Description
Small vessels represent an increasing percentage of the coronary lesions treated by coronary interventionalists. Recent studies have indicated that the size distributions of reference vessel diameters of treated coronary lesions are roughly as follows: 25% are 2.0- 2.5 mm; 40% are between 2.5 mm and < 3.0 mm and 35% are > 3.0 mm. Smaller vessel diameters are more common in diabetic patients, women and in patients of certain ethnicities. Although clinically relevant lesions in small vessels (reference vessel diameter < 2.75 mm) can exist throughout the coronary tree, they are more frequently observed in mid to distal segments of major epicardial vessels or in side branches.
Prior to the development of stenting, balloon angioplasty was performed utilising the lowest inflation pressures required to adequately dilate coronary lesions. The intent was to reduce baro-trauma and limit the number and size of dissections. Balloon expandable stenting requires high pressure inflations (> 14 atm) to ensure adequate stent expansion and correct apposition of stent struts to the arterial wall. Comparisons of balloon expandable stenting vs. self-expanding (Radius, BSC) stenting have demonstrated the advantages of lower balloon inflation pressures in deployment of self expanding stents. The use of lower pressures resulted in fewer edge dissections in the self expanding group. Inherent advantages in conformability of self-expanding stents may be particularly valuable in tapering vessels.
CardioMind’s .014” guidewire based lumen delivery system is designed to improve access to distal and branch locations through tortuous, calcified, diseased or recently stented segments and may provide the operator greater procedural success and the need for fewer equipment changes. The deployment of self expanding stents can be accomplished with less balloon baro-trauma translating into fewer edge dissections. These thin strut self-expanding stents provide an excellent chance of reducing restenosis rates in small vessels with their inherently favourable conformability and positive effects on remodelling, coated with antiproliferative drugs in a biodegradable polymer matrix and delivered and deployed in an atraumatic fashion. As a result, CardioMind’s Sparrow™ Stent Delivery System offers the unusual opportunity of expanding the number of lesions treated in vessels 2.75 mm and smaller in diameter.
Each new generation of stent design attempts to further delineate a positive balance of selected structural and performance traits that may oppose or complement one another: flexibility, side branch access, scaffolding support, radiopacity, radial strength, etc. The CardioMind Sparrow™ Stent Delivery System is a low profile coronary stent system wherein a self-expanding radiopaque coated nitinol stent is integrated within a 0.014” guidewire delivery system platform for maximum flexibility and fully compatible with the inner diameter of standard commercially available PTCA catheters (Figure 1).
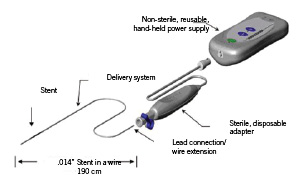
Figure 1. The CardioMind Sprarrow™ stent delivery system.
The potential benefits include the ability to access small diameter vessels in the distal and complex coronary anatomy via a stent delivery system that has the flexibility, pushability and low profile of a .014 inch guidewire.
History
CardioMind Incorporated is a U.S. based start-up medical device company whose charter since 2003 is to design and develop medical devices for use in cardiovascular, peripheral and neurological indications.
Technical specifications
The CardioMind Sparrow™ stent delivery system is comprised of the self-expanding nitinol CardioMind stent which is loaded into a 0.014” guidewire platform (Figures 2 and 3).
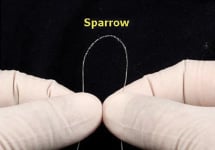
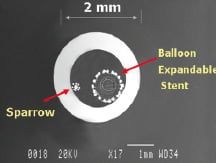
Figure 2. Close-up of the CardioMind stent on the wire platform (above) and in cross sectional comparison of the .014” delivery system to the commercially available balloon expandable stent delivery systems.

Figure 3. The self-expanding properties and the closed cell pattern of the CardioMind Sprarrow™ stent are designed to allow for atraumatic and even apposition against the vessel wall.
The delivery system has a 2-3 cm flexible radiopaque guidewire tip at the distal end to enable positioning within the vessel. The distal end of the stent is located at the proximal end of the guidewire tip while a second radiopaque marker indicates the proximal end of the stent. Both radiopaque markers highlight the pre-deployed position of the stent in the delivery system. The CardioMind stent is deployed through a proprietary mechanism within the Sparrow™ delivery system which enables the electrolysis of mechanical latches holding down each end of the CardioMind stent. Detachment of the stent is initiated and controlled by electrical energy delivered through a hand held battery source in a bipolar, closed loop circuit. A current of less than 0.5mAmp is delivered to each of the mechanical latches to enable electrolysis and stent release. A sterile adaptor/cable is provided which connects the delivery system to the non-sterile battery powered power source.
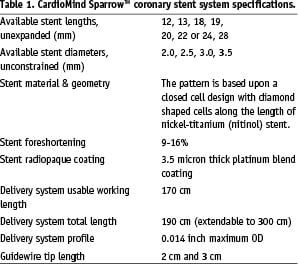
A drug-eluting stent (DES) version is under development, which would incorporate rapamycin in a biodegradable PLA/PGLA block copolymer matrix. The specifications are summarised on Table 1.
Indications for use
The CardioMind coronary stent delivery system is designed to be indicated in improving coronary luminal diameter in patients with symptomatic ischaemic heart disease due to discrete de novo native coronary artery lesions where reference vessel diameters are at least 2.0mm and no more than 2.75mm and < 24mm in length.
Tips and tricks for delivery (use)
The CardioMind Sparrow™ stent delivery system is designed to be used as a front-line guidewire for access to the lesion to be treated. The Sparrow™ is compatible with commercially available over the wire (OTW) or monorail/rapid exchange (RX) balloon catheters. The guidewire tip of the Sparrow™ is shapable and the system has the torque response and pushability of standard guidewires. The Sparrow™ can be navigated to the lesion and positioned with the use of the radiopaque coating and markers on the system indicating stent position. After pre-dilation of the lesion with the balloon catheter of choice, the catheter is withdrawn proximally such that the PTCA catheter tip marker is adjacent to the guiding catheter tip marker.
Once position of the stent to the lesion is confirmed, the Sparrow™ delivery system is then connected to a sterile adaptor which is then connected to a non-sterile power supply via sterile pouch. Final stent position relative to the lesion is verified under fluoroscopy and stent release can begin. Stent release is a two-step process where the distal end of the stent is deployed first, followed by the proximal end using separate buttons (one button for each end). Each side releases the mechanical latch in less than 20 seconds. Once complete, stent deployment is verified via fluoroscopy, the balloon catheter can be re-advanced to the lesion site for post dilation (see moving image). Additionally, the adaptor can be removed and the delivery system can function as a stand alone guidewire, enabling catheter exchanges for additional balloon dilatations or IVUS type imaging and/or stenting of proximal lesions with other PCI catheter systems.
Preclinical experience
Over 200 prototypes of the CardioMind stent delivery system have been evaluated in the swine normal coronary artery model. Target site delivery was primarily to the distal segments of the the LAD, LCX, and RCA epicardial vessels to test deliverability in challenging anatomy. The CardioMind stents that were evaluated ranged in diameter from 2.5 to 3.5 mm (median: 2.5 mm) using the 13, 19 and 28 mm lengths. The acute results of studies in animals demonstrated the deliverability of the CardioMind stent delivery system and the successful deployment of the CardioMind stent to the target vessels.
CardioMind conducted several chronic in vivo porcine animal studies with the purpose of demonstrating longer term safety of the Sparrow™ stent at 3, 28 and 90 days. The Sparrow™ stent can be delivered with minimal procedural injury to the vessel wall. The 28 day and 90 day data demonstrate that the CardioMind stents are well tolerated in the coronary vasculature with favourable histologic and histomorphometric parameters and no strut fractures were seen in single or overlapping stents (Figure 4).
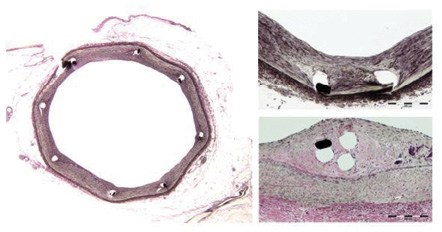
Figure 4. Different examples of low intimal thickness are observed in the porcine model at 90 days with the CardioMind stent whether implanted alone (left, bottom right) or with overlapping stents (top right). (Verhoeff Elastin stain. Courtesy of Groothuis, Bailey, Spognardi, Seifert, Edelman, CBSET).
Clinical experience
The clinical feasibility study, CARE I - using bare metal stent and the first generation delivery system - was recently completed in 2006 at two institutions (Institute Dante Pazzanese of Cardiology, Sao Paulo in Brazil and St Vincent’s Hospital in Melbourne, Australia). The CARE I study was a prospective, multicentre, feasibility study that enrolled 22 patients for assessment of this delivery system with the bare metal nitinol stent design. Patients who required treatment of a single de novo target lesion in a native coronary artery and who met eligibility criteria were enrolled into the study (Figure 5).
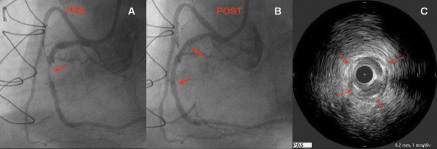
Figure 5. Panel A shows an important lesion in the mid portion of the right coronary artery (red arrow). Panel B illustrates the excellent result obtained after the deployment of a 2.5 x 20 CardioMind Sparrow™ stent. IVUS study immediate after stent deployment confirmed the excellent expansion and apposition of the stent along all the treated segment (panel C).
Clinical follow-up will be performed at 30 days, 6 months, 9 months, and 12 months and yearly for the years 2-3. Angiographic and IVUS follow-up will be conducted on all patients at 6 months, and a subset of patients will have angiographic and IVUS follow-up at 12 months. The CARE II trial, which will incorporate the DES (rapamycin) version of the CardioMind Sparrow™ stent delivery system, is expected to begin in the second half of 2007.
Online data supplement
This video demonstrates the principles of use for the CardioMind Sparrow™ stent delivery system. The CardioMind stent delivery system is navigated to the lesion site similar to a standard coronary guidewire. A coronary balloon catheter may ride on the stent delivery system to the lesion site. After predilatation with a balloon catheter, the balloon catheter is pulled proximal to the lesion. The CardioMind stent is then released from the delivery system in a controlled manner, distal to proximal. The CardioMind radiopaque nitinol stent is self-expanding and a 20% oversizing allows for stable positioning in the lesion with the thin struts conforming to the vessel wall. After stent release, the stented vessel is post dilated on the same guidewire platform and short balloons may be used to minimise ballooning of healthy vessel wall.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.

