Abstract
Aims: Excessive movement of coronary stents within the artery can make precise stent placement difficult. This study assessed the use of rapid right ventricular pacing to reduce stent motion to improve the accuracy of placement.
Methods and results: During percutaneous coronary intervention, if excessive stent movement prevented accurate stent placement, rapid right ventricular pacing was performed to reduce stent motion within the coronary artery during both stent positioning and deployment. Post procedural angiograms were analysed by two independent operators to measure stent movement during sinus rhythm and during rapid right ventricular pacing. Rapid right ventricular pacing was considered necessary in ten patients. No procedural complications occurred. Mean stent excursion for all patients in sinus rhythm was 2.9 mm (SD 1.6) and during rapid right ventricular pacing this was reduced to 0.8 mm (SD 0.6) (p<0.001). Movement assessed by individual operators ranged from 0.7 to 6.5 mm during sinus rhythm and from 0.1 to 2.4 mm during right ventricular pacing.
Conclusions: Rapid right ventricular pacing is an effective and safe method to reduce stent movement and facilitate accurate stent deployment. This simple technique can be easily applied in any interventional cardiac catheterisation laboratory without the need for additional training or equipment.
Introduction
Approximately 2 million patients undergo percutaneous coronary intervention (PCI) annually and a coronary stent is implanted in the majority of cases1,2. Accurate placement of intra-coronary stents is necessary to treat discrete segments of coronary artery disease, particularly at vessel ostia or bifurcations to avoid covering important side branches. Excessive movement of the stent within the artery due to myocardial contraction can make such precise stent placement difficult. Inaccurate stent placement may result in ‘geographic miss’ of the target lesion or may cover a previously uninvolved branch vessel. This pilot study assessed the use of rapid right ventricular pacing to reduce stent motion during deployment and improve the accuracy of stent placement.
Methods
The patients in this study were selected from a series of 965 patients undergoing PCI from December 2003 to June 2006. PCI was carried out via the right femoral approach using 6 Fr or 7 Fr guide catheters. Coronary stents (Taxus®, Boston Scientific, USA) were positioned across the target coronary stenosis and a coronary arteriogram was recorded using a flat plate imaging system to confirm stent position (Toshiba Corporation, Japan or Philips Electronics, Netherlands). If the operator considered that excessive stent movement prevented accurate placement, a 5 Fr temporary pacing wire was inserted via a femoral vein and advanced into the right ventricle under fluoroscopic guidance. Once a satisfactory pacing threshold was confirmed, the stent was positioned as accurately as possible in the conventional manner whilst the patient was in their native rhythm. Rapid right ventricular pacing was then used (rates varying between 120/min and 200/min) to reduce stent motion within the coronary artery. After final positioning a coronary arteriogram was recorded and the stent was deployed. Pacing was then discontinued and a final coronary arteriogram was obtained.
Following the procedure, digital angiograms were analysed by two independent operators using commercially available QCA software (Medcon Telemedicine Technologies™ USA). The system was calibrated against the known distance between the balloon markers on the stent delivery catheter. Movement of the stent relative to an anatomical reference point in the diseased vessel (usually a side branch) was measured in sinus rhythm and during rapid right ventricular pacing. Reproducibility between individual operator’s measurements was assessed using the method described by Bland and Altman3.
Results
Rapid right ventricular pacing to stabilise the coronary stent and facilitate accurate stent placement was carried out in ten patients, including eight with stable angina, one with unstable angina, and one with acute myocardial infarction. Coronary stents were placed in ostial left anterior descending artery (LAD) stenoses (6 cases), an ostial intermediate stenosis, a very proximal diagonal artery stenosis, and two mid LAD stenoses.
In six cases involving ostial left anterior descending stenoses, the left main stem was thought to be angiographically free of significant disease. Accurate stent placement was felt to be necessary to completely cover the stenosis, and to avoid covering the ostium of the circumflex artery. Although in the majority of the cases a coronary guidewire was placed in the circumflex artery, subsequent circumflex intervention was not required.
In one patient with ostial intermediate artery stenosis, the true circumflex vessel was small, and precise ostial stent deployment was necessary to avoid impinging on the left anterior descending artery. An initial attempt to direct stent this stenosis failed due to excessive stent motion, despite deep guide catheter engagement. A second stent, stabilised by rapid right ventricular pacing, was required to ensure that the ostial disease was completely covered.
In the case of the proximal diagonal stenosis, a left anterior descending artery proximal occlusion had just been successfully opened and a stent deployed just distal to a large diagonal branch. Improved flow revealed a tight proximal diagonal lesion. Marked stent movement prevented accurate placement of a short stent to cover this lesion without impinging on the left anterior descending artery, just proximal to its stented segment. Rapid right ventricular pacing allowed the stent to be positioned without the need for further bifurcation intervention.
In a further two cases, despite adequate stent length relative to length of lesion, excessive stent movement risked geographical miss of the stenoses with the potential to require a second stent to ensure adequate lesion coverage. The use of rapid right ventricular pacing to stabilise the stent and reduce movement in these cases allowed precise deployment of the stent, and complete lesion coverage without the need for an additional stent.
Examples of stent motion in sinus rhythm and during rapid right ventricular pacing are shown in Figure 1 and with moving images via the EuroIntervention website.
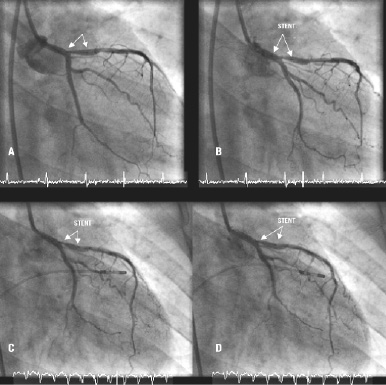
Figure 1. Stent motion in sinus rhythm (diastole – A, systole – B) and during rapid right ventricular pacing (systole – C and diastole – D).
The results of the QCA measurements for individual patients during sinus rhythm and rapid right ventricular pacing are shown in Table 1 and Figure 2.
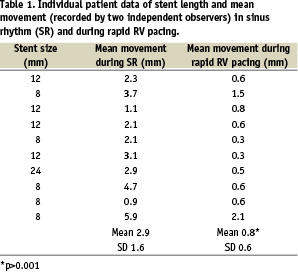
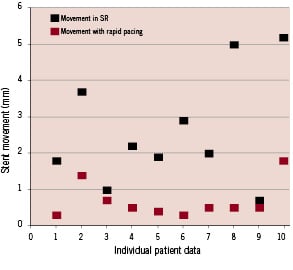
Figure 2. Individual patient data showing stent movement in sinus rhythm and during rapid right ventricular pacing.
Mean stent excursion for all patients in sinus rhythm was 2.9 mm (SD 1.6) and during rapid right ventricular pacing this was reduced to 0.8 mm (SD 0.6) (p<0.001). Movement assessed by individual operators ranged from 0.7 to 6.5 mm during sinus rhythm and from 0.1 to 2.4 mm during right ventricular pacing. In the most extreme case, there was 6.5 mm excursion of an 8 mm stent during sinus rhythm (83% of stent length). Mean difference in stent movement between sinus rhythm and rapid RV pacing was 2.1 mm (SD 1.3).
Inter observer differences were analysed using the method described by Bland and Altman3. For each subject the difference in stent movement between operators was plotted against the mean of the measurements recorded by both operators. The mean of the difference between the two operator measurements was 0.01 mm (SD 0.75) and only one measurement lay outside two standard deviations from the mean suggesting good inter-operator repeatability (Figure 3).
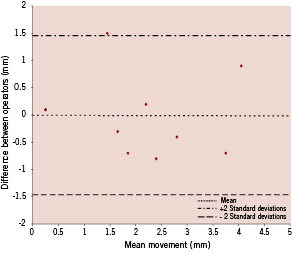
Figure 3. Bland Altman plot comparing measurements of stent movement between sinus rhythm and rapid RV pacing obtained by two operators.
Discussion
Our results suggest that rapid right ventricular pacing is associated with a clinically important reduction in stent motion before stent deployment, thus facilitating accurate stent placement. In this small series right ventricular pacing was not associated with arrhythmia, cardiac perforation or any other complication.
Our review of the literature has identified no reliable method of stabilising intra-coronary stents. Rapid right ventricular pacing has recently been used to stabilise devices in the heart during non-coronary procedures, such as percutaneous valve interventions4, but we are not aware of any previous reports suggesting the use of this technique for the placement of coronary stents.
It may be possible to reduce stent movement by guiding catheter manoeuvres such as deep coronary intubation but this may risk injuring the coronary vessel. Moreover, during deep guide catheter engagement the catheter tip may extend into the segment requiring stent coverage, especially in ostial left anterior descending or circumflex artery locations. Direct stenting of stenoses without pre-dilatation may also reduce stent movement, although this may not always be possible in very tight stenoses, thus limiting its application.
It has also been suggested that placement of the guidewire very distally in the coronary bed, may in fact increase stent movement due to an ‘anchoring effect’, and that withdrawal of the wire more proximally may, at least partially, resolve this issue. We did not routinely withdraw coronary guidewires to reduce any anchoring effect, but in some cases we have seen persistent excess stent movement despite this manoeuvre.
In one small series, the use of a ‘buddy-wire’ has been advocated to stabilise the stent and facilitate accurate stent positioning in ostial lesions5, although this technique is not widely used in routine practice.
In the drug eluting stent era, a lower incidence of in-stent restenosis means that the routine use of longer coronary stents to ensure adequate lesion coverage and to avoid geographical miss may reduce concern about minor stent movement. If a mid-vessel stenosis is ‘missed’ however, even with a relatively ‘long’ stent due to excessive movement, a second stent may be required, both increasing the procedural risk and financial cost of the intervention. Although this may potentially allow ‘less accurate’ stent deployment ‘mid-vessel’, this strategy may not be applicable in ostial lesions where failure to deploy the stent precisely could have significant adverse clinical consequences. An alternative strategy in ostial left anterior descending or circumflex stenoses is to electively stent back into the left main stem, covering the respective side branch. This strategy has the disadvantage of placing a stent in a non-diseased segment of left main stem and covering the ostium of a major coronary vessel with stent struts.
The use of overly long stents to compensate for excessive stent movement may reduce the need for very accurate stent placement but is not without risk, even in the drug eluting stent era, and a method for stent stabilisation to allow precise stent placement is therefore still clinically relevant.
We recognise the potential complications of rapid right ventricular pacing may include cardiac arrhythmias and cardiac perforation which can subsequently cause tamponade in patients with concomitant glycoprotein IIb/IIIa receptor antagonist use6,7. We used 5 Fr pacing wires, short duration of pacing, and removed the wire immediately after stent deployment. We also used the minimum pacing rate required to achieve visually acceptable stability.
Conclusion
This study demonstrates that rapid right ventricular pacing is an effective and safe method to reduce stent movement and facilitate accurate stent deployment. This simple technique can be easily applied in any interventional cardiac catheterisation laboratory without the need for additional training or equipment.
Online data supplement
Video 1. Stent motion in sinus rhythm
Video 2. Stent motion during rapid RV pacing

