Abstract
Aims: To assess the long-term safety and efficacy of the paclitaxel-eluting TAXUS moderate-release (MR) investigation-only stent for the treatment of long, complex coronary artery lesions.
Methods and results: TAXUS VI was a prospective, double-blind, multicentre trial wherein 446 patients were randomised between a TAXUS Express MR stent and an uncoated Express Control stent. At 5-years, the overall rate of major adverse cardiac events (MACE) was similar in the two groups at 27.8% in control and 31.3% in TAXUS (P=0.61), including similar rates for stent thrombosis. The target vessel revascularisation (TVR) rate was 23.7% in control and 22.2% in TAXUS (P=0.45) with a non-target lesion revascularisation (non-TLR) rate of 5.1% in control and 10.9% in TAXUS (P=0.0274) and a TLR rate of 21.4% in control and 14.6% in TAXUS (relative reduction, 32%; P=0.0325). Furthermore, subgroup analysis revealed that the TLR benefit of TAXUS was preserved among study groups including small vessels, long lesions and patients receiving multiple overlapping stents.
Conclusions: Treatment of complex coronary lesions with the TAXUS MR stent demonstrated similar MACE, similar TVR, and reduced TLR rates compared with control through five years. Based on these positive results, the aetiology of increased non-TLR TVR rate in TAXUS remains unclear.
Introduction
The advent of drug-eluting stents (DES) into clinical practice has been widely heralded as a significant evolution in the treatment of coronary artery disease. By combining the mechanical benefit of bare metal stents (BMS) with local pharmacologic action, DES use has resulted in a substantial reduction in the incidence of in-stent restenosis reducing the rate of repeat revascularisation compared with BMS1-4.
Previous randomised clinical trials demonstrated the safety and efficacy of DES in low-risk patient populations1-8. The positive outcomes of these trials and the ensuing expansive use of paclitaxel and sirolimus-eluting stents necessitated characterisation of DES use in high-risk patients commonly encountered in routine clinical practice. TAXUS VI was the first trial to evaluate the benefit of the polymer-based moderate release (MR) paclitaxel-eluting TAXUS Express MR stent in patients with a high risk of restenosis including patients with small vessels and long lesions often requiring the implantation of longer or multiple overlapping stents.
As previously reported, the TAXUS MR stent demonstrated a significant reduction in the primary endpoint target vessel revascularisation (TVR) rate from 19.4% in the control group to 9.1% in the TAXUS group (P=0.0027)9. Also, at nine months, patients who received multiple, overlapping stents (N=124) had a 94% reduction in the TVR rate from 25.0% in the control group to 1.6% in the TAXUS group (P<0.0001)10. This significant difference in the TVR rate was maintained at 2-year follow-up in the overall population (control 21.9%, TAXUS 13.9%; P=0.0335). Furthermore, subgroup analysis through two years revealed a significant reduction in target lesion revascularisation (TLR) rates ranging from 72 to 84% compared with control11.
The purpose of this report is to assess the long-term safety and efficacy of treatment with the TAXUS MR stent in TAXUS VI which had the longest mean lesion length (20.6±7.6) of any DES versus BMS trial12.
Methods
Device description
The TAXUS stent (Boston Scientific Corporation, Natick, MA, USA) consists of a balloon-expandable Express stent with a polymer coating containing 1 µg/mm2 of paclitaxel. Based on in vivo preclinical measurements, the MR polymer formulation used in this study produces a paclitaxel release rate approximately three-fold higher than the commercially available slow-release (SR) formulation. The TAXUS MR stent is pre-mounted on a high pressure balloon in a monorail delivery catheter. An uncoated Express stent served as control in the study.
Patient selection, procedure, and follow-up
TAXUS VI was a randomised double-blind trial conducted at 44 sites in 15 European countries. The study design and methods have been described previously9. Four-hundred and forty-six patients were randomised 1:1 between the TAXUS MR stent and control. The study protocol was approved by local ethics review committees, and all patients provided written informed consent.
Stents were implanted after balloon pre-dilatation as described previously9. Loading doses of clopidogrel (300 mg) and aspirin (75 mg) were administered before the procedure. Following stent implantation, all patients were prescribed clopidogrel (75 mg daily) and aspirin (≥75 mg daily) for at least six months.
Clinical follow-up was scheduled at one, three, six and nine months and yearly thereafter for five years. Quantitative coronary angiography (QCA) was performed at baseline, post-procedure and at 9-month follow-up for all randomised patients. An intravascular ultrasound (IVUS) sub-study was performed post-procedure and at 9-month follow-up.
Definitions
Major adverse cardiac events (MACE) were defined as cardiac death, myocardial infarction (MI; Q- and non-Q-wave) or clinically driven TVR. MACE were reviewed and adjudicated by an independent Clinical Events Committee. Non-TLR TVR was defined as any clinically driven repeat percutaneous intervention of the target vessel or bypass surgery of the target vessel for a lesion other than the target lesion. Stent thrombosis was defined per protocol as the clinical presentation of an acute coronary syndrome with angiographic evidence of stent thrombosis, acute MI in the distribution of the treated vessel, or death within 30 days without other obvious cause. Target vessel failure (TVF) was defined as any ischaemia-driven revascularisation of the target vessel, MI (Q- and non-Q wave) related to the target vessel, or death related to the target vessel. Incomplete stent apposition (ISA) was defined as a separation of at least one strut from the intimal surface of the arterial wall; incomplete apposition at nine months was considered ‘late-acquired’ if it was not present at the completion of the procedure.
Endpoints
The primary endpoint of the study was the rate of TVR at nine months post-procedure. Secondary endpoints included the rates of clinical procedural success, composite MACE (cardiac death, MI and TVR) at one, three six and nine months and annually for five years, TVF rate, and the rate of stent thrombosis.
Statistical methodology
All analyses were based on the intent-to-treat (ITT) population. For continuous variables, differences between treatment groups were evaluated by Student t-test. For discrete variables, differences were expressed as counts and percentages and were analysed with Fisher exact test or the chi-square test, as appropriate. The Kaplan-Meier method and log-rank test were used to assess time-to-event endpoints across study groups. Statistical significance was set at P<0.05.
Results
Between May and December 2002, a total of 446 patients were randomised to receive a control Express BMS (n=227) or a TAXUS MR stent (n=219). Follow-up at five years was available for 95.5% of patients in the control group and 95.9% of patients in the TAXUS group (Figure 1).
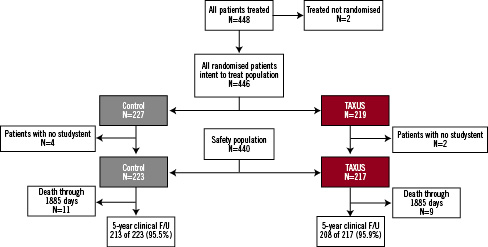
Figure 1. Patient flow through 5 years.
Baseline characteristics
Patients were well matched for baseline demographics, lesion and vessel characteristics between the two groups9. The mean lesion length was 20.6 mm and the mean stent-covered length was 33.4 mm. There was a 55.6% prevalence of type C lesions. Small vessel (<2.5 mm) disease was present in 27.8% and overlapping stents were used in 27.8% of patients.
Safety outcomes
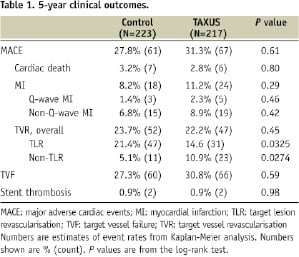
The components of MACE and other clinical outcomes are presented in Table 1.
The 5-year cumulative MACE event-free curves are presented in Figure 2.
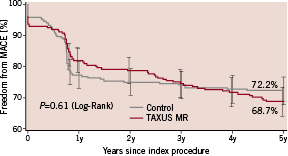
Figure 2. Freedom from major adverse cardiac events (MACE) through 5 years.
The overall rate of MACE at five years was similar in the two groups at 27.8% and 31.3% in the control and TAXUS groups respectively (P=0.61). Twenty patients died during follow-up (11 in the control group and nine in the TAXUS group). Thirteen deaths were due to cardiac events (seven in the control group and six in the TAXUS group).
The rate of TVF was similar in the two groups at 27.3% and 30.8% in the control and TAXUS groups respectively (P=0.59).
A total of four protocol defined stent thromboses were reported through 5-year follow-up (two in the control group and two in the TAXUS group). In three patients, the stent thrombosis was sub-acute (within the first 30 days post-procedure; two in the Control group and one in the TAXUS group). Between 1-and 2-year follow-up, one patient in the TAXUS group developed a stent thrombosis at day 408. No stent thrombosis occurred beyond 2-year follow-up in either study group.
Antiplatelet medication use
At hospital discharge, 98.7% of the control and 100% of the TAXUS patients were receiving dual anti-platelet therapy (P=0.25). A total of 70.5% of the control patients and 66.2% of the TAXUS patients (P=0.33) received dual anti-platelet therapy through the protocol-mandated 6-month duration. By nine months, the use of dual anti-platelet therapy had decreased to 34.1% for the control and 34.1% for the TAXUS patients (P>0.99); however, at five years 89.1% of the control and 91.0% of the TAXUS patients were taking aspirin (P=0.54).
Efficacy outcomes
The 5-year cumulative TVR, TLR and non-TLR TVR event-free curves are presented in Figures 3, 4, and 5 respectively.
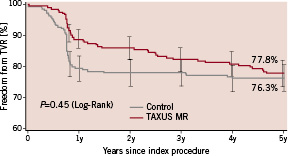
Figure 3. Freedom from target vessel revascularisation (TVR) through 5 years.
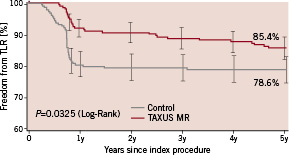
Figure 4. Freedom from target lesion revascularisation (TLR) through 5 years.
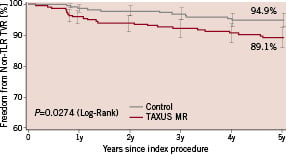
Figure 5. Freedom from non-target lesion revascularisation (non-TLR) target vessel revascularisation (TVR) through 5 years.
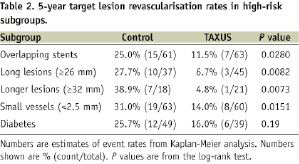
At five years, the TVR rate was similar in the two groups at 23.7% and 22.2% in the control and TAXUS groups respectively (P=0.45). The TLR rate was reduced from 21.4% in the Control group to 14.6% in the TAXUS group (relative reduction, 32%; P=0.0325). The TLR benefit with TAXUS was independent of the classic risk factors for restenosis; in overlapping stents 25.0% and 11.5% (relative reduction, 54%; P=0.0280); in long lesions (≥26 mm), 27.7% and 6.7% (relative reduction, 76%; P=0.0082); in longer lesions (≥32 mm), 38.9% and 4.8% (relative reduction, 88%; P=0.0073); in small vessels (<2.5 mm in diameter), 31.0% and 14.0% (relative reduction, 55%; P=0.0151). In the diabetes subgroup, the TLR rate was reduced from 25.7% in the control group to 16.0% in the TAXUS group (P=0.19). Subgroup analysis for TLR is presented in Table 2.
The 5-year non-TLR TVR rate was 5.1% in the control group compared with 10.9% in the TAXUS group (P=0.0274). Distribution of non-TLR TVR events is presented in Table 3.
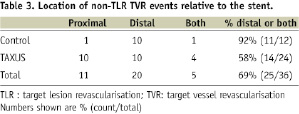
There were a total of 36 non-TLR revascularisations through five years. Of the 12 non-TLR TVR events in the Control group, 10 were distal to the stented segment, whereas of the 24 non-TLR TVR events in the TAXUS group, 10 were distal, 10 were proximal and four were both proximal and distal in relation to the stented segment.
Incidence of incomplete stent apposition
The incidence of ISA in the intravascular ultrasound (IVUS) subset is presented in Table 4.
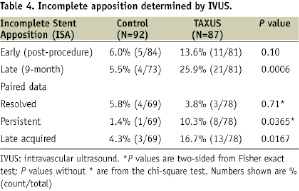
Paired post-procedure and 9-month analysis was completed in 147 patients. Of 11 instances of ISA observed post-procedure in the TAXUS group, three were resolved and eight were persistent at nine months. In the control group, four of the five instances of ISA were resolved and one was persistent. There were 13 instances of late-acquired ISA in the TAXUS group compared with three cases in the control group (P=0.0167).
Clinical outcomes of patients with late-acquired incomplete stent apposition
Two of the three patients with late-acquired ISA in the control group had MACE compared with two of the 13 patients in the TAXUS group. One of the two patients in the control group had a non-Q wave MI at days one, 20, and 204 post-procedure and a stent thrombosis on day 20. The second patient in the control group had a TLR at day 272 post-procedure. One of the two patients with late-acquired ISA in the TAXUS group had a non-TLR TVR event at day 923 and the second patient had a TLR at day 988 post-procedure.
Discussion
Randomised trials have demonstrated the safety and effectiveness of DES in less complicated de novo lesions. However, long-term data on their use in high-risk patients are lacking. This prospective, multicentre, double-blind randomised trial was the first trial to evaluate the benefit of DES in a very challenging patient population in clinical practice; implanted stent covered length of 33.4 mm; overlapping stents used in 27.8%, complex (ACC/AHA type C) lesions present in 55.6% of lesions, small vessel (<2.5 mm diameter) disease present in 27.8%.
At 5-year follow-up, the safety profile for the TAXUS MR stent was comparable to BMS control. There was no significant difference in cardiac death, MI, or stent thrombosis over time between the two study groups (Table 1). A protocol defined stent thrombosis rate of 0.9% in the TAXUS and control groups with no stent thrombosis beyond two years in both groups when patients were not mandated on clopidogrel beyond six months reinforces the safe use of the TAXUS MR stent. This finding is in agreement with a meta-analysis report that the overall rate of stent thrombosis during four years of follow-up was not significantly different between patients who had received one of two approved types of drug-eluting stents and those who had received bare-metal stents in randomised clinical trials13. However, TAXUS VI was not powered for analysing rare events such as stent thrombosis, and the very small number of cases limits conclusions about stent thrombosis rates between the TAXUS and control group.
We observed that the incidence of late-acquired ISA was higher in the TAXUS group compared with control, although there was no stent thrombosis associated with the late-acquired ISA in the TAXUS group through five years. Stented length has been identified as one of the main predictors of stent thrombosis, but the 5-year results of TAXUS VI with a mean stent covered length of 33.4 mm demonstrate that the safety benefit of the TAXUS MR stent is maintained in complex lesions14.
Besides the long-term safety of the TAXUS MR stent, the long-term TLR benefit of this stent is equally noteworthy. At five years, there was a significant reduction in the TLR rate in the TAXUS group compared with control (relative reduction, 32%; P=0.0325). However, there was no significant difference in the incidence of TVR between the two study groups through five years after stent implantation. Furthermore, non-TLR TVR was significantly increased in the TAXUS group. This finding could result from the uncharacteristically low rate of non-TLR TVR in the Control group compared to a 5-year rate of 11.5% reported previously in a meta-analysis of TAXUS trials15. Alternately, it could be due to a distant effect of the higher paclitaxel dose. However, the location of the non-TLR events (Table 3) indicates that there was no evidence for a downstream effect in the TAXUS group despite the higher local paclitaxel release from the TAXUS MR stent used in this trial compared with the commercial TAXUS SR stent.
Subgroup analysis revealed that the TLR benefit of the TAXUS MR stent was preserved among all subgroups including patients with small vessels, long lesions, and those receiving overlapping stents (Table 2). The 54-88% reduction in the TLR rate in the high-risk subgroups in the TAXUS group compared with control extends the proven benefit of this stent in simple lesions as seen in the MR arm of the TAXUS II trial6 to more challenging lesions and patient subsets commonly encountered in clinical practice. The TAXUS V trial investigated the safety and efficacy of the SR formulation in a patient population with complex lesions16. When the data become available, a comparison of the long-term outcomes from the TAXUS V and VI trials will allow a detailed analysis of the safety and efficacy of the two paclitaxel dose formulations in similar patient subsets.
These long-term results from the TAXUS VI trial are unique and cannot be extrapolated to other paclitaxel-eluting stents. This is supported by the results of previous studies conducted on non-polymeric paclitaxel-eluting stents to optimise dosing strategies which have demonstrated discrepant results. The ASPECT trial demonstrated a dose-dependent reduction in the rate of in-segment restenosis in patients assigned to a non-polymeric paclitaxel stent. However, this finding did not correlate with any improvement in the incidence of TLR17. Likewise, the ELUTES trial also showed a significant reduction in the rate of restenosis in the largest dose of paclitaxel with no significant benefit from intermediate doses18. The DELIVER trial, with a paclitaxel dose density of 3.04 µg/mm2, failed to demonstrate better angiographic and clinical outcomes compared with the bare stent19. It is important to note that in spite of the same drug, the discrepancy in the results appears to be due to the differing kinetic release profiles at similar doses which seem to have a significant impact on efficacy20.
The TAXUS MR stent has demonstrated promising long-term results for the treatment of simple and complex coronary lesions. However, due to the fact that TAXUS SR is the minimum effective formulation for treatment of coronary lesions, the TAXUS SR stent will remain the commercial product. Although the TAXUS MR stent is investigation-only and does not have any immediate commercial implications, we believe that trials such as TAXUS VI are an important step in the evolution of DES as a safe and effective treatment option for a large and growing population of patients with complex coronary artery disease.
Study limitations
Limitations of this study include the fact that it was underpowered to assess low frequency endpoints including stent thrombosis. Also, subgroup analyses were not adjusted for multiple comparisons. Furthermore, the inclusion of lesion length ≥18 mm and ≤40 mm in the trial hampers generalisation of these results to shorter lesion lengths.
Conclusions
Treatment of complex coronary lesions with the non-commercial TAXUS Express MR stent demonstrated similar MACE, similar TVR, and reduced TLR rates compared with control through five years. Based on these positive results, the aetiology of increased non-TLR TVR rate in TAXUS remains unclear.
Acknowledgements
The authors thank Tricia Larson, MS of Boston Scientific Corporation for statistical analysis of the data.

