Description
The treatment of coronary bifurcation lesions remains a challenging area in interventional cardiology1. Compared to non-bifurcating lesions, they have a lower rate of procedural success and a higher rate of restenosis2. By employing drug-eluting stents (DES) the restenosis in the main branch (MB) can be effectively reduced, however at the side branch (SB) ostium it is still a problem3. Indeed several two stent bifurcation techniques using conventional stents have been fashioned in an attempt to circumvent this problem4. But they can be unpredictable especially when it comes to recrossing the side branch. In addition, the occasional poor apposition due to the mismatch between cylindrical stents and Y-shape bifurcations can alter the shear stresses at the ostium and increase the thrombotic risk5. Consequently, the gauntlet is set to create an ideally suited dedicated bifurcating stent, which is simple to use in the majority of bifurcating lesions whilst offers good mechanical support at the carina with minimal disruption of the side branch anatomy.
The recently published Nordic bifurcation randomised study revealed that complex stenting of bifurcations does not offer any significant advantage over stenting the main vessel with the optional stenting of the side branch6 a concept adopted in the Stentys Coronary Bifurcation Stent (Stentys SAS, France) design. The stent is a provisional, self-expanding, drug-eluting stent designed to treat Y-bifurcations various calibrations. The procedure is very similar to a provisional technique with the commercially available workhorse DES. The novelty is that the stent shape is adapted to the bifurcation anatomy after implantation. This is due to the stents’ unique self-expanding nitinol Z-shaped mesh that is linked by small interconnections. It offers the stent’s struts the ability to be disconnected by an angioplasty balloon to create the side branch access and to achieve optimal ostial scaffolding. The stent struts have this property all around and along the stent so that the initial positioning is irrelevant, solving a problem often encountered in the more cumbersome dedicated bifurcating stent designs.
The procedure entails (Figure 1):
1. The placement of Stentys Stent Delivery System over the bifurcation via a standard 0.014” guidewire. The self-expanding stent is then deployed in the main branch across the bifurcation by retracting the covering sheath and the Delivery System is withdrawn.
2. The guidewire is passed into the side branch through the stent wall in the cell closest to the carina. A standard angioplasty balloon is then passed through the stent (Figure 1A).
3. The angioplasty balloon inflation disconnects some of the stent struts and allows the stent to expand completely into the bifurcation, giving full access to the side branch. The self-expanding properties of the stent allow optimum scaffolding of the walls in the complex bifurcation anatomy (Figure 1B-D).
4. The procedure can be complemented by the addition of a workhorse stent in the side branch if needed. The geometry of the Stentys stent enables the positioning of the side branch stent without a significant gap or excessive metal overlap.
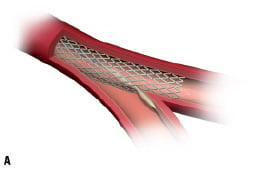
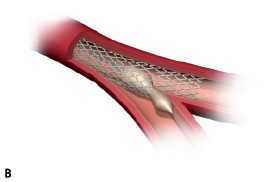
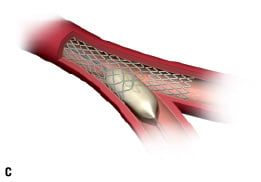
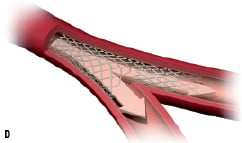
Figure 1. Illustrating the principle of the Stentys bifurcation stent deployment.
History
A review of bibliography on bifurcation stenting shows that the best results are currently obtained with self-expanding drug-eluting stents7. But currently available bifurcating stents, self-expanding or balloon-expandable alike, are limited by the need for precise positioning of the side branch segment8. In order to circumvent this limitation the Stentys stent was conceived and a company created in Paris, France in 2005. As such, the Stentys system is designed to be used effectively regardless of initial deployment position and without a significant learning curve.
Technical specifications
The stent
The Stentys Coronary Bifurcation Stent is made from nitinol, a nickel titanium alloy that can self expand to adopt the desired shape of the bifurcation. The stent itself is 18 mm long and has the potential to expand to a maximum of 7.5 mm diameter at the carina. It is recommended however to be deployed in vessels with diameters at the carina in the order of 6.0-6.5 mm to maintain its physical properties. The unique Z-shape mesh cells are composed entirely of nitinol and are not welded together. At full expansion the ‘step’ height (strut and bridge) has a dimension of 1.2 mm in length. On the abluminal side of the stent a currently undisclosed anti-restenotic drug is incorporated in a durable polymer matrix (PESU), a polysulfone, that permits controlled drug elution. The stent has three highly visible markers distally and one proximal to facilitate accurate placement (Figure 2A and B).


Figure 2. A: The Stentys stent showing its markers and B: after deployment in a silicon bifurcation model.
The delivery system
The user friendly design of the stent is further extended to the delivery system. A 5 Fr rapid-exchange delivery system delivers the stent in position in the main vessel by withdrawing a retractable sheath. The same single 0.014” guidewire that was used in the main vessel can now be used to cross the ostium. This allows access for a standard angioplasty balloon into the side branch to disconnect the stent struts and anatomically reconstruct the bifurcation shape. The entire delivery system is compatible with 7 Fr guiding catheters and there is a marker on the end of the sheath and on the stent stopper to aid the deployment. Following deployment, a commercially available DES can be effectively deployed in the side branch to treat any residual disease without compromising the carina.
Indications for use
The stent is at its final stages of development and is not yet currently available for sale. Animal testing is however near completion and first-in-man studies are due imminently. The suggested indications are in symptomatic patients with discrete de novo bifurcation lesions in the major coronary arteries having diameters of 3.0 mm to 4.0 mm in the proximal main branch and 2.5 mm to 3.0 mm in the side branch. It is designed to encompass the majority of bifurcating angulations (30° to 70°) and to be used in conjunction with other drug-eluting stents in the side branch.
Tips and tricks for delivery
It may be necessary to prepare the bifurcation by firstly pre-dilating the lesion as is frequently performed when the bifurcation lesion is calcific or if there is a substantial plaque burden.
Pre-clinical experience
The device has been successfully evaluated in porcine coronary and renal arteries (Figure 3).
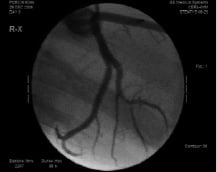
Figure 3. Stentys stent after implantation in a porcine bifurcating artery.
In addition to determining the most suitable vessel diameters, the study achieved the primary endpoints of acute device success and acute procedure success. The acute device success was graded (0 to 3) for insertion, deployment of the stent and creation of the side branch with grade 2 being the benchmark. The acute procedure success was defined as achieving TIMI 3 flow in the main and side branch following deployment. The device achieved excellent results (95%) in the both areas evaluated (Table 1).

The optimal apposition of the stent in the main vessel and side branch was confirmed with intravascular ultrasound (IVUS) (Figure 4).
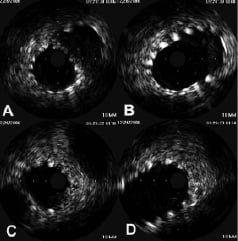
Figure 4. A: IVUS image of the MB, just distal to the carina showing good apposition of the stent in the distal MB with some of the struts expanded into the SB ostial wall; and B: just proximal of the carina showing the self-expanding stent adopting the oval shape of the bifurcation; C: IVUS image of the SB, just distal to the carina showing the ostium wall is scaffolded by the stent and D: just proximal to the carina.
The overall results clearly demonstrate that this frangible stent can ‘split’ (Figure 5) only when it is needed, whilst still offering excellent trackability, positioning and deployment.
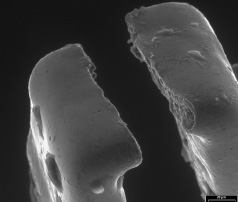
Figure 5. A scanning electron microscopy view of a ‘split’ strut.
Online data supplement
Video 1. Porcine angiogram
Video 2. IVUS MB after kissing
Video 3. IVUS SB after kissing

