the itemised outcome parameters: all-cause death, cardiac death and death from cancer, post-operative MI, TVR and stent thrombosis. Survival data for all patients were obtained from municipal civil registries on a yearly basis for each of the three patient cohorts. The most recent follow-up was performed in October 2007. Causes of death were obtained from the Central Bureau of Statistics, The Hague, The Netherlands. Causes of death were classified according to the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10).18 For the present analysis, death from ischaemic heart disease (I-20 - I-25), sudden cardiac death (I-46), sudden death undefined (R-96), or death from heart failure (I-50) were considered to be cardiac. Death from cancer was defined as any death from malignant neoplasms (C-00 - C-97). All the remaining deaths were classified as being due to other causes and no further distinctions were made. Follow-up was complete for 98.7% of the BMS patients, 100% of the SES patients and 98.4% of the PES patients. Target vessel revascularisation was defined as a re-intervention driven by any lesion located in the same epicardial vessel.19 Myocardial infarction at follow-up was diagnosed by a rise in creatine kinase-MB fraction (CK-MB) of three times the upper limit of normal, according to American Heart Association/American College of Cardiology guidelines.20 Stent thrombosis (ST) was defined as angiographically defined thrombosis with TIMI grade 0 or 1 flow or the presence of a flow limiting thrombus, accompanied by acute symptoms, irrespective of whether there had been an intervening reintervention.21 The timing of ST was categorised as early (within 30 days after implantation), late (between 30 days and 1 year) or very late (more than 1 year).22 Additionally, a difference was made between primary stent thrombosis (occurring directly after the index procedure) and secondary stent thrombosis (stent thrombosis occurring following a repeat target vessel revascularisation).
Statistical analysis
Continuous variables are presented as mean ±standard deviation. Categorical variables are expressed as percentages. Comparisons among the three groups were performed by the F-test from an analysis of variance for continuous variables and Pearson’s Chi-Square test for categorical variables. All statistical tests are 2-tailed. The incidence of events over time was studied with the use of the Kaplan-Meier method, whereas log-rank tests were applied to evaluate differences between the treatment groups. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. Cox proportional-hazards regression analyses were applied to further study treatment effects, adjusting for potential confounders listed in Table 1. The number of co-variables in the final model was limited to variables (p<0.10) in Cox multivariable regression, and variables considered clinically relevant for each specific endpoint. Final results are presented as adjusted hazard ratios with 95% confidence interval. Subsequent analyses were performed to evaluate possible heterogeneities in treatment effects on mortality and TVR according to the following clinically relevant characteristics: age, gender, diabetes, clinical presentation, treated vessel, multivessel disease, AHA lesion class, bifurcation, in-stent restenosis, average stent diameter≤2.5 mm and total stented length >30 mm. Treatment effects were evaluated with the use of Cox regressions that included a term for the interaction between each characteristic of interest and the assigned treatment, adjusted for the previously defined clinically relevant characteristics.
Given the differential follow-up in the three treatment cohorts, additional stepwise logistic regression analyses were performed on the 2-year endpoints of all-cause mortality and TVR to check whether these results were in line with the Cox proportional hazards regression analyses on the 4-year endpoints.
Results
Baseline and procedural characteristics
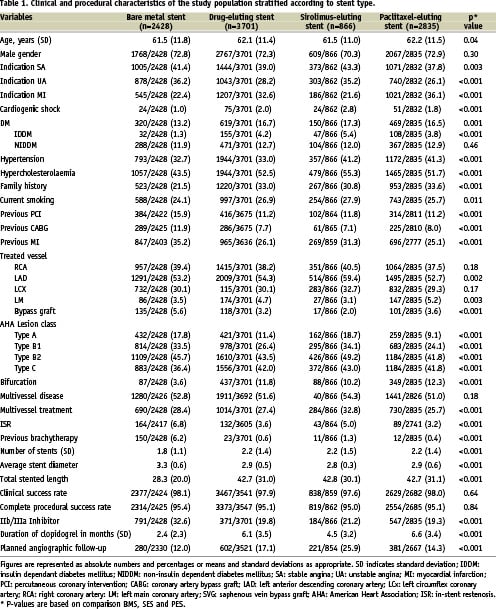
Both baseline and procedural characteristics are depicted in Table 1. Mean age increased slightly over time from 61.5±11.8 in the BMS group to 62.2±11.5 in the PES group (p=0.04). Treatment for acute MI increased from 22.4% in the BMS group to 36.1% in the PES group (P<0.001). Procedural complexity increased over time, illustrated by an increase in the treatment of type C lesions, bifurcations and left main stem lesions. Over time, total stented length and number of stents increased, while the average stent diameter decreased.
Clinical outcomes
At thirty days, the cumulative incidence of all-cause mortality was 3.5% in both the DES and BMS groups (adjusted HR 0.84, 95% CI 0.63 - 1.13). However, there was a trend towards a lower 30-day mortality rate in the SES group (2.2%) compared to the PES group (4.0%) (adjusted HR 0.73, 95% CI 0.44 - 1.22). At four years, the mortality rates in the DES and BMS group remained remarkably similar (13.5% vs. 13.4% respectively; adjusted HR 1.10, 95% CI 0.90 - 1.34); however, a trend remained towards a lower mortality rate in the SES group as compared to the PES group (11.2% vs. 14.0% respectively; adjusted HR 1.16, 95% CI 0.88 - 1.53) (Table 2, Figure 2).
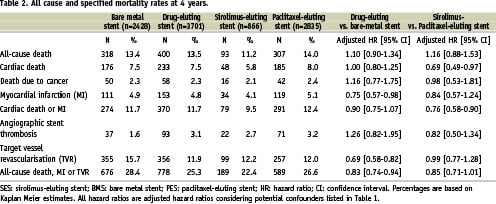
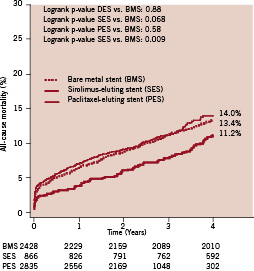
Figure 2. Kaplan Meier all-cause mortality curves for all patients receiving bare-metal stents (BMS), sirolimus-eluting stents (SES) or paclitaxel-eluting stents (PES).
The majority (57%) of all deaths were due to cardiac causes, 15% were due to cancer and 28% of the patients died of other causes. While cardiac mortality was similar in the overall DES group as compared with the BMS group, the cardiac mortality rate was significantly lower in the SES group as compared with the PES group (5.8% vs. 8.0% respectively, adjusted HR 0.69 95% CI 0.49 - 0.97). Death due to cancer occurred at a similar rate in both DES groups as in the BMS group (Table 2).
Although the cumulative incidence of post-operative MI was similar among the DES and BMS groups (4.8% vs. 4.9% respectively) adjusting for independent predictors resulted in a significantly lower risk for post-operative MI in the DES group (adjusted HR 0.75, 95% CI 0.57 - 0.98). No statistically significant differences were observed between the SES and PES group (adjusted HR 0.84, 95% CI 0.57 - 1.24). (Table 2)
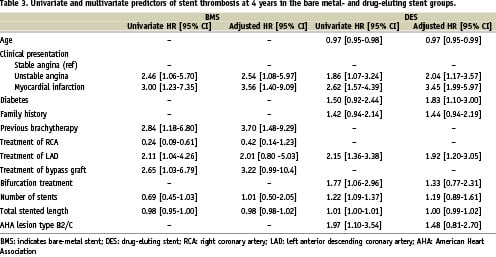
The cumulative incidence of angiographic stent thrombosis was significantly higher in the DES group as compared to the BMS group (3.1% vs. 1.6%; HR 1.80, 95% CI 1.23 - 2.64)(Figure 3). Cox multivariable regression analysis revealed that in the BMS group previous brachytherapy and MI at presentation were significant predictors of stent thrombosis while in the DES group, MI at presentation, diabetes, treatment of the LAD and age significantly increased the risk for stent thrombosis (Table 3). When correcting for independent predictors of stent thrombosis, the adjusted risk for stent thrombosis in the DES group decreased to 1.26 (95% CI 0.82 - 1.95). Additionally, there were no significant differences in the occurrence of stent thrombosis between both DES groups (adjusted HR 0.82, 95% CI 0.50 - 1.34). Among patients with stent thrombosis, secondary stent thrombosis (stent thrombosis occurring after a target lesion revascularisation) occurred in 10.8% of the BMS patients compared to the 4.3% of the DES patients (p=0.22). None (0%) of the patients in the BMS group vs. one (0.1%) patient in the SES group and eight (0.3%) patients in the PES group experienced a second episode of stent thrombosis. In the BMS group 22/111 (19.8%) post-operative MIs were due to ST versus 59/153 (38.6%) in the DES group (p=0.016).
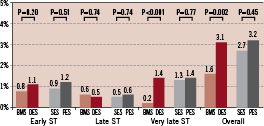
Figure 3. Chart depicting the cumulative incidence of early (<30 days), late (>30 days, <365 days) and very late (>365 days) angiographic stent thrombosis in the bare-metal stent group (BMS), sirolimus-eluting stent (SES) group, paclitaxel-eluting stent group (PES) and drug-eluting stent (DES) group (combined SES and PES). P values are based on the Logrank test.
TVR was performed significantly more often in the BMS group (15.7%) as compared to the two DES groups (12.2% vs. 12.0% in the SES and PES groups, respectively)(Table 3, Figure 4). The use of DES was associated with a 31% lower risk for TVR at 4-years compared to BMS (adjusted HR 0.69, 95% CI 0.58 - 0.82). As compared to PES, the use of SES was associated with an equal risk for TVR at 4-years (adjusted HR 0.99, 95% CI 0.77 - 1.28).
Finally, given the differential follow-up between the three treatment cohorts, a stepwise logistic regression analyses with follow-up truncated at two years was used to test the estimated 4-year treatment effect using Cox proportional hazards regression analyses. At two years, the adjusted HR for all-cause mortality in the DES group was 0.92, 95% CI 0.77 - 1.10, which was comparable to initial adjusted HR of 1.04, 95% CI 0.80 - 1.34 derived from a Cox proportional hazards regression model including all univariate significant (p<0.1) predictors of all-cause mortality. Similarly, for TVR, the adjusted HR at two years was 0.55, 95% CI 0.46 - 0.65, which was comparable to initial adjusted HR of 0.60, 95% CI 0.50 - 0.73 derived from a Cox proportional hazards regression model including all univariate significant (p<0.1) predictors of TVR.
Stratified analysis among subgroups
When more specifically analysing the heterogeneity of the treatment effect (DES vs. BMS) on the 4-year TVR rates, a trend towards heterogeneity was observed among patients presenting with MI (p heterogeneity 0.086).(Figure 5a) When assessing the treatment effect of SES vs. PES, which was remarkably similar in the overall population (Figure 4), significant heterogeneity was observed in patients with diabetes (p heterogeneity 0.045), and bifurcation lesions (p heterogeneity 0.036).(Figure 5b)
A stratified analysis to detect heterogeneity in the treatment effect between DES vs. BMS and SES vs. PES did not reveal any significant differences in the 4-year all-cause mortality rates.
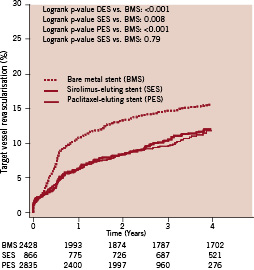
Figure 4. Kaplan Meier curves for target vessel revascularisation up to 4 years for all patients receiving bare-metal stents (BMS), sirolimus-eluting stents (SES) or paclitaxel-eluting stents (PES).
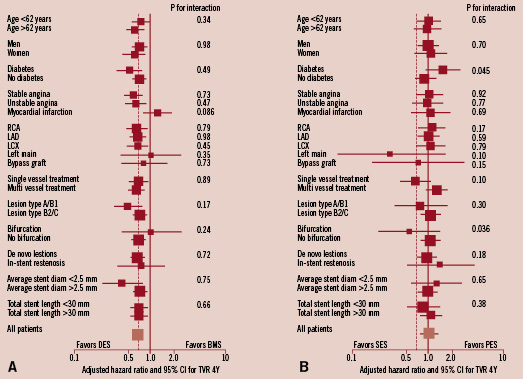
Figure 5. Exploratory analyses to evaluate possible heterogeneity in treatment effects on 4-year target vessel revascularisation rates according to drug-eluting vs. bare-metal stent cohort (A) and according to the sirolimus- vs. paclitaxel-eluting stent treatment cohort (B) and the following clinically relevant characteristics: age, gender, diabetes, clinical presentation, treated vessel, multivessel disease, AHA lesion class, bifurcation, in-stent restenosis, average stent diameter≤2.5 mm and total stented length≤30 mm. The size of the squares corresponds to the amount of statistical information. For the continuous variables (age, average stent diameter and total stented length), medians were used as cut-off. Results of tests for heterogeneity in treatment effect were considered significant if P was <0.05. SES indicates sirolimus-eluting stent; BMS: bare metal stent; PES: paclitaxel-eluting stent; AHA: American Heart Association; LAD: left anterior descending coronary artery; LCx: left circumflex coronary artery; RCA: right coronary artery.
ST segment elevation MI subgroup
While in patients presenting with stable or unstable angina, the risk for TVR at 4-years was 38% lower in patients treated with DES as compared to BMS (adjusted HR 0.62, 95% CI 0.51 - 0.75), the risk for TVR in patients presenting with MI was 26% higher (adjusted HR 1.26; 95% CI 0.82 - 1.93) in patients treated with DES as compared with BMS (p heterogeneity 0.086). There was no difference between SES and PES at four years (Figure 5b). The cumulative incidence of all-cause mortality was 16.9% in the DES group vs. 18.7% in the BMS group (Adjusted HR 1.15, 95% CI 0.76 - 1.74) with no significant difference between the SES and PES groups (14.8% vs. 17.0% respectively; adjusted HR 0.82, 95% CI 0.53 - 1.29). Furthermore, there was no difference in the combined endpoint of cardiac death or post-operative MI in DES (18.6%) and BMS (17.9%) group (adjusted HR 1.05, 95% CI 0.60 - 1.84). Stent thrombosis however, occurred in 5.0% of the DES (SES: 4.9%, PES 4.7%) patients as compared to 2.4% of the BMS patients (p=0.06) and very late stent thrombosis (>1 year) was significantly more frequent in the DES as compared to the BMS group (2.7% vs. 0% respectively; p=0.0007).
Diabetes subgroup
Although the 4-year cumulative incidence of TVR in the diabetic subset was significantly lower in the overall DES group as compared to the BMS group (16.2% vs. 24.6%; Adjusted HR 0.53, 95% CI 0.36 - 0.78) significant heterogeneity in the treatment effect was found between SES and PES.(Figure 5b) While in the non-diabetics, the risk for TVR in the SES group was 11% lower than in the PES group (adjusted HR 0.89, 95% CI 0.67 - 1.19), the risk was 41% higher in the patients with diabetes (adjusted HR 1.41, 95% CI 0.85 - 2.35) (p heterogeneity 0.045). In diabetics, the cumulative incidence of TVR at 4-years was 20.9% in the SES patients as compared with 13.9% in the PES group (Logrank p-value 0.048). There were no significant differences in the hard clinical endpoints between SES and PES treated patients: four-year all-cause mortality was 19.4% in the SES group as compared to 18.4% in the PES group (Adjusted HR 1.38, 95% CI 0.86 - 2.19), whilst the cumulative incidence of cardiac death or post-operative MI was 17.7% in the SES group as compared to 14.6% in the PES group (Adjusted HR 1.09 95% CI 0.64 - 1.83) and stent thrombosis occurred in 6.5% of the SES patients as compared to 4.1% of the PES patients (adjusted HR 1.41; 95% CI 0.56 - 3.56).
Bifurcation lesions
Finally, significant heterogeneity in the 4-year TVR rates between SES and PES was observed in patients treated for bifurcation lesions. While in patients without bifurcations there was no difference between the TVR rates in both DES groups (12.7% in the SES group vs. 11.7% in the PES group; adjusted HR 1.04, 95% CI 0.80 - 1.36), in patients with bifurcation lesions conversely, there was a strong trend towards a lower TVR risk in patients treated with SES as compared to PES (7.1% vs. 14.3% respectively; adjusted HR 0.56, 95% CI 0.23 - 1.36) (p heterogeneity 0.036).(Figure 5b) The difference in all-cause mortality did not reach statistical significance (SES: 6.2% vs. PES: 15.2%; adjusted HR 0.62, 95% CI 0.21 - 1.84). However, the cumulative incidence of cardiac death or post-operative MI was significantly lower in the SES group as compared to the PES group (4.5% vs. 14.2%; adjusted HR 0.30, 95% CI 0.10 - 0.88). Additionally, the cumulative incidence of stent thrombosis was lower in the SES group (1.3%) than in the PES group (5.2%) (adjusted HR 0.21, 95% CI 0.03 - 1.67).
Discussion
The results of the present study show that in a real world patient population, after four years, the overall use of DES was associated with similar all-cause mortality rates and a significantly reduced risk of post-operative MI and TVR as compared to BMS. As compared to patients treated with PES, the use of SES was associated with a significantly lower cardiac mortality and a strong trend towards lower all-cause mortality as compared to PES. Although the cumulative incidence of stent thrombosis was significantly higher in the DES group, adjustment for confounders resulted in a non-significant 26% increased risk for stent thrombosis at four years in the DES group.
The findings of the present study need to be interpreted in the context of a tertiary referral centre that decided to adopt a policy of default DES use for all-comers (including acute MI at presentation, post-CABG, in-stent restenosis etc.) since the first day of commercial availability of the first approved drug-eluting (Cypher®) stent in Europe on April 16, 2002. On February 23, 2003, for financial reasons, our institution replaced the Cypher® stent by the second CE-mark approved drug-eluting stent (TAXUS™).23 These two sequential cohorts were complemented by an equally sized cohort of consecutive patients treated with BMS in the two years preceding the commercial introduction of the Cypher® stent.
With the exception of a slightly longer follow-up in the pivotal randomised trials of the two DES24, the follow-up of the present registry (mean 3.8 years) exceeds that of previously reported registries.5,6,25-28 Another unique feature of this registry is that it was conducted in a small European country with a sedentary population and a very accurate registration of the vital status and cause of death of its citizens by a well-organised governmental administration. These features reinforce the strength of our observations.
Comparing our results to a recently performed network meta-analyses of 38 drug-eluting stent trials revealed a significantly lower absolute risk reduction for TVR at four years in the present study (3.8% vs. approximately 12% in the meta-analysis). Additionally, a higher overall mortality rate was observed in the present study as compared to the network meta-analysis (13.3% vs. approximately 7.5%, without significant differences between DES and BMS).4 Yet, it is difficult to compare our results to other published meta-analyses and registries for three reasons. First, meta-analyses using patient level data of the pivotal randomised trials included only highly selected patients, and are representative of only ~40% of the clinical population of a tertiary medical centre.29 Secondly, in comparable registries, the use of either a DES or BMS was often operator and procedure dependent, resulting in an even greater degree of heterogeneity of the patients treated with DES or BMS, thereby introducing a major potential for selection bias. For example, whereas diabetics would be likely to receive a DES because they are at increased risk of restenosis following BMS placement, patients presenting with acute MI are generally more likely to receive a BMS. It is unlikely that extensive regression and propensity analyses can completely compensate for this inherent type of bias. Thirdly, the vast majority of these registries pooled the outcomes of different devices into one “DES” group, despite the widely acknowledged differences between different types of DES.4,10-12
Recently, at least six large-scale real world registries demonstrated similar to significantly lower mortality rates in patients treated with DES compared to BMS.5-8,30,31 Considering the different features of the present study, our findings demonstrated a similar safety profile for DES and BMS with a significantly lower risk of cardiac death (or post-operative MI) in patients treated with SES compared to PES. The survival benefit in patients treated with SES was already apparent at one week and remained so at one month. Exploring clinical- and procedural success rates, including mortality due to cardiogenic shock at presentation could not account for this difference, and clopidogrel was mandated for at least one month in all patients. Of note, both the short- and long-term survival in the PES group was remarkably similar to the BMS. Several other large-scale registries have also found a similar survival benefit with DES in the first six months which sustained in the longer term.6,7,25 Our sequential registry analysis does not eliminate the possibility of confounders, but sheds additional light on the late survival after BMS, SES and PES implantation in all comers and demonstrates that pooling the outcomes of different types of DES may not always be appropriate. This is an important lesson as new DES, eluting different drugs form different polymers over different periods of time, enter the market.
We performed a stratified analysis to assess the relative safety of DES. The safety of DES appeared to be consistent among several pre-selected high-risk patient subsets, without a significantly superior safety profile, as expressed by all-cause mortality, between SES and PES. However, we found a strong trend towards heterogeneity in the 4-year TVR rates between DES and BMS in patients presenting with MI. As compared to BMS, the adjusted risk for TVR was 26% higher in patients treated with DES. Although this difference in performance did not reach statistical significance, the observation was in clear contrast to the non-MI population, in which the adjusted risk for TVR in the DES group was significantly (38%) lower. Pivotal randomised controlled trial data revealed that the use of SES was equally safe and more efficacious in reducing TVR in this setting as compared to BMS at 1-year, however, PES failed to demonstrate a superior performance as compared to BMS at one and two years.32-34 These latter controversial findings, together with the fact that MI at presentation appeared to be a strong predictor of stent thrombosis in patients treated with DES12,27,35,36, inevitably leading to repeat revascularisations, together with the results of the present study including 1,752 MI patients (DES group with over 80% PES use), makes the use of DES in this high-risk patient subset disputable.
There was significant heterogeneity in the 4-year TVR risk between SES and PES in patients with diabetes and bifurcation treatment. While both drug-eluting devices had a similar safety profile, there was a trend towards a 41% higher risk for TVR in diabetic patients treated with SES. Several smaller subgroup analyses of randomised controlled trials and registries concur with our findings. In the 1-year results from the SOLACI and MILAN registry and the REALITY trial, the use of PES was associated with non-significantly lower rates of target lesion revascularisation as compared to SES in diabetics.37,38 The Kaiser Permanente and TC-Wyre registries conversely, even demonstrated a significant difference between SES and PES in reducing target lesion revascularisation in diabetics, in favour of PES.39,40 Indirect evidence of a possible superiority of paclitaxel as compared to the limus family drugs was derived from a pooled analysis of the randomised SPIRIT-II and III trials, which showed a strong trend towards lower major adverse cardiac events rates in patients treated with PES as compared to the Everolimus-eluting XIENCE V stent. (FDA Executive Summary Memo. FDA Panel 29 November 2007; http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4333b1-00-index.html) The sole randomised ISAR-Diabetes trial did not show any significant differences in the clinical endpoints with SES and PES.41 Large-scale randomised controlled trials are needed to assess the possible superiority of PES as compared to SES in diabetics. The randomised controlled FREEDOM trial (using PES and SES) vs. coronary artery bypass surgery will shed additional light on this issue. 42
Finally, significant heterogeneity in the treatment effect was observed in patients treated for bifurcation lesions, in which there was a strong trend towards a lower TVR risk and a significantly lower risk (70%) to suffer from cardiac death or post-operative MI when treated with SES as compared to PES. These findings confirm the results of a randomised trial by Pan et al evaluating the safety and efficacy of SES vs. PES in bifurcation lesions.43 The authors concluded that the overall system of the SES is better than the PES in terms of main vessel restenosis rates, late loss, and neointimal proliferation assessed using intravascular ultrasound.
The present single centre study has several limitations. First, the clinical and procedural complexity increased over time, which resulted in substantial differences in clinical and procedural characteristics between the sequential patient cohorts. Despite the use of extensive regression models, it remains uncertain whether we were able to completely adjust for the differences between the groups. However, the likelihood of a randomised trial comparing BMS with DES in an all-comer population is already remote and will become even more unlikely with the advent of the second and third generation of DES.
A substantial amount of pivotal experiences with DES in several high risk patient and lesion subsets were reported based on the RESEARCH and T-SEARCH registries. Subsequently, late angiographic evaluation was eventually obtained from “complex” patients, typically with DES implanted in bifurcations, left main coronary, chronic total occlusions, very small vessels, long stented length (>36 mm), and acute myocardial infarction (in total, 25.9% patients in the SES group had angiographic follow-up between six and 12 months).44-50 In the BMS and PES groups, planned angiography was performed in 12.0% and 14.3% respectively. In all other cases, coronary angiography during follow-up was obtained as clinically indicated by symptoms or documentation of myocardial ischaemia. Of note, planned angiographic re-evaluation was used as a co-variable in the Cox proportional hazards regression models.
Due to the sequential nature of the three patient cohorts in the present study, the follow-up in the PES group was shorter than the follow-up in both the BMS and SES groups resulting in a lower number of patients at risk at three years in the PES group. Kaplan Meier survival analyses were performed to reconcile this limitation.
The per patient clinical- and procedural risk profile was linearly associated with time. Given the sequential nature of the three patient cohorts in our study, propensity analyses were considered inappropriate. However, the overall risk profile was more favourable for the BMS group than for either DES group and might even underestimate the real difference.
Finally, the findings derived from the stratified analyses to detect possible heterogeneity in the treatment effect of the different devices should be seen as hypothesis generating. The non-randomised nature of our study precludes any definite statements about the true superiority of one DES above the other in several high-risk subgroups. However it was remarkable that our findings concurred with the few comparative data available in these high-risk subgroups, despite the longer follow-up and subsequent higher event rates in the present study. With the exception of the FREEDOM trial, there are currently no comparative randomised controlled trials ongoing comparing either SES or PES or one of both with BMS in patients with acute MI, bifurcations and/or diabetes properly powered for hard clinical endpoints to confirm our findings.
Conclusion
The results of the present study show that in a real world patient population, after four years, the overall use of DES was associated with similar all-cause mortality rates and a significantly reduced risk for post-operative MI and TVR as compared to BMS. This finding appeared to be consistent among several high-risk patient subsets, with the exception of patients presenting with MI. Furthermore, the use of SES resulted in significantly lower rates of cardiac death and post-operative MI as compared to PES.
Acknowledgement
Dr. Serruys assumes overall responsibility for the integrity of the data, the accuracy of the data analyses, and the completeness of the material reported.
The contribution of the authors is as follows: Conception and design: Daemen, van Domburg, Serruys. Analysis and interpretation of data: Daemen, van Domburg, Boersma, Serruys. Drafting of the manuscript: Daemen, van Twisk, Kukreja, Serruys. Critical revision of the manuscript for important intellectual content: van Twisk, Kukreja, van Domburg, de Jaegere, Daemen, Serruys. Statistical expertise: Boersma, van Domburg, Daemen. Administrative, technical, or logistic support: van Twisk, van Domburg, Serruys. Acquisition of data: van Twisk, Kukreja, van Domburg, Daemen.

