Abstract
Aims: Paclitaxel is a potent and effective inhibitor of neointimal proliferation after coronary stenting. The Conor stent loaded with Paclitaxel can be programmed with multi-parameter matrix of dose, temporal release profiles and release pathways. The aim of this study was to determine the most efficacious dose and release pattern of Paclitaxel in a porcine model and parallels the PISCES trial.
Methods: 32 farm pigs were implanted with Conor stents loaded with 10 or 30 µg of Paclitaxel with, 10 or 30 day and mural or bidirectional release patterns. Angiographic and histomorphometric analysis was performed at 30 and 90 days.
Results: All doses of Paclitaxel were angiographically superior to control (P < 0.01). At 30 days, intimal thickness was similar between Pisces D4 (30 µg/10 days, bidirectional release), D5 (10 µg/30 day mural) and D6 (30 µg/30 day, mural) with D4 having the lowest intimal thickness (167±59 µm). There was a significant increase in the mural injury associated with D4 in comparison to all other doses (P < 0.00001). At 90 days D4 was significantly worse in comparison to Pisces D5 and D6; (P < 0.01) and Pisces D5 and D6 were similar to controls.
Conclusions: 10 day release of Paclitaxel may be too short a period to inhibit neointimal proliferation after coronary stenting, or the rapid release of Paclitaxel may induce chemical injury causing secondary insult to the artery resulting in a rebound increase in intimal thickness at 90 days. These data parallel clinical findings in the PISCES trial.
Background
As compared to balloon angioplasty, bare metal stents reduce but do not eliminate the incidence of restenosis1-4. Drug eluting stents are replacing bare stents in clinical practice because of their capacity to further reduce restenosis rates5. Paclitaxel, a potent antineoplastic compound that promotes the assembly of microtubules and inhibits the tubulin disassembly process, has been used as a locally delivered agent for the prevention of restenosis. Not withstanding the potency of paclitaxel, it has not been uniformly efficacious in clinical trials. The TAXUS trials have provided excellent angiographic and clinical evidence of inhibition of neointimal hyperplasia6-9. The DELIVER trial which used a stent loaded with a higher dose of paclitaxel than did the TAXUS trials, failed to show statistically significant reduction of restenosis10. The reasons for such discordant results are unclear but suggest that the method and kinetic profile of paclitaxel delivery may be important.
The Conor stent is a novel vascular drug delivery stent, the struts of which are endowed with small holes that function as drug reservoirs. Each of these holes can be inlaid with paclitaxel and polymer. This system allows for manufacture of a stent with pre-determined drug release kinetics as well as the ability to control the direction (luminal, mural, both) of paclitaxel elution11. In its’ present embodiment, the Conor stent uses fully erodable polymer and paclitaxel is released by a combination of polymer erosion and paclitaxel diffusion.
The Conor stent has been used clinically in the PISCES trial. This multi-center registry was designed to determine the optimal dose and pharmacokinetic profile for paclitaxel release from a stent12. In the PISCES study, 2 doses and 3 kinetic release formulations were tested in a total of 6 patient cohorts. The present manuscript describes the pre-clinical evaluation of the PISCES dose groups. Its’ goal is to describe the angiographic and histomorpohometric observations in the porcine model for bare stents, stents with polymer only and for 5 of the formulations used in the PISCES trial.
Methods
Conor stent
The stent is made of 316 L stainless steel and is balloon expandable. 17 mm long, by 3.0 mm diameter devices were used in this study as shown in figure 1. The design incorporates struts linked to sinusoidal bridges by contoured features called ductile hinges. The hinges serve to concentrate the stress bearing aspect of the struts onto themselves and as such render the remainder of the strut as passive with no stress bearing function. This allows for the cutting of holes along the length of the struts without compromise of stent strength or crush resistance. The holes can be inlaid with polymer and drug. The unique geometry of the stent is such that the holes do not deform and there is no deformation or extrusion of the polymer during stent expansion11.The strut thickness is 127 µ (range 122-132 µ).
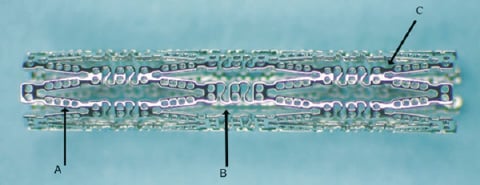
Figure 1. The Conor MedSystems DepoStent™. A. Polymer/Drug wells. B. Sinusoidal bridges™ C. Ductile Hinges™
Each stent is loaded with paclitaxel within a bioresorbable polylactide-co-glycolide (PLGA) matrix. An automated micro-jet system is used to evenly load the polymer/paclitaxel combination by depositing individual drops within each hole. The paclitaxel is released by erosion of the polymer and by diffusion. Pre-determined release kinetics can be “programmed” by varying the method and concentration of paclitaxel deposition in the holes. At the end of the release period, neither polymer nor paclitaxel is retained on the stent11.
Dose and kinetic release profiles
This study evaluated 5 different release formulations which varied in dose (10 or 30µg) and in-vitro elution release kinetics (10 days and 30 days), direction (mural, luminal) as well as stents loaded with polymer only and bare stents. The release profiles for the formulations are shown in table 1.
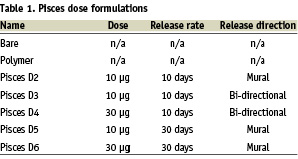
Study design and endpoints
The study was designed to evaluate the effect of dose and kinetic release variation on efficacy as measured by histomorpohometric indices of neointimal proliferation including intimal thickness, area and lumen dimensions at both 30 and 90 days following stent implantation as well as angiographic late loss, percent diameter stenosis and lumen dimensions at the same time intervals.
Sample size calculation
Neointimal thickness (NIT) is a direct measurement of tissue proliferation after stenting. Prior studies in this model for bare metal stainless steel stents have shown mean±standard deviation values of approximately 300±50 um as measured using quantitative histomorphometry (13,14) A sample size calculation shows that N=7 stents per group will detect a 50% difference in NIT between the control and either paclitaxel group with a two sided alpha error of 0.05 and a power of > 90%. 7-8 stents of each group were implanted in the 30 day study. Each suitable vessel of the 3 primary coronary arteries (LAD, LCX, and RCA) was utilized in this study when size permitted. As such, each pig received ‘multi-vessel’ stent implantation. The 90 day study included 9 pigs as it was primarily intended to provide safety data with respect to histology.
Stent implantation
Animal experiments were approved by IACUC and conformed to the AHA guidelines. The porcine stent injury model was used (13). The coronary arteries of juvenile farm pigs (age 3 mo) N=32 were implanted using previously described techniques (11,13,14) with Conor Stents inlaid with paclitaxel 10 µg per 17 mm or paclitaxel 30 µg per 17 mm and mural (M) or bidirectional [mural and luminal],(BI) release patterns. Bare and polymer controls were also implanted. Stents were implanted at balloon to artery (B/A) ratios of approximately 1.0 to 1.1 times the reference vessel diameter (RVD). The animals were pre-treated for 3 days with Clopidogrel (75mg) and Aspirin (325mg) and maintained on both until sacrifice. Nineteen animals were sacrificed at 30 days ± 3 days and 6 animals were sacrificed at 90 ± 7 days. At necropsy the hearts were removed and examined visually for any sign of infarct. The coronary arteries were perfusion fixed at 100-120 mmHg with approximately 500ml of formalin. The hearts were stored in formalin and shipped to the Biomedical Research Foundation of South Texas were the histomorphometry was performed.
Data acquisition and analysis
Angiographic analysis
Quantitative Coronary Angiography (QCA) was performed to obtain the mean RVD pre and post implantation. The minimum luminal diameter (MLD) pre and post implantation, proximal reference diameter and distal reference diameter pre and post implantation and at follow up were also measured. At the time of implantation the mean balloon diameter was measured by QCA to determine the B/A ratio (RVD pre/mean Balloon diameter). Angiography was only performed at implantation and at 30 days follow-up. The pigs were too large at 90 days to accommodate angiography.
Histological analysis
The hearts were examined for evidence of infarction and the stented arteries were identified, dissected from the hearts and processed for embedding in methyl methacrylate plastic blocks. Serial cross-sections were obtained from the proximal to the distal end. The sections were polished and stained with metachromatic stain prior to mounting in immersion oil for viewing under the microscope. Three sections representing the proximal, middle and the distal regions of the stented segment were studied in addition to sections from each of the adjacent non-stented ends.
Area measurements of the artery, stent/IEM and lumen were obtained using a Nikon Labophot compound microscope. The microscope field of view was integrated to an Intel based computer with a digitizing tablet through a drawing tube attachment and a pin-point LED-lit puck. The Sigma-Scan scientific measurement software from SPSS was utilized for the calculation of area measurements, area percent occlusion or stenosis and various other ratios and dimensions. The sections were also evaluated for mural injury, inflammation, vascularization, intimal fibrin, intimal smooth muscle, adventitial fibrosis and endothelialization.
Statistical analysis
The mean angiographic and histologic results for were compared by ANOVA. Post hoc analysis was preformed between groups using Tukey’s HSD test for uneven N (Spjotvoll-Stoline test). Non- parametric statistics were performed on mural injury, inflammation, intimal fibrin and endothelialization scores using Kruskal-Wallis ANOVA (K-W ANOVA) and Median test. The number of samples did not allow for direct comparison between groups. Significant values were expressed as P values < 0.05. All analysis was performed using Statisitca version 5.0 and the values are expressed as mean ± SD except where stated.
Results
Thirty-two pigs were implanted with Conor stents (n=23 in the 30 day cohort and n=9 in the 90 day cohort. Each animal received at least 2 stents in different arteries. Nineteen pigs survived to follow up in the 30 day group and 6 in the 90 day group. Two deaths in the 30 day group were presumed arrhythmic at 3 days, 1 was secondary to anesthetic complication (hyperthermia) and 1 was secondary to air embolus during implantation. In the 90 day group, 2 animals did not receive anti-platelet therapy secondary to viviaurm error and one animal died the day prior to sacrifice of a presumed arrhythmia.
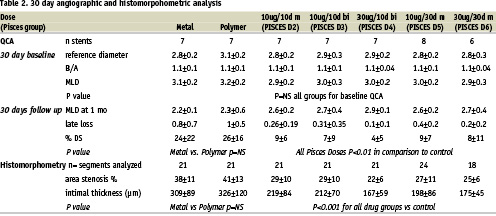
Table 2 shows the QCA and the histomorphometry results for the 1 month experiments. The balloon injury was equivalent in all groups. (P=NS). There were no differences between bare metal and polymer controls in any of the angiographic or morphometric endpoints. All doses of paclitaxel resulted in a significant reduction in late loss (LL) and diameter stenosis (DS) as compared to either bare stents or polymer only controls (P < 0.01). There was a trend towards greater reduction of these indices at the 30 µg doses though this did not reach statistical significance. Histomorphometry in the 1 month pigs demonstrated results consistent with the angiography. The area stenosis (AS) and mean intimal thickness (IT) for all the drug groups were significantly lower than in the control animals (p < 0.001 and p < 0.0006 respectively). The mean intimal thickness was the least in the Pisces D4 (30 µg/10 day) group (167±59 µm), with significantly less intimal thickness as compared to Pisces D2 (219±84 µm, P < 0.05). There was no significant difference in MIT or AS between Pisces D3, D4, D5, and D6.
The histological appearance of typical segments from the 1 month group is shown in Figure 2. In the polymer only group (2B), there was complete filling of the drug reservoirs with neointima which had replaced the erodable polymer. All groups demonstrated endothelialization at 30 days. Arteries that received stents with the 10 day release, both at 10 µg and 30 µg displayed scattered mural hemorrhage and possible medial injury (2C, 2D, 2E). The long release groups had no such findings and appeared indistinguishable from the control stents (2F, 2G).
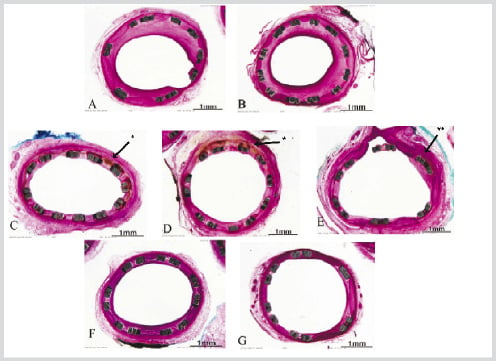
Figure 2. 30 Day Stent Histo-pathology
A. Bare stent (B/A 1.08). B. Polymer only in holes (B/A 1.01). C. Pisces D2- 10µg/10d m (B/A 1.07). D. Pisces D3-10µg/10d Bi (B/A 1.04). E. Pisces D4- 30µg/10d Bi (B/A 1.04). F. Pisces D5- 10µg/30 d m (B/A 1.04). G. Pisces D6- 30µg/30d m (B/A 1.09).
* Both figures 2C and 2D show evidence of scattered eosinophilic deposits within the intima and mild inflammatory infiltrate.
** Figure 2E demonstrates intimal fibrin deposits and mild inflammation.
B/A=Balloon to Artery ratio, Bi=bidirectional release, m=mural release, d=days for full release in vitro.
Figure 3 shows the results of blinded scoring of several qualitative parameters. In the fast release groups (D2, D3, D4), the injury score was significantly increased in comparison to control stents and the long release groups, D5 and D6. Within the fast release groups there was more injury (1.71±0.7) in the 30 µg group D4, as compared to the 10 µg doses D2 (0.81±0.98) and D3 (0.86±0.96), (P < 0.00001 by K-W ANOVA). The injury was independent of the balloon to artery ratio. There were no significant differences with respect to inflammation or endothelialization. Intimal fibrin was increased in all stents, consistent with drug effect and was greater in the 10 day release groups.
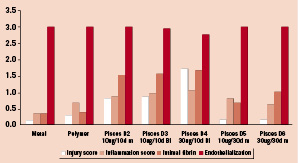
Figure 3. Qualitative Histology Scoring at 30 days. Mural injury is increased in 10 day release groups. Initmal fibrin scores are increased in comparison to bare controls consistent with a Paclitaxel effect. Inflammation and Endothelialization is equivalent among all groups. Bi=bidirectional, m=mural.
Table 3 demonstrates the quantitative measurements in the 90 day animals. The MIT was significantly increased in D4 in comparison to metal, polymer, D5 and D6 (P<0.01). There was no difference between control stents, D5 and D6 in MIT or AS (P= NS). Figure 4 demonstrates the MIT at 30 and 90 days was similar in the 30 day release groups P=NS by ANOVA in comparison to 10 day release groups which displayed a significant rebound in intimal thickness at 90 days.
Figure 5 demonstrates that the injury score and inflammation score in D3 at 90 days was greater in comparison to the other groups but due to small sample size did not reach statistical significance. All other groups appeared to have healed well and had no other significant differences in the qualitative indices (P=NS by K-W ANOVA). Figure 6 shows the histology sections for the respective groups at 90 days. There is evidence of neovascularization within the intima in all the fast release groups. The slow release groups are histologically similar to the control stents. The greatest amount of neoinitma is seen in D4 and is significantly greater than D5, D6 and control stents (P < 0.01).
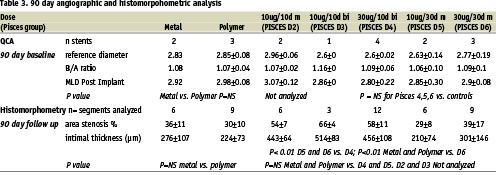
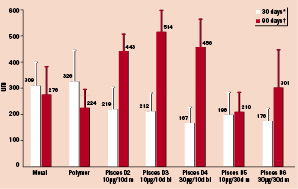
Figure 4. Mean Intimal Thickness at 30 and 90 days * P < 0.0002 all Pisces doses vs. controls at 30 days. P=NS for all Pisces doses at 30 days. † P<0.01 Pisces D5 and D6 vs. Pisces D4. P=NS Pisces D5 and D6 vs. controls at 90 days. Bi=bidirectional, m=mural
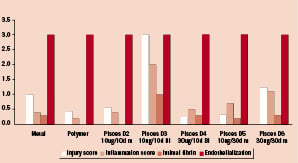
Figure 5. Qualitative Histology Scoring at 90 days. Bi=bidirectional, m=mural.
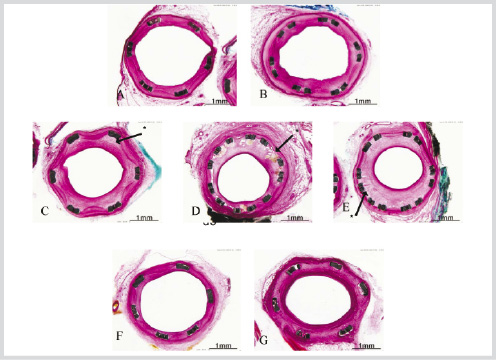
Figure 6. 90 day stent histo-pathology A. Bare- B/A 1.09. B. Polymer- B/A 1.12. C. Pisces D2- 10 µg/10d m B/A 1.08. D. Pisces D3 -10 µg/10d bi B/A 1.14. E. Pisces D4 -30 µg/10d bi B/A 1.09. F. Pisces D5- 10 µg/30d m B/A 1.14. G. Pisces D6 -30 µg/30d m B/A 1.14. Bi=bidirectional, m=mural, B/A=Balloon to Artery ratio. * Figures 6C, D, and G display 10 day release groups with greater amounts of intimal proliferation and some neo-vascularization. Control stents and slow release stents (D5 and D6) are qualitatively similar.
Discussion
The findings of this study confirm the potent anti-proliferative action of paclitaxel when delivered locally in the porcine coronary artery from a stent. The principal original contribution of this study is the demonstration, for the first time, that apparently subtle variations in drug release rates can have profound impact on outcomes in the porcine model. This has potential significance with respect to understanding mechanisms of action and optimization strategies for drug eluting stents.
The findings of this study can be summarized as follows. Paclitaxel is undoubtedly a potent inhibitor of neointima in the porcine model as studied at 1 month. The PLGA polymer itself appears to be well tolerated. Both the 10 µg and the 30 µg doses are effective at this time period. Efficacy was seen across all drug groups regardless of kinetic release characteristics with a trend toward greater efficacy in the 30 µg groups. Notably, there were signs of mild chemical injury in the 10 day release groups though there was complete endothelial coverage at both 30 and 90 days. It appeared that the Pisces D4 (30 µg/10 day) group had the most prominent findings with respect to chemical injury. In the 30 day release groups, the histopathologic findings with respect to injury, inflammation and fibrin were indistinguishable from controls. The findings at 90 day follow-up are markedly different. The histomorphometry demonstrated that the Pisces D4 (30 µg/10 day) group had the most rebound or catch-up in neointimal (Figure 2). In fact, this group was significantly worse than either the control or the 30 day release groups.
Paclitaxel appears to have a rather narrow therapeutic window. Previously reported studies in rabbit iliac arteries with polymer based Paclitaxel at 21 µg and 42 µg in a 2 week release period, showed inhibition of neointima at 1 month. An 8 µg dose was not effective. In that study however, there was no sustained inhibition of neointima at 90 days with either the 21 µg or the 40 µg doses15. Drachman et al. showed sustained neointimal inhibition at 30, 90, and 180 days with 200 µg of Paclitaxel16. This was at the apparent cost of complete suppression of neointima and endothelialization.
Clinical trials with Paclitaxel have yielded strikingly different results. The TAXUS trials using either moderate or long release have been consistently positive with low rates of angiographic and clinical restenosis9. The DELIVER trial, which used a higher dose of Paclitaxel than did the TAXUS trials, failed to show significant reduction in restenosis10. In DELIVER, the drug delivery period was probably shorter than in TAXUS as no polymer system was applied to the stent surface.
One can only hypothesize with respect to the underlying mechanisms responsible for our findings. At 30 days follow-up, neointimal suppression is seen with all groups but with the expense of some chemical injury to the artery. In fact, the 30 µg/10 day release was the most potent inhibitor but potentially the most injurious as well. At 90 days, this same dose is actually the least effective. Though the numbers were not sufficient to establish statistical analysis, the 10µg/10 day release stents also showed a significant quantity of neointima. We believe that at least two mechanistic explanations may be proposed.
The first explanation invokes dual injury. The first injury is caused by the stent implantation. The second injury is caused by the drug itself as it is released from the stent. In this theory, the drug which is intended to mitigate the injury induced by stent implantation, actually induces a second injury which in and of itself, obviates this potential benefit. This is consistent with the greater degree of chemical injury seen in the fast release groups and the poor results seen at 90 days follow-up; especially in the 30 µg/10 day group. The second possible explanation invokes the concept that 10 day release is simply not long enough to be effective for long term efficacy. As such, delivery duration may play a pivotal role. Of course, some combination of these two mechanisms may be operative.
These theories may cast insight into the apparent differences between the TAXUS and DELIVER data. Perhaps the higher dose delivered too rapidly in DELIVER was injurious and/or unavailable for sufficient time. Serruys et al., in the PISCES trial have shown results consistent with the animal data presented in the present study12. In that study of 191 patients treated with the same product presented in this evaluation, the 10 µg/10 day release groups were marginally effective at 4 month follow-up. The 30 µg/10 day group was slightly more effective but both the 10 µg/30 day and 30 µg/30 day release groups were very effective with respect to angiographic and IVUS indices of restenosis. All groups had excellent safety profiles. The animal data, at least at 90 days, may have predicted the human data.
This study is subject to a number of limitations. First, despite the large number of pigs studied, each group is relatively small though sufficient power is available to make important comparisons but not necessarily within all the drug groups at both follow-up periods. Second, insufficient numbers of pigs in the 10 µg/10 day group were available for statistical assessment at 90 days. Finally, the method of tissue sectioning does not permit sophisticated histochemical staining which may have provided further insights into cellular and molecular mechanisms as had been described by the Vermani group15. Such studies, in the future, may be useful to further explore the cellular and biochemical mechanisms underlying the present observations.
In summary, we have demonstrated that paclitaxel release kinetics play a potentially important role in the underlying tissue response to paclitaxel as delivered from a drug eluting stent. A release period of 30 days was more important than dose (either 10 µg or 30 µg) as a determinant of both chemical toxicity and 90 day efficacy. These finding may have important implications for the optimization of drug eluting stents using paclitaxel or other compounds.

