Abstract
Aims: To assess the effectiveness and safety of sirolimus-eluting stents (SES) in de novo native coronary lesions in small vessels (≤ 2.5 mm).
Methods and results: PORTO was a multicentre, prospective registry, performed in 274 patients. Of these, 120 (43.8%) patients were diabetic. A total of 324 lesions were treated with 347 SES. The primary end-point was 6-month in-stent late luminal loss (LL); secondary end-points included in-stent and in-segment binary restenosis (BR), target lesion revascularisation (TLR), target vessel revascularisation (TVR) and major adverse cardiac events (MACE) rate at six months and one-year. Pre-specified subgroup analysis included the comparison between diabetic and non-diabetic patients, and patients with stents of different diameter (2.25 mm vs. 2.5 mm) and length (≤ 18 mm vs. ≥ 23 mm).
The mean (SD) reference vessel diameter of the treated segment was 2.08 (0.33) mm and lesion length 11.04 (6.0) mm. After six months, LL was 0.07 (0.37) mm. BR was 5.1% in-stent and 9.1% in-segment. At one year, TLR was 5.6% and TVR was 9.0%. MACE rate was 2.6% at six months and 8.6% at one year with 2.3% cardiac death and 1.5% non-fatal myocardial infarction. Stent thrombosis rate at one year was 0.8% per protocol. There were more MACE in diabetic patients (12.8%) than in non-diabetic (5.4%, p=0.046), but no other significant differences in clinical and angiographic parameters were noted between the subgroups analysed.
Conclusions: The use of SES for lesions in very small coronary arteries proved to be safe and efficacious, irrespective of the size and length of the stents, with low restenosis and repeat revascularisation rates at one year.
Introduction
Percutaneous revascularisation of small coronary arteries represents a continuing major challenge in interventional cardiology, particularly in diabetic patients and in long lesions. Several randomised clinical trials have shown the superiority of bare metal stents (BMS) over balloon angioplasty in small coronary arteries, either as a provisional or an elective treatment option.1,2 However, rates of angiographic restenosis and repeat revascularisation procedures remain high and continue to reflect the high propensity of small arteries to develop restenosis.
The introduction of drug-eluting stents (DES) established important improvements in reduction of restenosis.3,4 Physicians started using DES in subsets of patients for whom data from the randomised trials were limited. Accordingly, initial data from small coronary arteries were mainly derived from subgroup analyses of these trials, or from single-centre registries5-7.
Only more recently, randomised clinical trials and registries were published comparing either DES with BMS, or different DES types between each other in small coronary arteries and in diabetic patients.8-15 Accordingly, small vessels and long lesions requiring longer stent lengths continue to be predictors of restenosis in diabetic patients.16
When the present multicentre, prospective registry was initiated, there was no information available regarding safety and efficacy of sirolimus-eluting stents in very small coronary arteries from studies having investigated a sufficient sample size with significant numbers of diabetic patients and different lesion lengths.
Methods
Patients
The study was initiated and overseen by the Portuguese Society of Cardiology and included nine Portuguese and one Brazilian centre. The ethics committees of the Portuguese Society of Cardiology and of all participating hospitals approved the study protocol. Written informed consent was obtained from all patients.
Patients were eligible for inclusion into the study if they had stable or unstable angina pectoris (excluding Braunwald Classification A I-II-III) or documented silent ischaemia and were acceptable candidates for coronary artery bypass grafting (CABG). They should have at least one de novo lesion with ≥50% stenosis, TIMI flow ≥ I, less than 33 mm in length in a native artery with a vessel diameter ≤ 2.50 mm (visual estimate). Patients were considered diabetic if they were under treatment with either insulin or oral anti-diabetic drugs for at least three months.
Patients were excluded if they had an acute ST- on non-ST segment elevation myocardial infarction (STEMI and NSTEMI), impaired renal function (creatinine >3.0 mg/dl), pregnancy, known allergies (aspirin, clopidogrel, ticlopidine, heparin, stainless steel or sirolimus), were pregnant, were currently participation in another study, or had a life expectancy of less than 12 months.
Angiographic exclusion criteria were ejection fraction ≤30%, angiographic evidence of thrombus, chronic total occlusion, in-stent restenosis within target lesion, heavily calcified lesions, unprotected left main coronary disease, significant stenosis or prior stent proximal or distal to the target lesion, location in an arterial or venous by-pass graft and previous brachytherapy treatment in any vessel.
Adjunctive drug therapy, interventions and follow-up protocol
All patients were medically treated according to routine local clinical practice, but the recommendation was to use pre-procedure aspirin 100 mg (at least 12 hours before) and clopidogrel (300 mg loading dose or 75 mg, 3 days before) or ticlopidine (2x250 mg, 24 hours before). During the procedure, intravenous heparin was given to maintain ACT>250 seconds, and glycoprotein IIb/IIIa inhibitors were left to the discretion of the investigator. Recommended post-procedure antiplatelet therapy included aspirin 100 mg/daily indefinitely and clopidogrel 75 mg/daily or ticlopidine 2 x 250 mg/daily for at least eight weeks.
Balloon predilatation of the target lesion was recommended. Stent implantation deployment should achieve an angiographic stent/vessel ratio of 1.1:1. The treatment of two or more small vessels was permitted, as well as the treatment of other lesions in vessels of larger diameters (with sirolimus-eluting stents or other available stents).
Post-procedure, routine laboratory evaluation (CK, CK-MB, troponins) were performed every six hours and ECG at hospital discharge. Patients were followed through hospital discharge and up to 12 months after the index procedure. This involved physician visits and/or phone contacts to obtain information regarding clinical status, use of cardiovascular drugs, hospitalisations, as well as invasive and non-invasive diagnostic tests. The 6-months visit – or earlier in case of angina – included repeat coronary angiographic assessment of the treated target vessel and an ECG.
All patient data were entered at the participating hospitals into an electronic case record form (CRF) provided by to an independent data coordinating service (Eminent-PPD, Minnesota, USA), while monitoring and data analysis were performed by an independent contract research organisation (KeyPoint, Portugal).
Quantitative coronary angiography
Coronary angiography was performed at baseline, post-procedure, and after six months. All angiograms were analysed by an independent angiographic core lab (Cardialysis, Rotterdam, The Netherlands) using quantitative coronary analysis including edge detection techniques. In particular, the following parameters were measured: reference vessel diameter (RVD), minimal luminal diameter (MLD), % diameter stenosis, and late luminal loss (defined as the difference between the postprocedural MLD and the MLD at 6-month follow-up). The analysis used measures for in-stent, as well as in-lesion parameters, where the in-lesion category includes 5 mm margins on either side of the stent.
Study endpoints and definitions
The primary endpoint of the study was in-stent late luminal loss at 6-month follow-up. Secondary endpoints included: in-stent and in-segment binary restenosis; rates of target lesion and target vessel revascularisation (TLR and TVR); and major adverse cardiac events (MACE) at six months and one year follow-up. MACE was defined as a composite of cardiac death, non-fatal myocardial infarction (MI) and TLR. The diagnosis of MI required the detection of new electrocardiographic Q-waves and/or an elevation of CK or CM-MB above at least three times the upper limit of normal.
Stent thrombosis (ST) was defined as an acute coronary syndrome with angiographic confirmation of target vessel occlusion. ST was defined as acute within the first 24 hours, sub-acute between 24 hours and 30 days, and late when occurring later than 30 days after the index procedure.
The protocol pre-specified subgroup analyses comparing diabetic vs. non-diabetic patients, long (≥ 23mm) vs. short (≤ 18mm) stented lengths, and stents of 2.25 mm vs. 2.5 mm diameter.
Statistical analysis
As PORTO was an observational registry there was no formal statistical hypothesis. The data are presented as means ±SD, counts or percentages. The differences between groups were assessed using a two-sided χ square test or Fisher’s exact test for categorical data, and Student’s t-test for the comparison of continuous variables. A P-value of <0.05 was considered statistically significant. For the analysis of late lumen loss, frequency histograms were created and the distributions were tested for normality using the Kolmogorov-Smirnoff test.
Results
Between January 2003 and September 2004, a total of 274 patients were included in the study. Table 1 shows the baseline demographic and clinical characteristics of the whole population together with a breakdown into diabetics and non-diabetics.
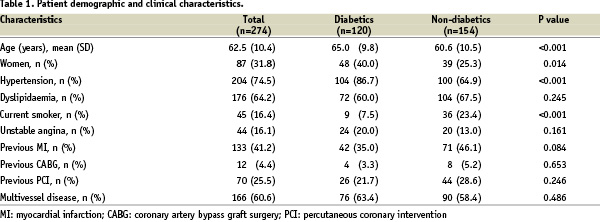
Of note, 32% of the study population were female, 75% had arterial hypertension, and 64% had hyperlipidaemia. Multivessel coronary disease was present in 61%, and the clinical indication for PCI was predominantly stable angina or silent ischaemia in 84%.
In the subgroup of 120 diabetic patients, 33 (27.5%) were insulin-dependent. In comparison with non-diabetics, diabetic patients were older (65 vs. 61 years), more frequently female (40 vs. 25%), had more often arterial hypertension (87 vs. 65%), and were less frequently smoking (8 vs. 23%). Moreover, diabetics were more frequently obese (25 vs. 15%), and had more often peripheral arterial disease (6 vs. 2%).
The angiographic baseline characteristics are shown in Table 2.
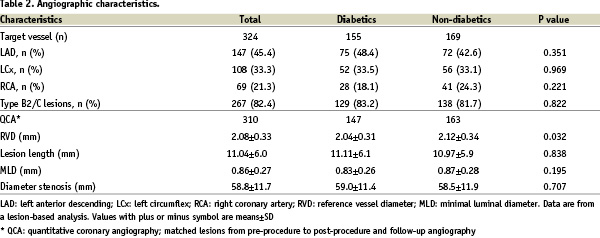
Most importantly, the average RVD was only 2.08 (0.33) mm confirming that the intention was achieved to study a population with very small vessels. While there was no significant difference between diabetics and non-diabetics in terms of the involvement of the different coronary arteries, and the distribution of lesion types, diabetics had a significantly smaller RVD (2.04 vs. 2.12 mm, P=0.03).
The procedural characteristics are shown in Table 3.
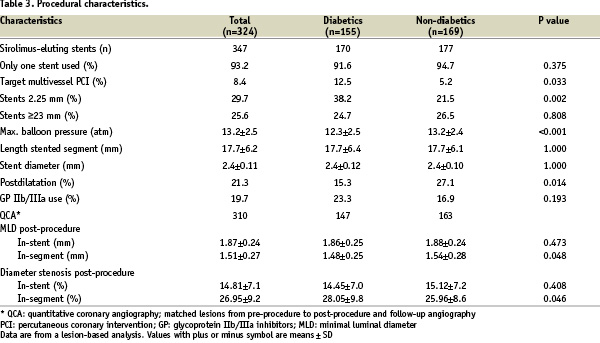
A total of 347 sirolimus-eluting stents were implanted, mainly during single vessel PCI and with a single stent used. There were 81 patients (29.6%) with multilesion/multivessel PCI at baseline. Of these, 38 patients were diabetic, and 10 had multilesion PCI with SES ≤ 2.5 mm, while 28 had multivessel PCI with non-study stents. Out of the 43 non-diabetic patients, five had multilesion PCI with SES ≤ 2.5 mm, and 38 had multivessel PCI with non-study stents.
In 133 lesions (41%), stents were implanted without pre-dilatation. In diabetics, maximum balloon pressure was inferior and post dilatation was less frequent than in non-diabetics. The majority of the stents used were 2.5 mm diameter and 18 mm or less length. Direct stenting was significantly more frequent in patients receiving stents ≤ 18 mm compared with stents ≥ 23 mm (45.1% vs. 29.9%, p<0.01). However, multivessel stenting and use of 2.25 mm sirolimus-eluting stents were significantly more frequent in diabetics. Post-procedure in-segment minimal luminal diameter was inferior and diameter stenosis higher in diabetics.
Follow-up angiography was performed in 244 (89%) patients 226±69 days after the index procedure. The primary end-point, in-stent late luminal loss, was 0.07±0.37mm in the total cohort, 0.09±0.37mm for diabetics, and 0.04±0.37mm for non-diabetics. The frequency histograms for diabetic and non-diabetic patients are shown separately in Figure 1.
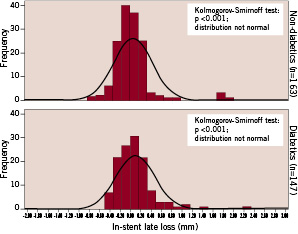
Figure 1. Histograms of in-stent late luminal loss comparing diabetic with non-diabetic patients.
Both distributions are positioned similarly with a mean value close to zero, and both failed to show normality in the Kolmogorov-Smirnoff test. The distribution for diabetic patients is obviously right skewed by visual estimate. It is important to mention that the standard deviation for both distributions is very close to the variability of the two serial QCA measurements (MLD post, MLD follow-up) needed to determine late loss. Accordingly, the distribution pattern shown in Figure 1 is dominated by the variability of the measurement methodology.
More details including the rates of binary restenosis are given in Table 4.
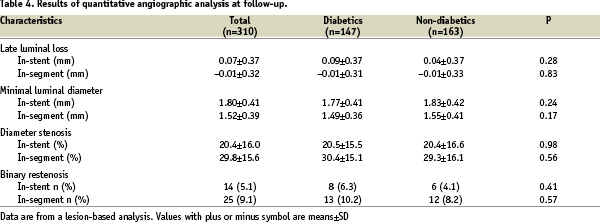
The type of in-stent restenosis found in 14 cases, is described in the Table 5 using the Mehran classification17 together with the treatment performed. In all cases angiography was elective and there were 5 cases (35.7%) of occlusive restenosis.
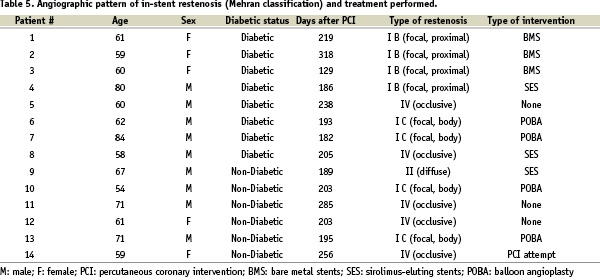
Results of quantitative angiographic analysis at 6 month follow-up by length and diameter of the implanted stents are shown in Table 6.
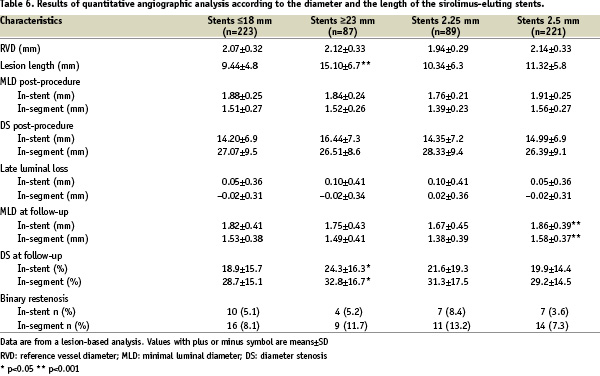
While average values of late loss and rates of binary restenosis were similar for shorter stents (≤ 18 mm) in comparison with longer stents (≥ 23 mm), significantly higher values for longer stents were found for the degree of diameter stenosis for both in-stent (24.3 vs. 18.9%) and in-segment (32.8 vs. 28.7%) analyses.
Clinical outcome
The duration of hospital stay for the PCI procedure was on average 1.5±3.1 days. Medication at discharge included double antiplatelet therapy with aspirin and clopidogrel (81%) or ticlopidine (19%) recommended to be maintained per protocol for two months. Other medications included statins (75%) and beta-blockers (65%) in the overall population.
In-hospital events included only one case of NSTEMI. Within 30 days there were two non-cardiac deaths and one case of STEMI caused by subacute stent thrombosis requiring target lesion revascularisation – all of these patients were diabetic. The subacute thrombosis occurred at day nine, and was confirmed to be due to premature complete discontinuation of antiplatelet therapy.
The 6-month clinical follow-up was completed in 98% of the patients (Figure 2).
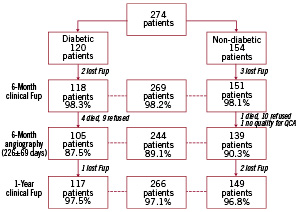
Figure 2. Flow chart of patients included in the study.
There were three additional sudden cardiac deaths, two in the diabetic subgroup and one in non-diabetics. One additional case of NSTEMI as a consequence of late stent thrombosis occurred at day 44 in a non-diabetic patient who was still under double antiplatelet therapy. There were one additional TLR and 3 new TVR in the diabetic subgroup. PCI of non-target vessels was performed in 9 patients, 6 diabetics and 3 non-diabetics, and 2 cerebrovascular accidents (CVA) occurred in diabetic patients. The total cumulative incidence of MACE at six months was 2.6% (7/269) with no significant differences (p=0.27) between diabetic (4.2%) and non-diabetic (1.3%) patients.
The 1-year clinical follow-up was completed in 97% of patients. The majority of events occurred between six and 12 months. There were 6 additional deaths (3 cardiac, 2 in diabetics), one NSTEMI (in diabetics), 13 TLR (8 in diabetics), 6 TVR (1 in diabetics), 18 PCI of non-target vessels (5 in diabetics), two CVA (one in diabetics), and no cases of CABG.
The cumulative incidence of clinical events at one year is shown on Table 7.
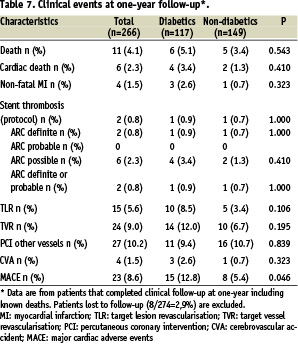
MACE was 8.6% for the total cohort, 12.8% for diabetics and 5.4% for non-diabetics, a difference which reached statistical significance (P=0.046). TLR was also higher for diabetics in comparison with non-diabetics (8.5% vs. 3.4%, p=0.106), albeit non significant.
Stent thrombosis was an infrequent finding in PORTO. Only two patients (one diabetic and one non-diabetic) had stent thrombosis according to the per-protocol definition, as well as to the new “ARC definite” category.18 One of these events was directly associated with premature discontinuation of antiplatelet therapy. There were no “ARC probable”, and six “ARC possible” late stent thromboses, all representing unexplained deaths after 30 days. Five of these patients were off double anti-platelet therapy at the time of the event.
Discussion
To our current knowledge, this registry represents the largest multicentre experience with sirolimus-eluting stents for small coronary arteries, so far published. With 274 patients and 347 stents implanted in 324 target lesions it included 120 (44%) diabetic patients and a large number of small stents and long lesions. The average RVD of this registry is only 2.08 (0.33) mm, which is even below the average RVD of the SES Smart study10 of 2.20mm (0.28), and is thus the smallest RVD so far studied in a clinical trial or registry. The high percentage of diabetics together with the small vessel size makes the population studied in PORTO exceptionally complex.
Since small vessels – in contrast to larger arteries – can accommodate only small amounts of luminal renarrowing19-20, it is important to note that in-stent late luminal loss – the primary end-point of this study – was only 0.07±0.37 mm for the total small vessel population (347 stents). This finding is consistent with the results from the small vessel stratum of the RAVEL trial5 (0.01±0.25 mm), and the RESEARCH registry7 (0.07±0.48 mm). It is also in line with the findings of other randomised clinical trials and registries evaluating the sirolimus-eluting stent in small vessels8-11,15, with in-stent late loss averaging from 0.12 to 0.25 mm.
In diabetic patients, in-stent late loss was 0.09±0.37 mm and similar to non-diabetics (0.04±0.37 mm). This finding correlates well with results from the DIABETES trial12, (0.09 mm), and the ISAR- DIABETES trial13 (0.19 mm). Of note, in-stent late loss was higher in the diabetic substudy of the SIRIUS trial20 (0.29 mm), but this might have been due to a protocol-mandated high rate of post-dilatation of the proximal stent margin, which was discouraged in our study. Very similar values for in-stent late loss were observed for the smallest stent diameter (2.25 mm) used in PORTO, as well as for stented lengths exceeding 23 mm.
The clinical efficacy of sirolimus-eluting stents was assessed by target lesion or target vessel revascularisation rates. Our study confirms that the reduction of further revascularisation procedures following implantation of sirolimus-eluting stents is preserved in patients with small vessel disease. Despite a high rate of angiographic follow-up (89%), which artificially drives repeat interventions, TLR was only 5.6% for the total population at one year. These findings correlate well with the TLR rate in the SES-SMART10 trial (7.0% at 8-months), with E-SIRIUS8 and C-SIRIUS9 (4.0% at 9 months), and with ISAR-SMART 311 (6.6% at 1-year).
For diabetic patients in PORTO, TLR at 1-year was 8.5%, which again compares well with the TLR rate of 8.8% observed in the diabetic cohort of the RESEARCH registry14 at 1-year, the 6.3% in the DIABETES trial12 at 9-months, and the 6.4% observed in ISAR-DIABETES13.
Safety - Progression of MACE and stent thrombosis
Total MACE, defined as a composite of cardiac death, non-fatal MI, and TLR at 1-year, was 8.6% in our study. There were, however, five additional noncardiac deaths, and four strokes not included in our MACE definition. While the total MACE was also 8.0% in the E-SIRIUS trial8 and 9.3% in the SES-SMART10, total mortality (4.1%) was higher than in previous trials8-11. A possible explanation for the high incidence of MACE might be the complexity of this patient cohort characterised by a high percentage of diabetic patients, as diabetes mellitus is known to have a negative impact on long-term outcome after percutaneous or surgical revascularisations22, in particular in combination with very small target vessels for stenting. Three out of the four strokes occurred in diabetic patients.
Another important aspect of our study is the progression of MACE from six months to one year. In detail, 6-month MACE was only 2.6% (1.1% cardiac death and non-fatal infarction and 0.7% target lesion revascularisation), while the rate observed at 1-year climbed to 8.6%. Not only the importance of a longer follow-up, but also the presence of diabetes should be stressed, as the majority of the late events occurred in the diabetic group.
The re-adjudication of stent thrombosis according to the new ARC definitions resulted in very similar findings as the per-protocol analysis. This is due to the fact that the “per-protocol” and “ARC definite” categories are indeed almost identical. There were 0.8% of patients – i.e., one diabetic and one non-diabetic patient – in this category, the first counting as sub-acute, and the second as late stent thrombosis. While no events were noted for the “ARC probable” category, there were six unexplained death at longer follow-up counted as “ARC possible” stent thromboses. It is, however, unclear if stent thrombosis was indeed the cause of death in these patients. In comparison, the RESEARCH registry14 reported 2.1% stent thrombosis – defined per protocol – within the first month after sirolimus-eluting stent in unselected diabetic patients. This somewhat higher rate may be due to the “all-comer” population studied in RESEARCH.
Study limitations
The main limitation is that PORTO was not a randomised trial. The cohort studied may also not reflect a real-world population as patients with STEMI, NSTEMI, acute coronary syndromes, as well as the angiographic presence of thrombus in the target vessel were excluded by protocol. Therefore, the study results do only apply to stable clinical situations. The small number of patients treated with insulin in the study, precluded further subgroup analysis in this important subset of diabetic patients.
Conclusions
This large multicentre registry demonstrated the efficacy and safety of sirolimus-eluting stents for routine percutaneous coronary interventions of very small native coronary arteries.
Acknowledgements
We are very grateful to Dr. Hans-Peter Stoll and David Barrow for revising the manuscript and writing assistance and to Dr. Pedro Araújo Gonçalves for helping with the data analysis.
Appendix
Study organisation
Scientific Committee - R. Seabra-Gomes (Principal Investigator), J. Eduardo de Sousa (co-Principal Investigator), Amanda Sousa, Helder Pereira (Chairman, Working Group of PCI, PSC), Lino Gonçalves (Director, Data Collection Centre, PSC)
Steering Committee - Scientific Committee and one representative from each participating Hospital
Clinical Events Committee - Damião Cunha, Maria Júlia Maciel, João Morais, António Nunes Diogo, Paulo Pinho
Data Coordinating Centre - Eminent-PPD (electronic CRF)
Angiographic Core Laboratory - Cardialysis (Rotterdam)
Monitoring and Data Analysis - KeyPoint (Portugal) and P.A. Gonçalves
Participating centres and investigators
Hospital Santa Cruz (Carnaxide): R. Seabra-Gomes, R. Teles, M. Almeida, F.P. Machado; Instituto Dante Pazzanese (S. Paulo, Brazil): J.E. Sousa, A. Sousa, Áurea Chaves; Hospital Garcia de Orta (Almada): Helder Pereira, Rui Caria; Hospital Fernando Fonseca (Amadora): Pedro Farto e Abreu, José Loureiro; Hospital Pulido Valente (Lisboa): Manuela Adão, Pedro Cardoso; Centro Hospitalar de Coimbra: A. Leitão Marques, Hilário Oliveira, V. Matos; Centro Hospitalar de Vila Nova de Gaia: V. G. Ribeiro, M. Gonçalves, P. Braga; Hospital Curry Cabral (Lisboa): L. Mourão, Ramiro Sá de Carvalho, L. Brizida; Hospital S. Bernardo (Setúbal): Ricardo Santos; SMIC (Porto): Henrique Carvalho, Paulino Sousa.

